 Short Communication
Short Communication
On Phase Behavior of Mixtures of Non-Mesogenic Metal Complexes and Their Mesogenic Parent Ligand
H Hakemi, University of Plastic Liquid Crystal Technology, Italy.
Received Date:July 03, 2025; Published Date:July 14, 2025
Abstract
The phase diagrams of mixtures of two non-mesogenic alkyl/alkoxy-zobenzene palladium (L1Pd-acac) and alkyl/alkoxy-zobenzene mercury (L1HgCl) metal complexes with their mesogenic parent ligand were studied in order to evaluate the phase behavior of their mixutres for potential application. Although the studied metal complexes belonging to the metallomesogen (MOM) class were not mesogens, but their parent ligand HL1 exhibited an enantiotropic nematic phase. The phase behavior of L1Pd-acac/HL1 mixtures indicated mesogenic miscibility where the nematic phase of ligand extended through the whole concentration range of their phase diagram. This means that, although L1Pd-acac was not mesogen, the L1Pdacac/ HL1 mixtures could be utilized for as potential mixture for application. In contrast, the L1HgCl/HL1 mixtures were only partially miscible, where the HL1 nematic phase was only stable at the low concentration range of L1HgCl.
Keywords: Metal-complex; Mesogen; Ligand; Phase diagram; Nematic; Miscibility.
Introduction
The organometallic liquid crystals known as “metallomesogens” (MOMs) are organic mesogenic structures including the metal-complex centers. The presence of metal complexation in the chemical structures of liquid crystals could add many physical and optical features that are not present in organic mesogenic materials. Such features have been the main motivation for potential applications of MOMs, which offers the possible combination of liquid crystal supramolecular order with new properties of metal- complexation. In the past few decades, in addition of extensive studies on chemistry and chemical structures, MOMs have been also studied as potential materials for technological applications [1-8]. There exist some scientific and patent literature on potential applications of MOMs as photo and electro luminescence [9-13], magnetic and electric properties [14-18], as well as more general dichroic dyes, non-linear optics, thermal recording, thermo-chromism, optical filters, photo-sensing, laser addressing, optical and thermal recording, polarizing flms, radiation absorbing films, ferroelectricity, ferromagneticity, electro-conductivity, reaction catalysts, intermediates, ink jet and security printing, medicinal and agricultural components [19-28].
In spite of extensive literature on chemical structure, characterization
and potential applications, MOMs have not yet been devel
oped for commercial utilization even in simplest guest-host electro-
optical systems. In fact, the major drawbacks of existing single
component MOM materials have been due to:
a) high crystal-mesogenic and mesogenic-isotropic transition
temperatures
b) small mesophase range
c) risk of decompositions at high temperatures
d) low chemicl stability.
Therefore, in order to develop qualified MOMs for commercial application, either to apply themolecular engineering approach to synthsize a totally new single-component MOM chemical structure with accessible and wide range mesogenic phase or to utilize a more practical approach of MOM/MOM and MOM/ligand mixing strategy to develop materials with low transition temperature, eutectic behavior and wide-range and accessible mesogenic phase. The successful proof of the mixing approach has been provided in our previous studies [29-33] on the phase behavior of MOM/MOM, MOM/ligand, as well as their mixtures with commercial liquid crystals, which provided mesogenic miscibilities, eutectic behavior and accessable temperature range that qualified MOMs for potential application.
However, as there are some MOM structures that do not exhibit liquid crysralline phase, until now their phase behavior of by mixing approach have not yet been studied. As the first experimental attempt, in this work we utilized two none-mesogenic L1Pd-acac and L1HgCl metal complexes based on alkyl/alkoxy-azobenzene structures and studied their phase behavior in mixtures with their mesogenic parent ligand HL1. Accordingly, in order to determine the effect of none-mesogenic metal-complexes on liquid crystalline phase of mesogenic parent ligand, we studied the crystal, mesogenic and isotropic transition temperatures within the total composition range of their phase diagrams. The results of this experimental study are described in the following sections.
Materials and Methods
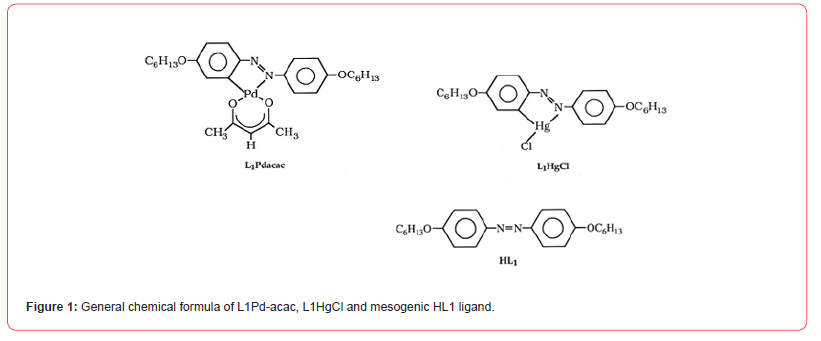
In Figure-1, we present the chemical structures of these non-mesogenic mono-ligand metal complexes and their mesogenic parent ligand (HL1), based on the common class of alkyl/ alkoxy-azobenzene palladium (L1Pd-acac) and mercury (L1HgCl) metal-complexes. The original synthetic procerdures of these metal- complexes and mesogenic ligand have been reported elsewhere [34-36], where they were synthesized through incorporation of ligand in Pd and Hg metal-complex chemical structures.
The transition temperatures of all materials and their mixtures were determined by a Perkin Elmer DSC7 Differential Scanning Calorimeter (DSC) and the phase behaviors were evaluated by Nikon Eclipse-50i polarizing optical microscope (OM) equipped with a temperature-controlled Mettler FP5 microscopic hot stage. The transition temperature of all mixtures were carried out by direct weiging of the components in DSC pan through repeated heating and cooling modes with scanning rates of 10 °C/min and 5 °C/min, respectively, until there were no change in their thermograms.
Results and Discussion
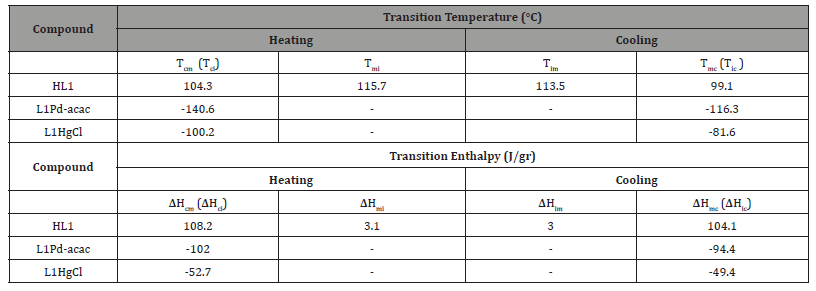
In Table-1, we tabulate the transition temperatures and enthalpies of HL1 ligand, L1Pd-acac and L1HgCl metal-complexes, including crystal-nematic (Tcn), nematic-isotropic (Tni) and crystal-isotropic (Tci) on heating mode, as well as the isotropic-nematic (Tin), nematic-crystal (Tnc) and isotropic-crystal (Tic) on cooling mode. The studied non-mesogenic L1Pd-acac and L1HgCl metal-complexes belong to the metallomesogens (MOM) class. In our previous studies, we reported on the phase transitions of other mesgogenic alkyl/alkoxy-azobenzene MOM mixtures with other terminal groups than the utilized -O-C6H13 in this study [29-33].
According to Table-1, the HL1 ligand exhibits an enantiotropic nemtic phase, high transition temperatures, two crystalline transitions and nematic range of 11.5°C and 14.2°C on heating and cooling modes, respectively. Both none mesogenic L1Pd-acac and L1HgCl complexes also exhibit high transion temperatures in the range of 100°C. With respect to transition enthalpies tabulated in Table-1, both HL1 ligand and L1Pd-acac exhibit high crustalline enthalpies in the range of 100 J/gr, whereas the crystalline enthalpy of L1Hg- Cl is around 50 J/gr, indicating its low crystal structure. The phase behavior of HL1/L1Pd-acac and HL1/L1HgCl binary mixtures are presented and discussed in the following sections.
Table 1:Transition temperatures (top) and enthalpies (bottom) of metal-complexes & patrent ligand.
L1 / L1Pd-acac Phase Behavior
In Figure-2, we present the phase diagram of nematic HL1 and none mesogenic L1Pd-acac mixrutres at heating and cooling modes within the whole composition range of the components. Accordingly, the phase diagram HL1/L1Pd-acac exhibits the extension of HL1 nematic phase up to 97% concentration of L1Pd-acac complex. The extention of nematic phase and quasi-linear trends of nematic- isotropic Tni and Tin transitions within the totlal phase diagrams of HL1/L1Pd-acac mixtures indicate the nematic miscibility of the components at both heating and cooling modes. Similar Tni and Tin transition trends and mesogenic miscibilities have been repored in the phase diagrams of metallomesogens and parent ligands [37], indicating according to Figure-2, the two-phase region of coexisting nematic and isotropic phases exhibit noticable Tni at 106.4°C and Tin at 102.1°C minimums at L1Pd-acac = 50% concentration. With regards to Tcn and Tnc transitions in HL1/L1Pd-acac phase diagrams, it is also noticed that, the mixtures exhibit an apparent eutectic point, particularly on cooling mode at L1Pd-acac=50% contcentration range (see Figure-2). At this concentration range, the nematic stability from 10.2°C on heating mode expands to 25.4C at cooling mode as a result of Tnc super-cooling of the mixture.
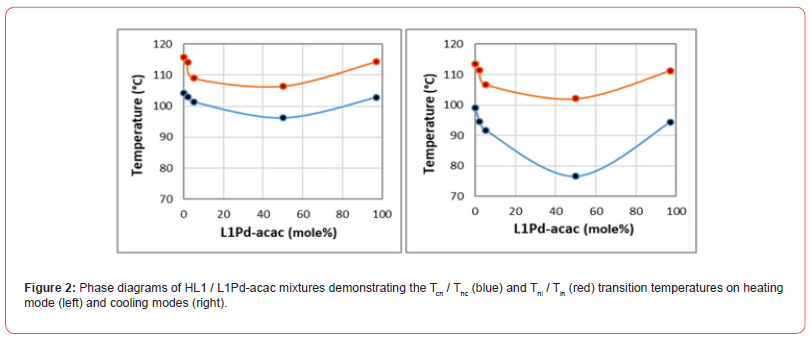
The phase behavior of HL1/L1Pd-acac binary mixtures exhibits sufficient nematic miscibility of the components, where the nematic phase of HL1 ligand persists within the whole concentration range of phase diagram. The eutectic behavior in HL1/L1Pd-acac binary mixture at around 50% concentration of L1Pd-acac on cooling mode and its 25°C nematic stability range also indicates that L1Pd-acac complex behaves as a metallomesogen and the two compnents are also miscible at their crystalline phase. According to Table-1, the crystal-nematic transition enthalpy of HL1 ligand and crystal-isotropic transition enthalpy of L1Pd-acac metal-complex are of the same order of magnitude of around 100 J/gr, which is also a further confirmation of their crystalline compatibility, appearance of eutectic behavior and extension of HL1 nematic range within the whole phase diagram (see Figure-2).
L1 / L1HgCl Phase Behavior
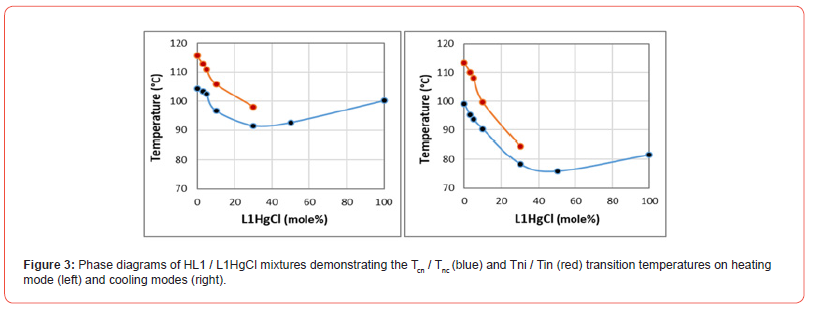
In Figure-3, we provide the phase diagrams and transition temperatures of HL1 / L1HgCl mixtures at heating and cooling modes. Clearly the nematic phase of HL1 ligand in this binary mixtures do not prolong over 30% of L1HgCl concentration range. The Tni and Tin transitions of the mixtures exhibit sharp linear decrease within this concentration range. At above L1HgCl 30% concentration range, the mixtures do not exhibit any mesogenic phase and only exhibits nonmesogenic Tci and Tic transition temperatures.
Also according to Figure-3, the phase behavior of HL1/L1Hg- Cl binary mixture shows that the HL1 and L1HgCl components are not miscible within the whole composition range of their phase diagrams. Clearly, the nematic phase of HL1 decreases sharply, survives only within 30% L1HgCl concentration range and disappears in the resmaing compositions of their phase diagrams on both heating and cooling modes. Another confirmation to immiscibility of HL1 and L1HgCl comonents is the sharper decrease of Tin and Tnc transitions on cooling with respect to Tni and Tcn transitions on heating below the 30% concentration range. Namely, in contrast to HL1/L1Pd-acac mixtures, by increasing the L1HgCl concentration, the nematic instability of HL1 from 14.4°C to 6.5°C at heating mode drops from 11.4°C to 6.0°C at cooling mode (see Figure-3).
As presented in Figure-3, above 30% L1HgCl concentration range the HL1/L1HgCl phase diagrams do not exhibit any nematic phase and the mixtures only exhibit crystalline and isotropic transition temperatures both on heating and cooling modes. Although due to immiscibility of HL1 and L1HgCl the Tci and Tic exhibit apparent minumum transitions within 30-50% L1HgCl concentration range, this does not provide any evidence of eutectic behavior in their phase diagrams.
With respect to Table-1 data, the crystal-nematic transition enthalpy of HL1 ligand and crystal-isotropic transition enthalpy of L1HgCl metal-complex exhibit different order of magnitudes, where the former is 100 J/gr and the latter is 50 J/gr. This difference is also another confirmation of incompatibility of HL1 and L1HgCl crystalline structures, which has resulted to disappearance of HL1 nematic phase of HL1 above the 30% concentration range of L1HgCl in their phase diagrams (see Figure-3).
Conclusion
The phase behavior of non-mesgnic metal-complexes L1Pdacac and L1HgCl in mixtures with their nematic HL1 parent ligands were studied. This sudy was part of the industrial development of metallomesogens and their parent ligands mixtures was to explore and qualify them as model materials for applications in electro-optical devices and liquid crystal displays. The choice of non-mesogenic metal-complexes in this study was to understand if the non-mesogenic metal-complexes could be also qualified as new materials for potential application. In conclusion, we found that the phase behavior of HL1/L1Pd-acac mixtures exhibited satisfactory mesogenic miscibility within the whole phase diagram, where the nematic phase of HL1 ligand survived up to 97% of L1Pd-acac concentration. This interesting outcome indicated that even a non-mesogenic metal-complex could stabilize the mesogenic phase of parent ligand and qualify such model mixture for application. On the other hand, the results in HL1/L1HgCl mixtures were different, where the components were not miscible and the nemtic phase of HL1 ligand did not survive above 30% L1HgCl concentration range. It should be noted that, further systematic studies will be required to provide better understanding of such structural materials for potential application.
Acknowledgment
The authors would like to thank the Electro-Optical Film Group of Snia Riceche, Snia BPD (Fiat Group), Via Pomarico, Pisticci Scalo (MT), Italy, who sponsored and funded the research and development projects of this study under collaboration with Prof. M. Ghedini’s group of the University of Calabria, Rende (CA), Italy, during 1993-1995 period.
References
- J L Serrano (1996) Metallomesogens: Synthesis, Properties and Applications, Wiley VCH, New York.
- B Donnio, D W Bruce, D M P Mingos (1999) Liquid Crystals II Metallomesogens pp. 95.
- B Donnio, D Guillon, R Deschenaux, D W Bruce (2003) Comprehensive Coordination Chemistry II. P. 6.
- R W Date, E F Iglesias, K E Rowe, J M Elliott, D W Bruce (2000) Dalton Transactions pp. 1914-1931.
- D W Bruce, R Deschenaux, B Donnio, D Guillon (2006) Comprehensive Organometallic Chemistry III, Elsevier Oxford U.K pp. 195.
- D W Bruce (2024) Cyanobiphenyls and metallomesogens – where it started and where it went. Liquid Crystals 51(8-9): 1311-1321.
- B Porta, J Khamsi, J C Noveron (2008) Current Organic Chemistry 12: 1298.
- Y Wang, J Shi, J Chen, W Zhu, E Baranoff, et al. (2015C) Chem, 3: 1993.
- Y Wang, J Fan, J Shi, H Qi, E Baranoff, et al. (2016) Dyes Pigments 133: 238.
- M Krikorian, S Liu, T M Swager, J Am (2014) Chem. Soc. 136: 2952.
- H Geng, K Luo, H Cheng, S Zhang, H Ni, et al. (2017) RSC Adv 7: 11389.
- S H Liu, M S Lin, L Y Chen, Y H Hong, C H Tsai, et al. (2011) Org. Electron 12: 15.
- Cristián Cuerva de Alaíz, PhD thesis (2018) Deprtment of Inorganic Chemistry, University of Madrid.
- M Seredyuk, M C Muñoz, V Ksenofontov, P Gütlich, Y Galyametdinov, et al. (2014) J Inorg Chem 53: 8442.
- A J Fitzpatrick, P N Martinho, B J Gildea, J D Holbrey, G G Morgan (2016) Eur J Inorg Chem 2025.
- A Ionescu, N Godbert, A Crispini, R Termine, A Golemme, (2012) Chem 22: 23617.
- P Y S Su, J C W Tseng, K M Lee, J C Wang, I J B Lin (2014) Inorg Chem 53: 5902.
- P Y S Su, S J Hsu, J C W Tseng, H F Hsu, W J Wang, (2016) Eur Chem J 22: 323.
- M Ginord-Godquin, P M Maitlis (1991) Angew Chem Int Engl 30: 375-402.
- D W Bruce (1992) Capter 8, Inorganic Materials, Wiley & Sons Ltd.
- M Blanca Ros (1996) Chapter 11, Metallomesogens - Synthesis, Properties & Applications, J. L. Serrano editor, VCH.
- H Hakemi, WO 95/01410.
- H Hakemi, M Caporusso, M Santangelo, EP 0747 461 A1.
- Roviello R, Centore B, Panunzi H, Hakemi, IT1394422.
- H Hakemi, A Lofer, E Peso; US62/065,805; PCT47459 WO2016/06327.
- H Hakemi, A Lofer, E Peso, D Gal-Fuss; US62/076,002.
- K Binnemans (2010) Inorganic Materials Series, J Wiley pp. 61-133.
- R G imenez, D P Lydon, J L Serrano (2002) Current Opinion in Solid State & Materials Science 6(1): 527.
- H Hakemi (2022) Journal of Materials & Polymer Science 2(2): 1-6.
- H Hakemi (2022) Journal of Materials & Polymer Science 2(4): 1-5.
- H Hakemi (2023) Material Science & Engineering 7(2): 108.
- H Hakemi (2024) Material Science Journal 6(2): 1-6.
- H Hakemi (2024) Phys Chem Journal 4(4): 447-457.
- M Ghedini, D Pucci, F Neve (1996) Chem Commun pp. 137.
- M Ghedini, S Morrone, F Neve, D Pucci (1996) Gazz. Chim. It 126: 511.
- M Ghedini, H Hakemi, D Pucci, I Aiello, S Pane (1994) SniaRicerche Internal Report.
- I I Tokmenko, T A Mirnaya, G G Yaremchu, Z Naturforsch (2011) 66a: 661-667.
-
H Hakemi*. On Phase Behavior of Mixtures of Non-Mesogenic Metal Complexes and Their Mesogenic Parent Ligand. Mod Concept Material Sci. 7(2): 2025. MCMS. MS.ID.000660.
-
Metal-complex, Mesogen, Ligand, Phase diagram, Nematic, Miscibility, Optical features, Ferroelectricity, Radiation, Temperatures
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






