 Review Article
Review Article
Polyvinyl Chloride (PVC) as a Robust Solid Phase for Efficient Metal Extraction from Aqueous Samples: A Comprehensive Review and Future Perspectives
Adrielli Cristina Peres da Silva, Valber Albuquerque Pedrosa, Margarida Juri Saeki and Gustavo Rocha de Castro*
Department of Chemistry and Biochemistry, Institute of Biosciences of Botucatu-UNESP, Brazil
Gustavo Rocha de Castro, Chemistry and Biochemistry Department, Institute of Biosciences of Botucatu, Brazil
Received Date:November 29, 2023; Published Date:December 13, 2023
Abstract
Polyvinyl chloride (PVC) is a widely used polymer with diverse applications, and enhancing its surface properties has been a subject of considerable research. This review explores wet methods in which surface modification occurs through classic chemical reactions in the presence of solvents, with a particular emphasis on the pivotal role played by the chlorine atom. Dechlorination emerges as a crucial pathway for numerous surface modification reactions, facilitating the attachment of diverse molecules to the surface. Depending on the nature of these molecules, the surface exhibits varying degrees of hydrophilicity or hydrophobicity. These insights contribute to a comprehensive understanding of PVC surface engineering for applications in metal adsorption and removal from aqueous environments.
Keywords:Ligand molecule; Toxic solvent; Environmental; PVC; Heavy metal
Introduction
The population growth, along with the demand for manufactured goods, ends up exerting pressure on productive sectors. These sectors, aiming for greater profit and productivity, seek to meet the demand, but still rely on unsustainable means today. Such ways (of meeting demand at any cost) no longer fit in this modern society, which already suffers from serious problems related to contamination of environmental compartments. Water resources, in particular, serve as one of the main receptors for both organic and inorganic substances, such as heavy metals, for example.
Water, as an indispensable component for sustaining life and ecosystems, faces an escalating threat due to the pervasive contamination by heavy metals. Anthropogenic activities, spanning industrial processes, agricultural practices, and urban development, have significantly contributed to the release of metals such as lead, copper, mercury, cadmium, and arsenic into aquatic environments [1-5]. The consequences of this contamination extend beyond the immediate aquatic ecosystems, impacting the health and wellbeing of both wildlife and human populations. Heavy metals exhibit persistence, accumulating in water bodies over time, and their toxic effects can lead to severe ecological disruptions, posing serious risks to biodiversity and public health [1,2,4-6]. Metal pollutants, once introduced into aquatic ecosystems, undergo complex interactions with sediments and biota, leading to bioaccumulation and biomagnification within the food web [7,8].
Every day, the need to improve our technologies becomes more evident, whether to reduce the amount of contaminants in effluents or to treat already contaminated water resources. Up to now various techniques have been employed for metal removal such as liquid-liquid extraction [9,10], ion exchange, which involves the exchange of metal ions with similarly charged ions on a solid resin and adsorption onto diverse materials such as activated carbon, zeolites, and bio-sorbents, exploiting their high affinity for heavy metals in a solid-phase extraction process (SPE) [11-15].
Solid-phase extraction involves the use of sorbents, such as activated carbon, zeolites, or specialized resins, to selectively adsorb metal ions from the aqueous phase. This technique is characterized by its versatility, allowing for the adjustment of sorbent material and properties to suit the specific requirements of the metal contaminants and the unique composition of the water sample. SPE offers advantages such as high selectivity, rapid kinetics, and the potential for automation, making it a preferred method in environmental analysis. The sorbed metals can then be eluted from the solid phase for subsequent analysis, providing a concentrated and purified sample for accurate determination. The continued refinement and application of solid-phase extraction techniques underscore their pivotal role in the arsenal of methods employed for the efficient removal of heavy metals from aqueous matrices.
Considering this context, many materials have been developed in recent decades aiming at the removal/adsorption of metallic species from aqueous samples. Among these, synthetic materials (based on silica and cellulose) can be highlighted, which have been surface-modified to increase their efficiency and/or adsorption capacity [11,16], as well as natural materials or biosorbents [13,17,18]. The first type has advantages such as high adsorption capacity and the possibility of developing an adsorbent for a specific target. However, it comes with a high cost, and modification reactions produce toxic residues. Biosorbents are mostly sustainable, have lower production costs, but may undergo degradation. The largescale production of biosorbents is conditioned by the availability of raw materials. Thus, in pursuit of contemporary concepts and more sustainable practices, numerous researchers have explored the application of a familiar material, polyvinyl chloride (PVC), as a starting point for the development of new adsorbents [19-22] or its use without previous treatment.
Polyvinyl chloride (PVC) is a widely used polymer in the plastics industry, ranking among the most globally produced polymers. The annual production of PVC in 2016 was approximately 61 million metric tons, and it is estimated that this market will continue to grow in the coming years [23]. Its versatility, durability, and resistance to various elements make it a popular material in a variety of applications, including pipes, profiles, laminates, films, and medical products. PVC’s popularity can be attributed to its exceptional properties, contributing to its widespread global use. Its versatility stems from the material’s ability to be rigid or flexible, depending on the desired application. Additionally, PVC exhibits excellent chemical resistance, making it suitable for applications where exposure to various substances is a concern. Its durability and low maintenance requirements further enhance its appeal, especially in construction and infrastructure projects.
However, the large-scale production and extensive use of PVC raise environmental concerns. PVC manufacturing can involve the emission of toxic compounds, such as chlorine gas, and the generation of hazardous waste. Additionally, improper PVC waste management can contribute to environmental problems, such as soil and water contamination [24,25]. As of now, predominant approaches to tackle the PVC waste dilemma encompass mechanical recycling (25.5%), incineration (9.3%), and landfill disposal (36.0%) [26,27]. Nevertheless, the mechanical recycling process is still in its early stages, mainly applicable to PVC waste that has been separated at the source. Both incineration and landfill disposal of PVC waste commonly lead to the release of perilous compounds to the environment.
Discussions on sustainability in the PVC industry have intensified due to environmental concerns. The search for more eco-efficient alternatives, the implementation of recycling practices, and the development of cleaner production methods are focal points to mitigate the negative impacts associated with PVC production and disposal. In this context, the integration of PVC into solid-phase extraction strategies offers an innovative and eco-efficient approach, contributing to the pursuit of more sustainable solutions at the intersection of the chemical industry and environmental preservation.
Characteristics of PVC
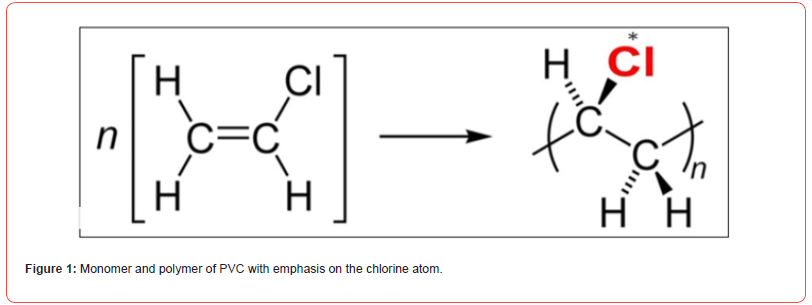
A principal thermoplastic material with a wide range of applications, polyvinyl chloride relies on essential components derived from oil and salt. The vinyl chloride monomer (VCM) is created through the combination of ethylene (sourced from oil) and chlorine (generated via the electrolysis of saltwater). Monomer molecules undergo polymerization to produce PVC resin, to which specific additives are introduced for the formulation of a tailored PVC compound. Figure 1 depicts a scheme representing the monomer and its corresponding polymer.
The importance of the chlorine atom lies in the fact that dechlorination is the pathway through which a significant portion of surface modification reactions occurs [19-21,28]. Consequently, various types of molecules can be attached to the surface. Depending on these molecules, the surface can become either more hydrophilic or hydrophobic. Thus, for studies where PVC will be applied in the adsorption/removal of metallic species from aqueous samples, it is necessary that its surface be somehow modified to make it more hydrophilic. This will enhance its interaction with target metals, which are typically present in their hydrated ionic form [21, 29]. Some treatments, such as hydrothermal treatments, may increase the hydrophobicity of the surface, which is consistent with a reduction in its adsorption capacity once metal species are in its hydrated form.
PVC Surface Modification and Adsorption of Metal Ions
The modification of PVC surface can be achieved in different ways, where the goal is often to introduce new functionalities to the polymer surface [27]. Among the main treatments performed, plasma treatments stand out, in which energetic particles (electrons, radicals, ions) can interact and modify the polymer surface without changing their bulk properties [20,30-32]. Other methods used in modifying the surface of PVC involve the use of high-energy irradiation, ultraviolet and hydrothermal treatment [20]. Xu et al. [27] conducted a surface modification of PVC (obtained from pipes) using a two-step hydrothermal method at a relatively low temperature of 250°C. In the second step, the material underwent sulfonation reaction with sulfuric acid and was subsequently utilized for the adsorption of Cu(II) and Cr(VI) ions. The findings propose an electrostatic interaction mechanism for Cu(II) ions and indicate a distinct mechanism for Cr(VI) ions. Other methods applied in PVC modification include classical approaches, where chemical reactions between a substance and the PVC surface take place in the presence of a solvent.
This last method has been widely used because it does not require sophisticated equipment and yields satisfactory results in relation to the final product obtained [21,22,33,34]. Mbarki et al. [33] conducted adsorption studies of Cr3+, Co2+, Cd2+, and Pb2+ using PVC modified with amino (diethylenetriamine) and dichlorodiethyl ether groups and achieved a high removal percentage using the batch method. The confirmation of the anchoring of the molecule of interest can be done through different techniques, with infrared spectroscopy being the most commonly used [21,33-35]. As the modification reactions often occur at the chlorine atom via a nucleophilic substitution mechanism, it is possible to monitor the progress of the reaction through the band located in the 700 cm-1 region of the spectrum, which may be attributed to the axial deformation of the C-Cl bond [21,33,34]. A similar study was conducted by Mbarki & Ammari [34], starting with commercial PVC (pipes and tubing) and emphasizing the need for a pre-treatment step to eliminate additives. Despite the promising results and the use of waste as raw material, the synthesis and modification stages can also generate toxic residues when solvents such as dimethylformamide (DMF), tetrahydrofuran (THF), and others are employed. According to Yousif et al. [36], the surface modification of PVC through chlorine atom substitution, depending on the anchored ligand, may lead to an enhancement in the photostability of the polymer. These characteristics, when combined, can improve the applicability and lifespan of the adsorbent.
The widespread use of highly toxic solvents in producing environmentally oriented adsorbent materials demands careful scrutiny. While these materials are designed to address specific issues, it is imperative to recognize and proactively manage the potential environmental and health challenges that may emerge during their creation. Thus, some authors proposed a surface modification route for PVC using a mixture of ethanol/water as a solvent. Castro et al. [21] anchored the molecule of the ligand 4-amino-5-hydrazino-1,2,4-triazole-3-thiol onto the surface of PVC through a simple and environmentally sustainable route. The results imply that the reaction took place through the SH group via an SN2 mechanism, consistent with previous studies on PVC modification [21,37]. Beyond the reaction pathway, the involvement of amine groups in the sorption process of metallic species was notable, presumably through coordination with the pair of free electrons [21].
These studies emphasize the vital role played by organically anchored groups in the adsorption and removal of metallic species from aqueous samples. Depending on the nature of the anchored groups, the material’s affinity for specific species can vary significantly. Figure 2 provides a conceptual illustration of diverse bonding possibilities and mechanisms that may occur during the sorption process.
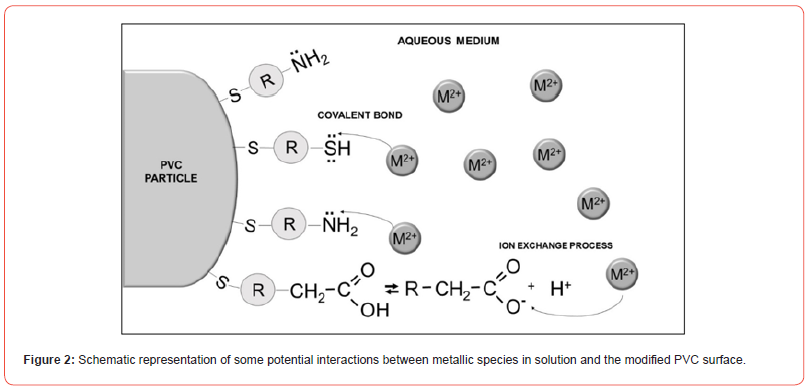
Through the visualization of this image, it becomes evident why PVC needs to be modified to effectively engage in the complexation of metallic species. The surface modification by molecules containing Lewis bases not only enhances its hydrophilic nature but also imparts an affinity for metallic species (Lewis acids).
Among the various PVC-based materials used in the removal of metallic species from water samples, composites (formed by the combination of two or more materials with distinct properties) also stand out. Rajaei et al. [38] produced a nanocomposite membrane using PVC and magnetite nanoparticles, which was applied in the removal of Cd(II) and Pb(II) ions from aqueous samples. The results indicated an increase in the surface area after modification with magnetite nanoparticles. The enhancement of the surface area in adsorbent materials is always beneficial when the goal is the removal/adsorption of metallic species, allowing greater interaction with the liquid phase and generally resulting in a higher adsorptive capacity. In a similar vein, Khan et al. [39] developed a composite material based on PVC and graphene oxide modified with p-Phenylenediamine, which was employed for the removal of Pb(II) ions from aqueous samples. The material modification was monitored by infrared spectroscopy, revealing the reduction of -COOH, -OH, -C-O-C- functionalities on the graphene oxide surface and the emergence of bands associated with -C-NH2. The specific surface area was also enhanced (312 m2 g-1), indicating an improvement in adsorptive properties.
Youness et al. [40] fabricated a membrane from PVC modified with triethylenetetramine using the electrospinning method. Binding mechanisms of lead and triethylenetetramine were investigated by UV-visible spectrophotometry, and the results indicate the formation of a highly stable complex with a 1:1 stoichiometry. In addition to the observed high adsorption capacity for PVC-based materials, these adsorbents/membranes also have the potential for reuse through multiple cycles of adsorption/ desorption without significant loss of their effectiveness [41,42]. This characteristic is of utmost importance in adsorbent materials as it maximizes their utilization. The loss of adsorptive capacity is observed in most modified materials and may result from the elution process of metal species, where acidic solutions are often used. These solutions can cause oxidation on the surface and/or anchored molecules.
Based on the aforementioned materials, the following table (Table 1) highlights the adsorption capacity of some PVC-based materials used in the removal of metallic species from aqueous samples. These materials include both PVC and composite materials. Adsorption capacity is crucial for assessing the effectiveness of these materials in retaining metal ions, serving as a significant indicator for practical applications in water treatment and environmental remediation.
Table 1:Adsorption capacities of various PVC-based materials for heavy metal removal.
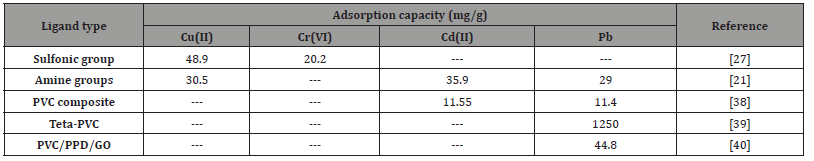
According to the data presented in Table 1, it is noticeable that the obtained values for the adsorption capacity of different metallic species are very similar to those obtained when using more commonly applied materials for this purpose, such as silica and cellulose, for example. Considering the possibility of working with recyclable material through a solvent-free route, its advantages become evident.
Final Consideration
In conclusion, the comprehensive review on the use of PVC in solid-phase extraction processes highlights the duality of this polymeric material. While PVC is extensively produced and globally utilized, raising significant environmental concerns, its application in solid-phase extraction methods emerges as a promising solution to mitigate the associated negative impacts. The ability of PVC to efficiently adsorb metal ions and organic pollutants, coupled with its chemical resistance and durability, makes it an ideal candidate for reuse in extraction processes, providing not only environmental benefits but also extending the lifespan of this material. The reuse of PVC not only aligns with the principles of the circular economy, reducing reliance on virgin resources but also represents a concrete step toward more sustainable practices in plastic waste management. In this context, the integration of PVC into solidphase extraction strategies offers an innovative and eco-efficient approach, contributing to the pursuit of more sustainable solutions at the intersection of the chemical industry and environmental preservation.
Future perspectives in this realm necessitate ongoing research and development to optimize PVC-based solid-phase extraction methods. This involves exploring novel modifications to enhance PVC’s adsorption capacity, investigating sustainable approaches for PVC waste management, and integrating advanced technologies to streamline the overall process. Collaborative efforts among the chemical industry, environmental scientists, and policymakers are paramount for establishing guidelines and regulations that promote the responsible use and disposal of PVC. The development of cleaner production methods and the widespread implementation of recycling practices will further contribute to reducing the environmental footprint associated with PVC.
In essence, the utilization of PVC in solid-phase extraction not only addresses the immediate need for efficient metal removal but also aligns with contemporary concepts of sustainability. As we progress, the exploration of PVC’s potential in environmental applications holds promise for fostering a more harmonious relationship between industrial progress and ecological preservation, paving the way for a greener and more sustainable future.
Acknowledgment
The authors thank CNPq for the scholarship and project support (Proc. 312361/2021-1).
Conflict of Interest
All authors state that there is no conflict of interest.
References
- Tchounwou PB, Tedjou CG, Patlolla AK, Sutton DJ (2012) Heavy Metals Toxicity and the Environment. Molecular, Clinical and Environmental Toxicology 101(1): 133-164.
- Briffa J, Sinagra E, Blundell R (2020) Heavy Metals Toxicity and the Environment. Heliyon 6(9): 1-26.
- Minello MCS, Paçó AL, Martines MAU, Caetano L, Santos A, et al. (2009) Sediment grain size distribution and heavy metals determination in a dam on the Paraná River at Ilha Solteira, Brazil. Journal of Environmental Science and Health, Part A 44(9): 861-865.
- Hou D, O’Connor D, Igalavithana AD, Alessi DS, Luo J, et al. (2020) Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nature Reviews Earth & Environment 1(1): 366-381.
- Rashid A, Schutte BJ, Ulery A, Deyholos MK, Sanogo S, et al. (2023) Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 13(6): 1-30.
- Neves RCF, Moraes PM, Ferrari JEM, Lima PM, Santos FA, et al. (2011) Levels of copper in Nile tilapia from Brazil. Food Additives & Contaminants: Part B 4(4): 238-243.
- Nnaji ND, Onyeaka H, Miri T, Ugwa C (2023) Bioaccumulation for heavy metal removal: a review. SN Applied Sciences 5(125): 1-12.
- Soliman MM, Hesselberg T, Mohamed AA, Renault D (2022) Trophic transfer of heavy metals along a pollution gradient in a terrestrial agro-industrial food web. Geoderma 413(1): 1-12.
- Li Z, Zhang Z, Smolders S, Li X, Raiguel S, et al. (2019) Enhancing Metal Separations by Liquid–Liquid Extraction Using Polar Solvents. Chemistry A European Journal 25(39): 9197-9201.
- Rodrigues GD, Silva MCH, Silva LHM, Paggioli FJ, Minim LA, et al. (2008) Liquid–liquid extraction of metal ions without use of organic solvent. Separation and Purification Technology 62(1): 687-693.
- Ferreira G, Caetano L, Castro RSD, Padilha PM, Castro GR, et al. (2011) Synthesis, characterization, and application of modified silica in the removal and preconcentration of lead ions from natural river water. Clean Technologies and Environmental Policy 13(1): 397-402.
- Ghasemi H, Afshang M, Gilvari T, Aghabarari B, Mozaffari S, et al. (2023) Rapid and effective removal of heavy metal ions from aqueous solution using nanostructured clay particles. Results in Surfaces and Interfaces 10(1): 1-10.
- Karimi F, Ayati A, Tanhaei B, Sanati AL, Afshar S, et al. (2022) Removal of metal ions using a new magnetic chitosan nano-bio-adsorbent; A powerful approach in water treatment. Environmental Research 203(1): 1-8.
- Mahmud HNME, Huq AKO, Yahya RB (2016) The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: a review. RCS Advances 6(1): 14778-14791.
- Zhao Y, Pan Y, Liu W, Zhang L (2015) Removal of Heavy Metal Ions from Aqueous Solutions by Adsorption onto ZIF-8 Nanocrystals. Chemistry Letters 44(6): 758-760.
- Daochalermwong A, Chanka N, Songsrirote K, Dittanet P, Niamnuy C, et al. (2020) Removal of heavy metal ions using modified celluloses prepared from pineapple leaf fiber. ACS Omega 5(10): 5285-5296.
- Priya AK, Gnanasekaran L, Dutta K, Rajendran S, Balakrishnan D, et al. (2022) Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 307(4): 1-12.
- Silva ACP, Jorgetto AO, Wondracek MHP, Saeki MJ, Schneider JF, et al. (2015) Characterization of corn (Zea mays) leaf powder and its adsorption properties regarding Cu(II) and Cd(II) from aqueous samples. Bioresource 10(1): 1099-1114.
- Wankasi D, Dikio ED (2014) Polyvinyl chloride waste as an adsorbent for the sorption of Pb2+ from aqueous solution. Journal of Chemistry 2014(1): 1-7.
- Asadinezhad A, Lehocký M, Sáha P, Mozetic M (2012) Recent Progress in Surface Modification of Polyvinyl Chloride. Materials 5(12): 2937-2959.
- Silva ACP, Jorgetto AO, Wondracek MHP, Saeki MJ, Pedrosa VA, et al. (2021) A global pollutant (PVC-polyvinyl chloride) applied as heavy metal binder from aqueous samples: green principles from synthesis to application. Environmental Technology 43(24): 3742-3754.
- Uyama Y, Kato K, Ykada Y (1998) Surface Modification of Polymers by Grafting. Advances in Polymer Science 137(1): 1-39.
- Lima R, Silva J, Vasconcelos M, Junior CAC, Pinto JC, et al. (2023) Bibliometric survey of the PVC production-Part I: the. Polímeros: Ciência e Tecnologia 33(2): 1-18.
- Barili S, Bernetti A, Sannino C, Montegiove N, Calzoni E, et al. (2023) Impact of PVC microplastics on soil chemical and microbiological parameters. Environmental Research 229(1): 1-12.
- Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Science Advances 3(7): 1-5.
- Yu J, Sun L, Ma C, Qiao Y, Yao H, et al. (2016) Thermal degradation of PVC: A review. Waste Management 48(1): 300-314.
- Xu X, Zhu D, Wang X, Deng L, Fan X, et al. (2022) Transformation of polyvinyl chloride (PVC) into a versatile and efficient adsorbent of Cu (II) cations and Cr (VI) anions through hydrothermal treatment and sulfonation. Journal of Hazardous Materials 423(1): 1-11.
- Edraki M, Sheydaei M, Alinia-Ahandani E, Nezhadghaffar-Borhani E (2021) Polyvinyl chloride: chemical modification and investigation of structural and thermal properties. Journal of Sulfur Chemistry 42(4): 397-409.
- Rehman M, Rehman W, Waseem M, Hussain S, Haq S, et al. (2019) Adsorption mechanism of Pb2+ ions by Fe3O4, SnO2, and TiO2 Environmental Science and Pollution Research International 26(19): 19968-19981.
- Zhianmanesh M, Gilmour A, Bilek MMM, Akhavan B (2023) Plasma surface functionalization: A comprehensive review of advances in the quest for bioinstructive materials and interface. Applied Physics Reviews 10(1): 1-59.
- Khorasani MT, Mirzadeh H (2006) Effect of oxygen plasma treatment on surface charge and wettability of PVC blood bag-In vitro assay. Radiation Physics and Chemistry 76(6): 1011-1016.
- Sant’Ana PL, Bortoleto JRR, Cruz NC, Rangel EC, Durrant SF, et al. (2020) Surface functionalization of polyvinyl chloride by plasma immersion techniques. Polímeros 30(4): 1-7.
- Mbarki F, Ammari F, Amor ABH, Meganem F (2017) Functional groups grafted on poly (vinyl chloride) – evaluation of new modified polymers in metal ions adsorption. Polimery 62(2): 109-117.
- Mbarki F, Ammari F (2021) Chemical modification of commercial and recovered poly (vinyl chloride) with amino groups – adsorption of heavy metals (Cr(III), Pb(II), Cd(II) and Co(II) by modified PVC polymers. Journal Marocain de Chimie Hétérocyclique 20(2): 80-94.
- Jorgetto AO, Pereira SP, Silva RIV, Saeki MJ, Martines MAU, et al. (2015) Application of Mesoporous SBA-15 Silica Functionalized With 4-amino-2-Mercaptopyrimidine for the Adsorption of Cu(II), Zn(II), Cd(II), Ni(II), and Pb(II) From Water. Acta Chimica Slovenica 62(1): 111-120.
- Yousif E, Hameed A, Rasheed R, Monsoor H, Farina Y, et al. (2010) Synthesis and photostability study of some modified poly(vinyl chloride) containing pendant benzothiazole and benzimidozole ring. International Journal of Chemistry 2(1): 65-80.
- Lu L, Li W, Cheng Y, Liu M (2023) Chemical recycling technologies for PVC waste and PVC-containing plastic waste: A review. Waste Management 166(1): 245-258.
- Rajaei GE, Khalili-Arjaghi S, Fataei E, Sajjadi N, Kashefi-Alasl M, et al. (2020) Fabrication and characterization of polymer-based nanocomposite membrane modified by magnetite nanoparticles for Cd2+ and Pb2+ removal from aqueous solutions. Comptes Rendus Chimie 23(9): 563-574.
- Khan ZU, Khan WU, Ullah B, Ali W, Ahmad B, et al. (2021) Graphene oxide/PVC composite papers functionalized with p-Phenylenediamine as high-performance sorbent for the removal of heavy metal ions. Journal of Environmental Chemical Engineering 9(1): 1-12.
- Youness F, Jaafar A, Tehrani A, Bilbeisi RA (2022) Functionalised electrospun membranes (TETA-PVC) for the removal of lead(II) from water. RCS Advances 12(1): 24607-24613.
- Hezarjaribi M, Bakeri G, Sillanpaa M, Chaichi MJ, Akibari S, et al. (2021) Novel adsorptive PVC nanofibrous/thiol-functionalized TNT composite UF membranes for effective dynamic removal of heavy metal ions. Journal of Environmental Management 284(1): 1-16.
- Sellami F, Kebiche-Senhadji O, Morais S, Fatyeyeva K (2022) PVC/EVA-based polymer inclusion membranes with improved stability and Cr(VI) extraction capacity: Water plasticization effect. Journal of Hazardous Materials 436(1): 1-18.
-
Adrielli Cristina Peres da Silva, Valber Albuquerque Pedrosa, Margarida Juri Saeki and Gustavo Rocha de Castro*. Polyvinyl Chloride (PVC) as a Robust Solid Phase for Efficient Metal Extraction from Aqueous Samples: A Comprehensive Review and Future Perspectives. Mod Concept Material Sci. 5(4): 2023. MCMS. MS.ID.000616.
-
Ligand molecule, Toxic solvent, Environmental, PVC, Heavy metal, Productive sectors, Organic, Inorganic, Metal pollutants, Solid phase
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






