 Research Article
Research Article
A Zirconium-Based Nanocoating for Corrosion Protection of the AA1230 as Clad Material for the AA2024-T3 Alloy assisted by PEG400
P K Lacava1, LL Kuhn1, E L Schneider2*, J Zoppas-Ferreira1
1LACOR - Laboratório de Corrosão, Proteção e Reciclagem de Materiais, Departamento de Engenharia de Materiais, Programa de Pós-Graduação em Engenharia de Minas, Metalúrgica e de Materiais – PPGE3M, Universidade Federal do Rio Grande do Sul – UFRGS
2LACAR - Laboratório de Caracterização de Materiais, Departamento de Engenharia de Materiais, Programa de Pós-Graduação em Engenharia de Minas, Metalúrgica e de Materiais – PPGE3M, Universidade Federal do Rio Grande do Sul – UFRGS
Eduardo Luis Schneider, Departamento de Engenharia de Materiais – DEMAT, Universidade Federal do Rio Grande do Sul – UFRGS, Brazil
Received Date: May 16, 2025; Published Date: July 21, 2025
Abstract
The corrosion protection of AA2024-T3 aluminum alloy, widely used in aerospace applications, remains a critical challenge due to its susceptibility to localized corrosion. The aluminum cladding layer from the 1XXX series (Alclad) provides a primary physical defense for the 2024 alloy, but its vulnerability to wear and damage from debris makes the application of supplementary chromate-based surface treatments, such as BONDERITE® M-CR 1200S AERO (Alodine 1200S), necessary to meet strict aerospace performance standards. However, due to the mutagenic and carcinogenic nature of hexavalent chromium (Cr6+), these treatments are being replaced by environmentally friendly alternatives. This study investigated the formation of a zirconium-based nanocoating by chemical conversion using polyethylene glycol (PEG)400 as a dispersing agent on the Alclad layer of 2024-T3. The results demonstrated that adding PEG400 at a molar ratio of 0.1036 PEG400/Zr, at pH 4 and zirconium concentrations of 0.1825 mol/L; 0.365 mol/L; 0.5475 mol/L and 0.73 mol/L, at room temperature, resulted in coatings with protective performance.
Introduction
The Alclad AA2024-T3 aluminum alloy, widely used in the aerospace industry, has been employed for decades in aircraft manufacturing. This alloy stands out due to its heat-treatable nature and characteristics such as high mechanical strength and low weight [1]. However, due to the high copper content (between 3.8 and 4.9%) and the presence of intermetallic precipitates, this alloy is more susceptible to corrosion in specific environments. Galvanic microcells form within the material, leading to the dissolution of alloying elements and resulting in localized corrosion such as pitting, crevice, and filiform corrosion [2-5]. To mitigate the corrosion susceptibility of the 2024 alloy, surface treatments such as cladding are applied, serving as the first protective barrier over the aluminium-copper (Al-Cu) alloy [6]. Thus, when the 2024 alloy is in sheet form, it receives a thin layer of 1xxx series aluminium, providing galvanic protection that enhances corrosion resistance of the (Al-Cu) alloy [7]. The clad acts as an effective physical barrier against corrosion, but it can wear away in aircraft, particularly in areas exposed to harsh conditions during operational service. In areas subjected to mechanical abrasion, such as fuselages near landing gear and takeoff zones, the clad may be gradually worn down by sand, gravel, or debris impacting the aircraft surface during takeoff and landing. Ice particles, bird strikes, or runway debris impacting the aircraft surface - either during flight or on the ground - can damage the cald [8]. This is particularly problematic in exposed areas, such as wing leading edges and control surfaces. In regions with relative movement between components (such as joints and fasteners), the clad become susceptible to fretting corrosion [9]. Corrosive environments, such as coastal areas or exposure to aggressive chemicals (like de-icing fluids), may degrade the integrity of the clad over time [10]. To address this issue, regular inspections are conducted to detect potential damage to the clad layer before it escalates into more serious problems. Damaged sections of clad sheets may undergo surface treatment or be replaced during scheduled aircraft maintenance to prevent damage propagation and preserve structural integrity [9,11].
Aircraft maintenance companies, known as MROs (maintenance, repair, and overhaul), follow the standards MIL-DTL-81706 and MIL-DTL-5541F for chromate-based chemical conversion processes such as BONDERITE® M-CR 1200S AERO (Alodine 1200S), a commercial product by Henkel® approved by manufacturers like Airbus, Boeing, and Embraer for corrosion protection of Alclad sheet metal structures [12]. This surface pre-treatment, based on hexavalent chromium, complex fluorides, and ferric compounds, is prepared by diluting a powder in pure water and applied by immersion, spraying, or brushing. Depending on the substrate alloy, application method, and exposure time, the coating color ranges to brown hues [13]. Known in the industry as Alodining, it forms a protective layer containing chromates on the metallic substrate, minimizing corrosion and enhancing the adhesive properties of subsequent coatings such as paints and primers [14-16]. However, Cr(VI)-based surface treatments have been limited or restricted by environmental regulations in the United States and the European Union due to the toxicity of Cr(VI) ions [17,18]. Eco-friendly, hexavalent-chromium-free surface treatments remain the focus of intensive research [13,19-21].
Chemical conversion coating (CCC) via brushing, also known as “Alodining”, is widely used in the aircraft maintenance industry due to its versatility and effectiveness in localized applications. This process allows the treatment of specific areas without the need for large volumes of solution or complex infrastructure. Among its advantages are material savings, the possibility of on-site application during maintenance, and reduced waste generation. In the last decade, zirconium-based salt coatings have emerged as promising candidates due to their environmentally friendly nature, strong adhesion, and excellent corrosion resistance, along with minimal mass incorporation to the substrate [22-25].
Significant progress has been made in the development of zirconium-based conversion coatings for aluminium alloys. Studies such as those by [26] and [25] have demonstrated that zirconium coatings exhibit high stability and corrosion resistance due to the formation of protective zirconium oxides and fluorides. [12,13,27] proposed zirconium coatings as sustainable alternatives to chromate-based treatments, aligning with environmental regulations from aviation authorities such as [17,18]. Studies such as [27-30] have detailed the protective mechanisms of zirconiumbased coatings, highlighting their ability to form dense oxide layers that act as physical barriers to chloride penetration cald [8]. and [31] present insights on the role of polymeric additives—such as polyethylene glycol (PEG)—in improving the homogeneity and properties of nanocoatings [33,34] demonstrated that the addition of stabilizers and modifiers can enhance protective properties. Despite advancements in surface treatment research, no scientific references were found regarding the application of zirconiumbased chemical conversion coatings using the brushing technique. This gap highlights the novelty and relevance of the present study, which applies this technique traditionally used in processes like Alodining to a sustainable alternative coating.
The objective of this study was to obtain and investigate the effectiveness of zirconium oxide nanocoatings on AA2024-T3 aluminum substrates clad with AA1230 (Clad-Material) through chemical conversion, using two different zirconium sources and adding PEG400 as a dispersing agent. The application was performed using the brushing technique and compared with the immersion method, both based on standards used in aerospace sector. The corrosion protection properties of the treated samples were investigated using electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDS) to evaluate surface characteristics. An epoxy coating was also applied to the treated substrate to assess adhesion properties using the pull-off test. By exploring the application of a PEG-modified zirconium nanocoating obtained via chemical conversion on aerospace-grade Alclad aluminum, this study aims to contribute to advancing knowledge in the field of protective coating technologies.
Materials and Methods
The Alclad 2024-T3 aluminum alloy, in laminated sheet form with a thickness of 1.2 mm, was used as the substrate. The sheet includes an Alclad cladding layer on both sides, with a thickness ranging from 60 to 80 μm. The cladding was composed of aluminum alloy 1230 (Al 99.3%, Si 0.7%, Cu 0.1%, Mn 0.05%, Mg 0.05%), analyzed by X-ray fluorescence using the Nilton XL3T GOLDD+ device from Thermo Scientific. The surfaces of test specimens measuring 50 mm × 50 mm were pre-cleaned to remove any surface contaminants, oils, and greases.
The procedure was based on the MIL-DTL-5541F standard, which specifies surface cleaning requirements prior to the application of chemical conversion coatings. This step aims to eliminate oxides, including native oxides and passivation films, which may interfere with the formation of the chemical coating. This step is essential to ensure coating adhesion and effective corrosion protection by providing a uniform surface and enabling an efficient chemical reaction between the substrate and the conversion solution. Initial degreasing, or pre-cleaning, was performed using deionized water (to avoid contamination by undesirable ions) and Scotch-Brite® for the removal of coarse particles. Subsequently, the substrate was decontaminated using two solvents, acetone (Sigma-Aldrich; 99.5% purity) and isopropanol (Sigma-Aldrich; 99.8% purity), to remove greases and adhesives. This precleaning is not specifically established in MIL-DTL-5541F, but is recommended as industrial best practice and in supplementary documents such as MIL-DTL-81706, Qualified Product Lists (QPL), AMS 1650, Society of Automotive Engineers (SAE) AMS 4037, and manufacturers’ guidelines for conversion coating solutions. These guidelines provide detailed descriptions of approved materials and procedures for surface preparation, widely adopted in the aerospace and defense industries.
Following pre-cleaning, a visual inspection was conducted to ensure that the surface of the test specimens appeared clean and uniform, with no visible residues. Next, the Water Break Test was performed, which is widely used in the industry to verify surface cleanliness prior to treatments such as chemical conversion. Although not specified in a single exclusive standard, it is described in various standards and guidelines as part of surface cleaning and preparation procedures. Based on acceptance criteria from standards such as MIL-DTL-5541F, ASTM F22, and Federal Test Method Standard 141C, Method 5331, surfaces should be hydrophilic, allowing water to spread evenly without forming droplets. The second decontamination step, or chemical pickling, is recommended for removing stubborn contaminants such as oxides and passivation films. Alkaline solutions compatible with the alloy were selected for this step. Immersion time and solution concentration were controlled to prevent excessive substrate attack. In this study, a commercial alkaline detergent, Salocin 667N®, was used at a concentration of 70 g·L⁻¹ in distilled water at 343 K for 10 minutes, followed by rinsing with distilled water. Once the specimen was properly cleaned, the chemical conversion processes followed.
Two zirconium sources were used: a standard analytical solution from Sigma-Aldrich (density 1.512 g·mL⁻¹, 50 wt% H₂ZrF₆, 3.65 mol·L⁻¹ Zr), and the commercial solution ZRC-23 from manufacturer Klintex. Solutions were prepared at concentrations of 0.1825, 0.365, 0.5475, and 0.73 mol·L⁻¹ Zr (approximately 5%, 10%, 15%, and 20% by mass of Zr), with PEG400 added at a molar ratio of PEG/Zr = 0.1036 (around 20% by mass). he pH was adjusted to 4, and tests were conducted at room temperature. The chemical conversion solution was applied using immersion and brushing methods, based on MIL-DTL-5541F specifications. Immersion was carried out using a beaker made of polypropylene, a material resistant to corrosion and non-reactive with the fluoride present in the conversion solution. To fully submerge the specimen in the chemical solution, the dip-coating technique was employed using a Marconi MA 765 disc lift, with 2 minutes of immersion at a rate of 420 mm·min⁻¹ for both immersion and withdrawal, at room temperature (between 20°C and 25°C). Samples were immersed in the conversion solutions for 3 minutes, the average time recommended by MIL-DTL-5541F for chemical conversion via immersion. After immersion, the specimens were thoroughly rinsed with deionized water to remove any residual chemicals and reaction by-products. All treated specimens were air-dried in a controlled environment to avoid contamination. The brushing application was carried out using cotton as the vehicle for applying the chemical conversion solution onto the substrate. The cotton was dipped into the conversion solution and gently squeezed to remove excess solution, preventing waste and dripping. The cotton was gently rubbed over the substrate using uniform and overlapping strokes to cover the entire surface, ensuring the solution formed a continuous layer without accumulating in specific areas. The solution was allowed to react with the substrate for a controlled period of 1 minute; after the reaction time, the surface was thoroughly rinsed to remove excess solution and reaction by-products. The treated specimens were air-dried in a controlled environment.
To characterize the nanocoating, electrochemical impedance spectroscopy (EIS) tests were conducted in 0.5 M NaCl (26 g·L⁻¹) at room temperature to evaluate corrosion resistance. In each electrochemical cell, the exposed area of the sample was 7.07 cm². A saturated KCl Ag/AgCl electrode (+0.207 V vs. SHE) was used as the reference electrode, and a platinum sheet served as the counter electrode. EIS spectra were acquired over a frequency range from 10⁵ Hz to 10² Hz, using a 10 mV (RMS) perturbation amplitude at the open-circuit potential (measured after a 2-minute stabilization period), employing an Autolab PGSTAT302 potentiostat and Nova software version 1.11.2. The tests were performed in duplicate, and samples were monitored over immersion periods of up to 696 hours. The contact angle (wettability) test was carried out using the sessile drop method with the SEO Phoenix Mini system from LACOR, and ultrapure water was used as the test liquid. Scanning electron microscopy (SEM), along with the pull-off adhesion test, were employed to assess the surface morphology and the adhesion strength of subsequent coatings such as paints and primers.
Results and Discussion
SEM and EDS analyses were carried out to assess the adhesion, chemical composition, and homogeneity of the coating. Top-view SEM images of the samples subjected to nanometric Zr oxide deposition, as described in Section 2, are shown in Figures 1–2. The EDS analyses at each marked point on the micrographs, presented in Tables 1–2, confirmed the presence of zirconium (Zr), oxygen (O), and fluorine (F), indicating the formation of fluorides and oxides on the substrate surface. However, the micrographs reveal an irregular distribution of zirconium within the nanocoating, with certain regions where the substrate remains exposed.
The surface morphology of the sample after surface treatment with hexafluorozirconic acid and PEG exhibited regions with more uniform deposition, as shown in Figure 2. By comparing the EDS analyses of the points marked on the micrographs in Figure 1 with those in Figure 2, a more homogeneous deposition is observed, corroborating the findings of [24], who obtained a similar coating on a 1XXX series alloy using the immersion process.
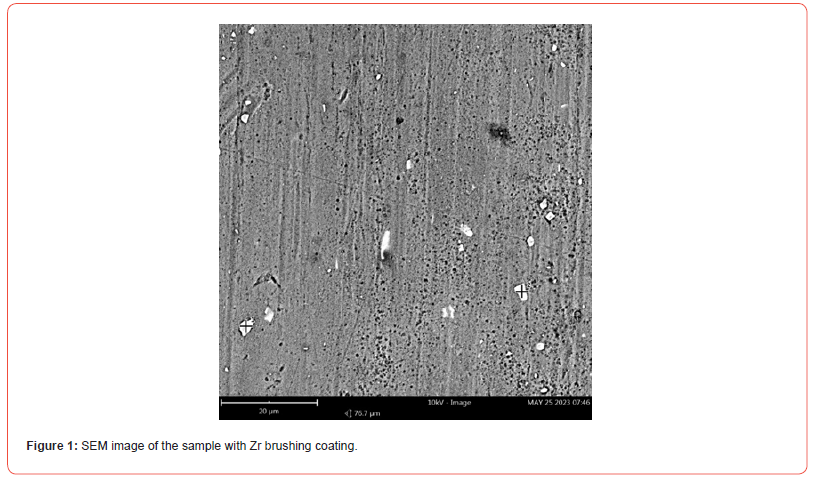
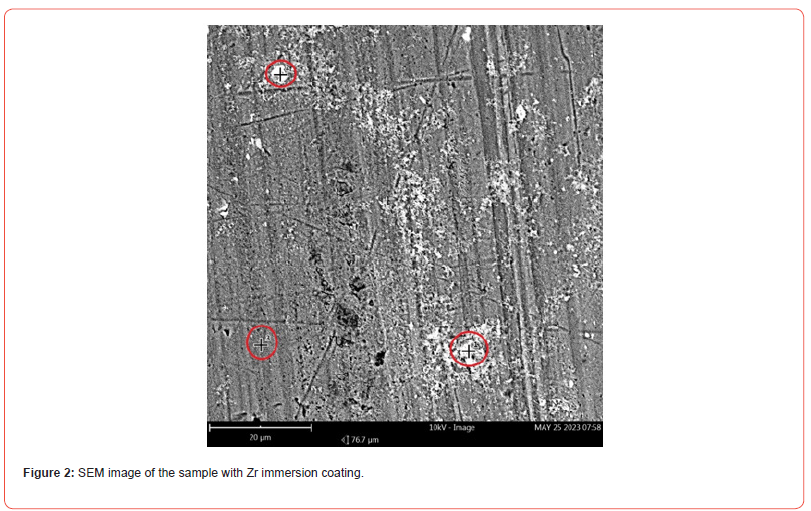
Table 1:Mass composition (%) of points 1 to 3 marked in Figure 1.
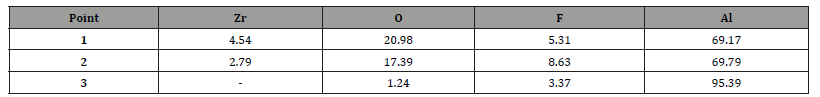
Table 2:Mass composition (%) of points 1 to 3 marked in Figure 2.
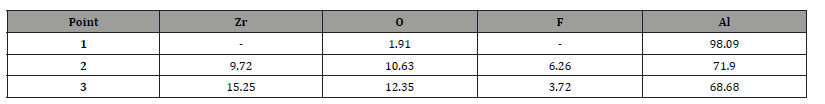
The presence of elements such as zirconium (Zr), aluminum (Al), fluorine (F), and oxygen (O) suggests the formation of a zirconium-based chemical conversion coating layer. Regions with lower zirconium concentration may indicate coating defects or areas of exposed substrate. Mass quantification revealed higher Zr density in well-coated areas, consistent with the findings of [26], who obtained zirconium–titanium-based chemical conversion coatings on AA6061 aluminum alloy. However, the detection of aluminum near the edges of the sample suggests reduced thickness, absence of coverage, or poor adhesion. These variations highlight the importance of pH, contact time, and solution concentration.
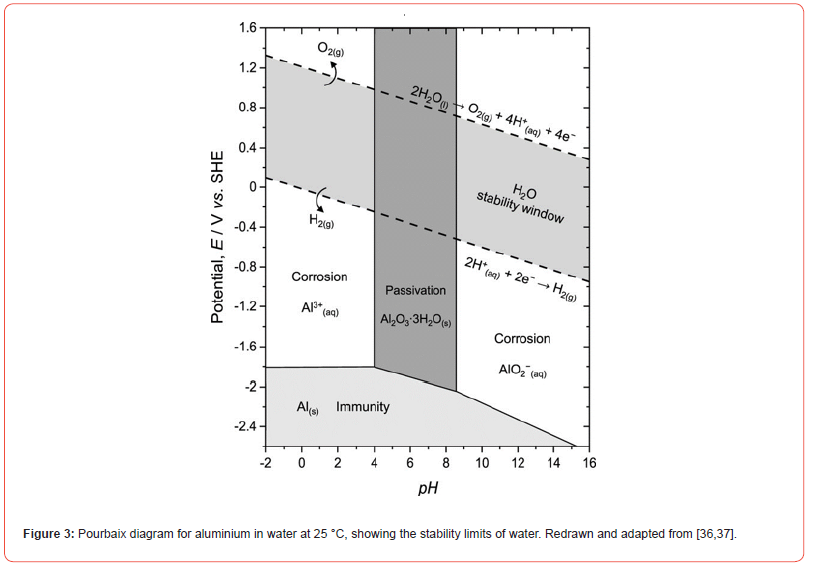
In a previous study conducted by the laboratory, [35] observed that a solution with pH 4 led to less effective formation of the Zr conversion coating, as the Zr contents measured by the author were lower compared to other pH conditions tested. However, there is a theoretical limitation to the usable pH range, since aluminium tends to corrode in both highly acidic and highly alkaline environments, as shown in Figure 3. The Pourbaix diagram Potential vs Standard hydrogen electrode x pH for aluminium in water at 25 °C [36,37] illustrates the regions where aluminium exhibits passivity, immunity, and corrosion. It is observed that at pH values below 4, aluminium is susceptible to acid corrosion, favoring the formation of Al³⁺ ions, while at pH above 9, aluminium undergoes alkaline corrosion, forming Al(OH)₄⁻ ions.
The electrochemical equations governing aluminum corrosion are:

Thus, aluminum could theoretically be processed at pH values below 4 or above 9, however, the hexafluorozirconate complex is stable under acidic to mildly acidic conditions, but may decompose at higher pH levels, releasing Zr(IV), which can then precipitate as zirconium hydroxide (Zr(OH)₄). Specifically, precipitation begins to occur around pH 4.5 and becomes more pronounced at pH ranges above 5.5 to 6.0. The precipitation reaction of zirconium hydroxide can be represented as:

Thus, only the acidic pH range would be viable for a hexafluorozirconate-based chemical conversion solution, with a pH range of 3.5 to 4.5 considered ideal for such solutions. This range is mildly acidic and beneficial for forming a uniform and adherent conversion layer, but not so acidic as to induce excessive corrosion of aluminum. These pH ranges are critical for designing chemical treatments and predicting the behavior of aluminum in different environments.
Higher zirconium concentrations—above 20 wt.% in the solution—and prolonged exposure times led to the formation of thick and brittle deposits, as illustrated in Figure 4. In these samples, the sessile drop contact angle was zero.

In the contact angle analysis, lower surface wettability was observed in the brushing process without PEG, indicating that H₂ZrF₆ and ZRC-23 induce changes in the surface properties of the substrate, rendering it hydrophobic, as evidenced in Figures 5–6.
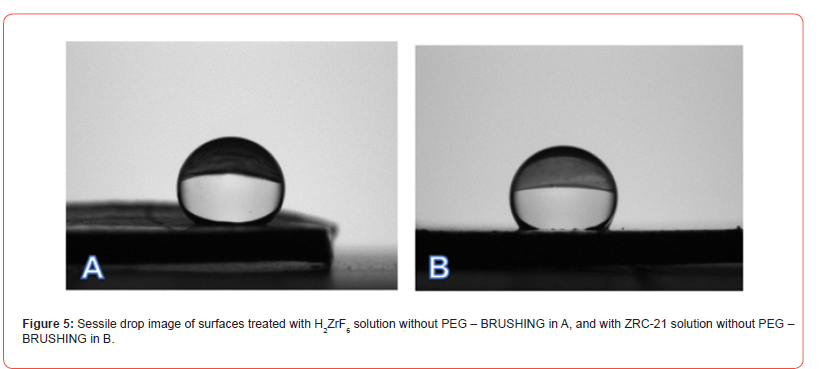
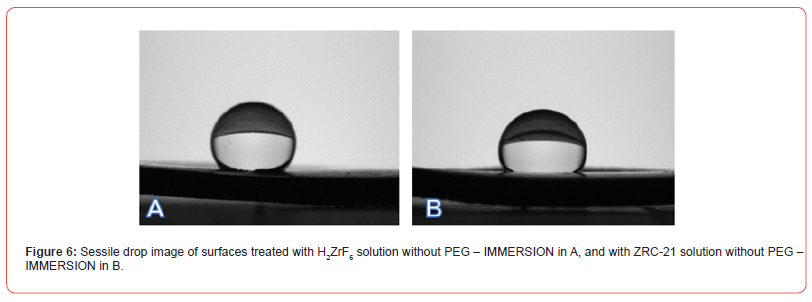
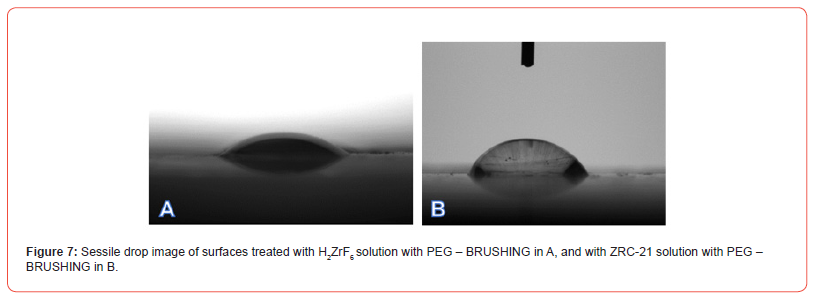
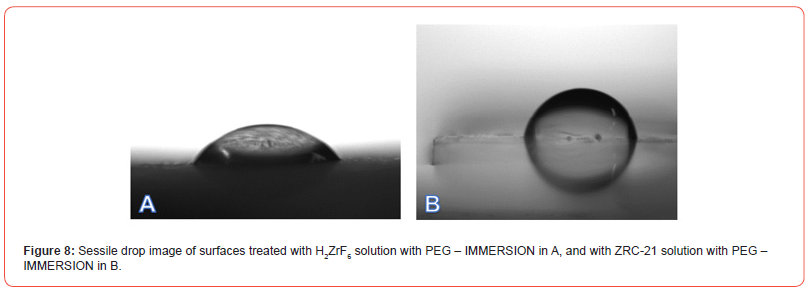
Figures 7–8 present comparative images of sessile drops on each of the evaluated surfaces, with the lowest contact angle observed in samples treated with PEG—regardless of Zr concentration— compared to those treated only with H₂ZrF₆ or ZRC-23, suggesting that the mechanism of hydration and precipitation of aluminum hydroxide on the Alclad surface was altered, indicating that the addition of PEG to the conversion solution changed the surface interaction with the chemical conversion bath, influencing surface tension and the ability to repel or absorb moisture. The lower contact angle observed in PEG-treated samples indicates greater wettability compared to those treated solely with H₂ZrF₆ or ZRC-23. This suggests that PEG reduces surface hydrophobicity, potentially due to the formation of more homogeneous and less porous layers, as observed by [38] and [39].
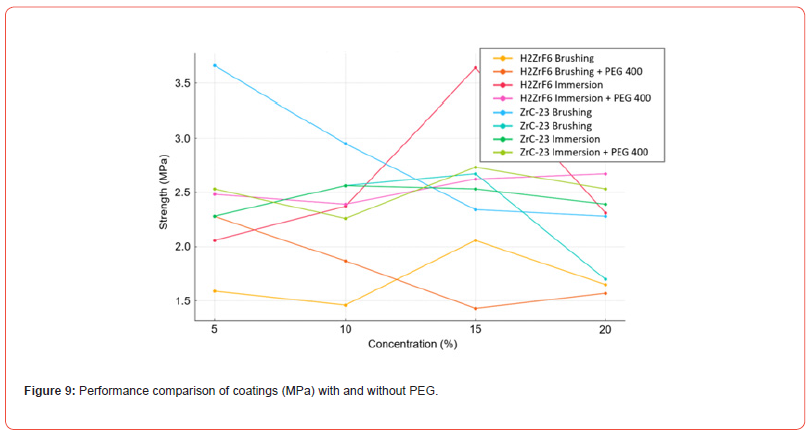
The adhesion test demonstrated that the application method has a significant impact on the mechanical strength of the coatings, as shown in Figures 9–10. When comparing the H₂ZrF₆ coating without PEG across both application methods, it was observed that the brushing method exhibited lower mechanical strength than immersion, particularly at higher concentrations (15% and 20%). In contrast, the immersion method demonstrated superior performance, with a peak strength at 15% concentration (3.64 MPa), attributed to the formation of more homogeneous zirconium layers.
The zirconium coating obtained using ZRC-23 via brushing achieved the highest initial strength (3.66 MPa at 5%), but showed a progressive reduction at higher concentrations. Immersion provided more consistent values, suggesting greater control over the coating deposition process.
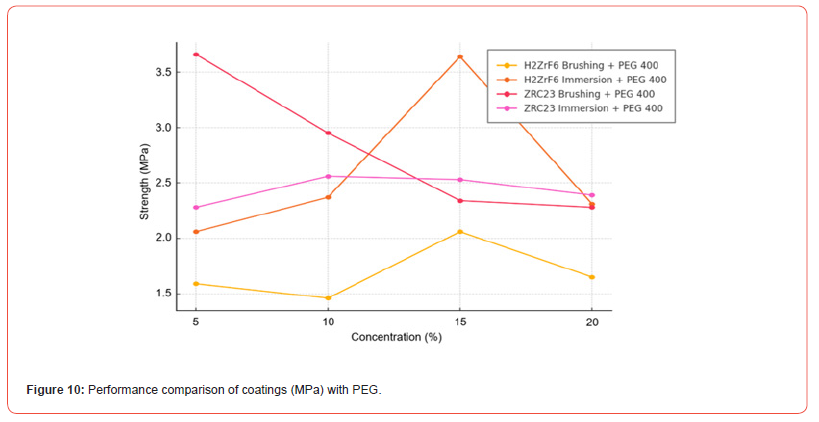
The presence of PEG 400 had a distinct influence on the performance of the coatings, depending on the precursor and application method. For coatings in which H₂ZrF₆ was used as the zirconium source and applied by brushing, the addition of PEG 400 reduced the mechanical strength at higher concentrations (15% and 20%), suggesting that PEG interferes with coating formation, in agreement with the findings of [34]. In the immersion process, however, PEG improved the results at intermediate concentrations (10% and 15%), promoting denser and less porous layers, with mechanical strengths ranging from 2.48 to 2.67 MPa.
For coatings that used ZRC-23 as the Zr source and were applied by brushing, PEG increased adhesion strength at concentrations of 10% and 15%, indicating a positive effect on coating uniformity. In contrast, for the immersion process, the values remained constant, suggesting that this application method is effective regardless of PEG 400 addition.
The highest overall performance of the zirconium coating was observed in the samples treated with H₂ZrF₆ via immersion at 15% concentration with PEG. This treatment achieved the highest mechanical strength value (3.64 MPa), standing out as the best combination of precursor, method, and concentration. Although the samples coated with ZRC-23 by brushing at 5% without PEG initially exhibited the highest value (3.66 MPa), their strength significantly decreased at higher concentrations.
As the main objective of this study was to evaluate the brushing application method, electrochemical impedance spectroscopy (EIS) tests were conducted on these samples. Impedance was measured as a function of frequency, and the results were analyzed to assess the corrosion resistance provided by each treatment. The graphs shown in Figures 11 and 12 illustrate the electrochemical impedance results of the coatings after 1 hour and 168 hours of exposure to a 0.5 mol/L NaCl aqueous solution.
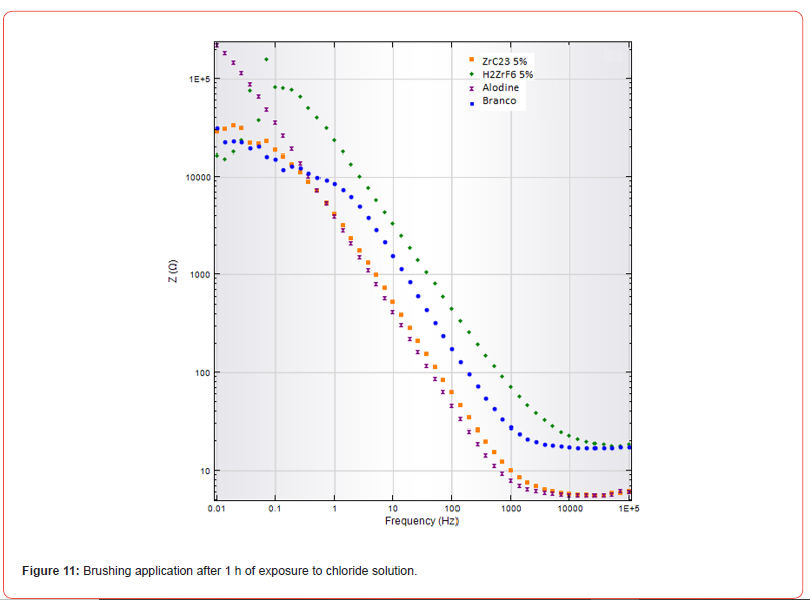
The graph in Figure 11 shows that the ZRC-23 at 5% and H₂ZrF₆ at 5% treatments exhibit higher impedance at low frequencies, suggesting good initial corrosion protection. As a comparative surface treatment, Alodine demonstrates relatively good protection, although slightly inferior to the zirconium-based treatments. The “Blank” sample (untreated) shows the lowest impedance, indicating significantly lower corrosion protection.
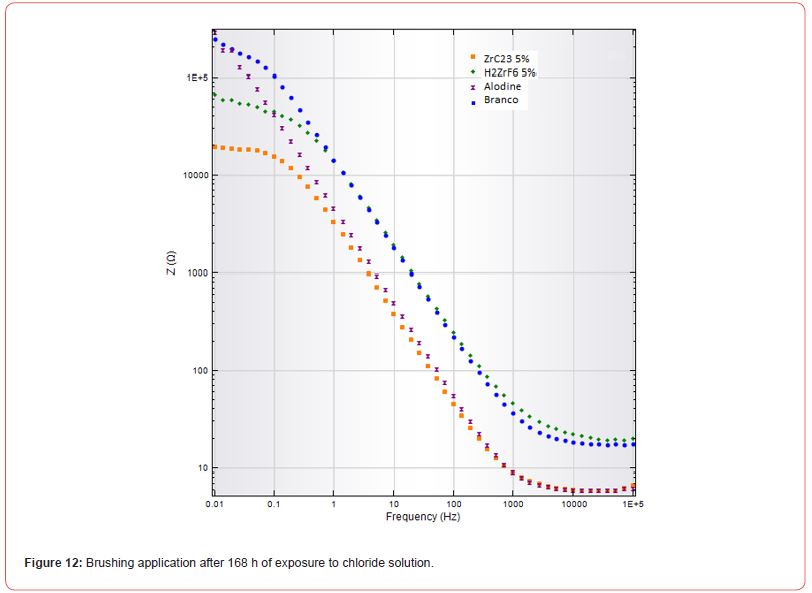
The electrochemical impedance test remained stable until 168 hours (Figure 12), when a significant reduction in impedance was observed for the ZRC-23 5% and H₂ZrF₆ 5% treatments, indicating a possible partial loss of protection. However, both treatments still maintained higher protection levels compared to the Alodine coating and the “Blank” sample, which continued to show lower impedance values, due to corrosion products of Al.
The increase in impedance in the uncoated (blank) sample at low frequencies is consistent with the formation of corrosion products that create a diffusional barrier. However, this increase does not necessarily indicate effective corrosion protection. The sample treated with Alodine maintained a higher impedance, reflecting the superior efficacy of chromate conversion coatings, confirmed by decades of industrial use and extensive scientific literature. The Nano Zirconium treatments showed slightly lower but stable impedance, suggesting an effective and long-lasting protection mechanism.
Samples that did not exhibit high mechanical strength were also evaluated in terms of corrosion behavior to understand whether the coating could serve as a standalone barrier layer without the addition of paints or primers. In this regard, the H₂ZrF₆ immersion treatment at 15% with PEG showed the highest electrochemical resistance observed in the EIS test, which aligned with its mechanical performance (3.64 MPa). This result suggests that immersion at 15% + PEG promotes a denser and more uniform coating deposition, corroborating the findings of [32] and [39].
Although brushing generated coatings with good initial performance, the EIS semicircles were smaller, indicating greater porosity and permeability. Despite the high initial mechanical strength (3.66 MPa) achieved by the ZRC-23 coating applied by brushing, the electrochemical behavior suggests limitations in longterm protection, attributed to the lower uniformity of the formed layer. This behavior was very similar to that observed in the ZRC- 23 immersion samples, suggesting that the performance of this Zr source remains relatively constant regardless of the application method.
Conclusion
This study demonstrated that zirconium-based nanocoatings assisted by PEG 400 exhibit performance that is significantly influenced by the combination of precursor, application method, and the presence of PEG 400. PEG appears to act as a morphology modifier of the coating layer, but its effectiveness depends on both concentration and application method. At higher zirconium concentrations, interferences may occur, leading to the formation of less corrosion-resistant layers. Immersion tends to promote a more uniform and controlled deposition, resulting in superior adhesion strength values.
Based on the results obtained, it can be concluded that H₂ZrF₆ applied via immersion produced the most stable performance, with less variation across different concentrations. This suggests that the method is more robust for this specific precursor. In contrast, the brushing application method showed greater variability, particularly when PEG was used, and seems to be suitable only at low concentrations (5%) for this precursor, due to performance limitations at higher concentrations. The addition of PEG should be carefully evaluated, as its effect depends on both the precursor and the application technique.
Further testing under extreme environmental conditions— such as prolonged exposure to marine atmospheres or elevated temperatures—would be valuable to validate the coating’s robustness. The incorporation of other polymeric additives or nanoparticles may potentially enhance both protective and mechanical properties of the coatings. Finally, studies addressing the economic and operational feasibility of large-scale application are essential for the industrial adoption of this process.
References
- Handbook Asm Specialty Aluminum and Aluminum Alloys (Asm Specialty Handbook) [Livro] / ed. Davis Joseph: Asm Intl, 1993.
- Ashby Michael F (2010) Materials Selection in Mechanical Design: Butterworth-Heinemann.
- Askeland Donald R, Fulay Pradeep P, Wright Wendelin J (2010) The Science and Engineering of Materials: CL Engineering.
- Callister William D, Rethwisch David G (2013) Materials Science and Engineering: An Introduction: John Wiley & Sons.
- Lytle F W, R B Greegor, G L Bibbins, K Y Blohowiak, R E Smith, et al. (1995) An investigation of the structure and chemistry of a chromium-conversion surface layer on aluminum. Corrosion Science 37(3): 349-369.
- Al Th Kermanidis, P V Petroyiannis, Sp G Pantelakis (2005) Fatigue and damage tolerance behaviour of corroded 2024 T351 aircraft aluminum alloy. Theoretical and Applied Fracture Mechanics 43(1): 121-132.
- Sahand Pourhassan, Paulo J Tavares, Pedro M G P Moreira (2017) Material properties of 2024-T3 ALCLAD and 2124-T851 aluminum alloys using 2D and 3D Digital Image Correlation techniques. Procedia Structural Integrity 5: 1355-1362.
- P V Petroyiannis, Al Th Kermanidis, R Akid, C A Rodopoulos, Sp G Pantelakis (2005) Analysis of the effects of exfoliation corrosion on the fatigue behaviour of the 2024-T351 aluminium alloy using the fatigue damage map. International Journal of Fatigue 27(7): 817-827.
- E Huttunen-Saarivirtaa, V T Kuokkalaa, J Kokkonena (2011) Paajanenb Corrosion effects of runway de-icing chemicals on aircraft alloys and coatings. Materials Chemistry and Physics pp. 138-151.
- L Ong C, Y Shu W, SB Shen (2003) The evaluation of non-tank surface treatments for aluminium bonding repairs. International Journal of Adhesion and Adhesives 12(2): 79-84.
- Park Sang Yoon, Choi Won Jong, Choi Heung Soap (2018) A review of the recent developments in surface treatment techniques for bonded repair of aluminum airframe structures. International Journal of Adhesion and Adhesives 80: 16-29.
- J Bautista-Ruiz, WA Bautista-Ruiz, Aperador W (2020) Anti-corrosive characterization of silicon, titanium, and zirconium oxide coatings deposited on aeronautical aluminum substrates via sol-gel. Journal of Physics: Conference Series.
- Yang X F, D Tallman, V J Gelling, G P Bierwagen, L S Kasten, et al. (2001) Use of a sol–gel conversion coating for aluminum corrosion protection. Surface and Coatings Technology 140(1): 44-50.
- Zhao J, L Xia, A Sehgal, D Lu, R L Mc Creery, et al. (2001) Effects of chromate and chromate conversion coatings on corrosion of aluminum alloy 2024-T3. Surface and Coatings Technology 140(1): 51-57.
- EASA EEA (2019) EUROCONTROL European Aviation Environmental.
- FAA U.S. DEPARTMENT OF TRANSPORTATION FEDERAL AVIATION ADMINISTRATION National Policy Flight Standards Service Chemical Safety and Hazard Communication Program 2020.
- Campestrini P, Westing E P M van, Hovestad A, Wit J H W (2002) Investigation of the chromate conversion coating on Alclad 2024 aluminium alloy: effect of the pH of the chromate bath. Electrochimica Acta 47(7): 1097-1113.
- Chidambaram Devicharan Clayton Clive R, Kendig Martin W, Halada Gary P (2004) Surface Pretreatments of Aluminum Alloy AA2024-T3 and Formation of Chromate Conversion Coatings: II. Composition and Electrochemical Behavior of the Chromate Conversion Coating. Journal of The Electrochemical Society 151(11): B605-B612.
- D Bryce Mitton Anna Carangelo, Annalisa Acquesta, Tullio Monetta (2017) Michele Curioni and Francesco Bellucci Selected Cr(VI) replacement options for aluminum alloys: a literature survey. Corrosion Reviews 35: 6.
- Fan Weijie, Li Weihua, Zhang Yong, Wang Wei, Zhang Xiaoying, et al. (2017) Cooperative self-healing performance of shape memory polyurethane and Alodine-containing microcapsules. RSC Advances.
- Proença C S, Pereira AM, Pigliaru L (2023) Alternative chemical conversion pre-treatment for aeronautical aluminium alloy: characterisation and anticorrosion performance. CEAS Space J.
- Merisalu Maido, Lauri Aarik, Jekaterina, Hugo Mandar, Aivar Tarre, et al. (2021) Effective corrosion protection of aluminum alloy AA2024-T3 with novel thin nanostructured oxide coating. Surface and Coatings Technology 411: 126993.
- Milošev I, Frankel G S (2018) Conversion coatings based on zirconium and/or titanium. Journal of The Electrochemical Society.
- Golru S Sharifi, Attar M M, Ramezanzadeh B (2015) Morphological analysis and corrosion performance of zirconium based conversion coating on the aluminum alloy 1050. Journal of Industrial and Engineering Chemistry 24: 233-244.
- Andreatta F, A Turco, I de Graeve, H Terryn, J H W de Wit, et al. (2007) SKPFM and SEM study of the deposition mechanism of Zr/Ti based pre-treatment on AA6016 aluminum alloy. Surface and Coatings Technology 201(18): 7668-7685.
- Wang SH, Liu CS, Shan FJ (2008) Corrosion behavior of a zirconium-titanium based phosphonic acid conversion coating on AA6061 aluminium alloy. Acta Metallurgica Sinica 21(4): 269-274.
- E Deltombe, M Pourbaix (1958) Corrosion 14: 16.
- Oi Man Leung, et al (2021) J Electrochem. Soc. 168 056509, Journal of The Electrochemical Society.
- Pinheiro J S, Regio G, Cardoso H R P, Oliveira C T, Ferreira J Zoppas (2019) Influence of Concentration and pH of Hexafluorozirconic Acid on Corrosion Resistance of Anodized AA7075-T6. MATERIALS RESEARCH 22: 1-11.
- Kraš Ana, Milošev Ingrid (2023) The Aqueous Chemistry of Zirconium as a Basis for Better Understanding the Formation of Zirconium Conversion Coatings: Updated Thermodynamic Data. Journal of The Electrochemical Society 170: 2.
- He Wei, Xiang Gao, Ru Yan, Yan Wang, Houyi Ma (2024) Zr-based conversion film fabricated on cold rolled steel by separately providing zirconium and fluorine ions: Performance and mechanism study. Electrochimica Acta 506: 145051.
- Mihalache Iuliana, Antonio Radoi, Cornel Munteanu, Mihaela Kusko, Cristian Kusko (2014) Charge storage and memory effect in graphene quantum dots – PEG600 hybrid nanocomposite. Organic Electronics 15(1): 216-225.
- Peng Zhili, Chunyu Ji, Yiqun Zhou, Tianshu Zhou, Roger M Leblanc (2020) Polyethylene glycol (PEG) derived carbon dots: Preparation and applications 20: 100677.
- Wijayanti R B, Rosmayanti I, Wahyudi K, Maryani E, Hernawan H, Septawendar R [Periódico] // Crystals 2021.
- Adhikari Saikat, Kinga A Unocic, Yanfen Zhai, Gerald S Frankel, W Fristad, et al. (2011) Hexafluorozirconic acid based surface pretreatments: Characterization and performance assessment. Electrochimica Acta 56(4): 1912-1924.
- Thangavelu Arunnellaiappan, Arun S, Hariprasad S, Gowtham S, Ravisankar B, et al. (2017) Fabrication of corrosion resistant hydrophobic ceramic nanocomposite coatings on PEO treated AA7075. Ceramics International 44(1): 874-884.
- Ognian Dimitrov, Irina Stambolova, Sasho Vassilev, Katerina Lazarova (2020) Tsvetanka Babeva e Mladenova Ralitsa Surface and Morphological Features of ZrO2 Sol-Gel. Materials Proceedings 2(1): 6.
- Hinsch Martin (2019) Industrial Aviation Management - A Primer in European Design, Production and Maintenance Organisations. Heidelberg Springer Berlim.
- Institut für seltene Erden und Metalle AG (2024) Institut für seltene Erden und Metalle AG.
- Jiao J, Shuai Y, Li J (2024) Identifying ESG types of Chinese solid waste disposal companies based on machine learning methods. Journal of environmental management 360: 12135.
- P V Petroyiannis, Sp G Pantelakis, GN Haidemenopoulos (2005) Protective role of local Al cladding against corrosion damage and hydrogen embrittlement of 2024 aluminum alloy specimens. Theoretical and Applied Fracture Mechanics 44(1): 70-81.
- Pantelakis Sp G, Chamos A N, Kermanidis Al Th (2012) A critical consideration for the use of Al-cladding for protecting aircraft aluminum alloy 2024 against corrosion. Theoretical and Applied Fracture Mechanics 57(1): 36-42.
- Smith CS Alumínio e Ligas de Alumí Em Alloy: Compreendendo o Básico (pp. 822-834). [Livro]. - 1985.
- Trzepieciński T, Kubit A, Dzierwa A, Krasowski B, Jurczak W (2021) Surface Finish Analysis in Single Point Incremental Sheet Forming of Rib-Stiffened 2024-T3 and 7075-T6 Alclad Aluminium Alloy Panels. Materials 14(7): 1640.
- Williams E Spencer, Julie Panko, Dennis J Paustenbach (2009) The European Union’s REACH regulation: a review of its history and requirements. Critical reviews in toxicology 39(7): 553-575.
-
P K Lacava1, LL Kuhn, E L Schneider*, J Zoppas-Ferreira. A Zirconium-Based Nanocoating for Corrosion Protection of the AA1230 as Clad Material for the AA2024-T3 Alloy assisted by PEG400. Mod Concept Material Sci. 7(3): 2025. MCMS. MS.ID.000662.
-
Zirconium Nanocoating, Chemical Conversion, PEG400, AA2024, Aircraft manufacturing, Aluminum, Surface, Zirconium coatings, Clad-Material
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






