 Research Article
Research Article
Light Fastness Testing of Cotton and Wool Samples Dyed with Natural Dyes and Correlation the Results with the Antioxidant Activity of the Plants
Tsouka Niki1*, Nikolaidis Nikolaos2, Lazari Diamanto3, Dimitriadis Kyriakos3 and Theodoropoulos Konstantinos1
1Laboratory of Forest Botany-Geobotany, School of Forestry and Natural Environment, AUTh, 54124 Thessaloniki, Greece
2Laboratory of Chemistry and Technology of Polymers and Colors, Chemistry School, AUTh, 54124 Thessaloniki, Greece
3Laboratory of Pharmacognosy, School of Pharmacy, AUTh, 54124 Thessaloniki, Greece
Tsouka Niki, Laboratory of Forest Botany-Geobotany, School of Forestry and Natural Environment, AUTh, 54124 Thessaloniki, Greece
Received Date: June 14, 2024; Published Date: July 02, 2024
Abstract
The present paper deals with the control of the color fastness given by aqueous extracts from selected plants, during the dyeing of samples of cotton and woolen fabric. Initially, during the experimental process, the tissue of each plant species underwent aqueous extraction. The antioxidant activity of the aqueous extracts was measured. This was followed by the dyeing of cotton and wool samples and, finally, the resistance of their color to light fastness was checked.
Keywords:Light fastness; natural dyes; %RSC; cotton, wool
Introduction
Colors are being used from the ancient times because the mood and activities of people are directly affected by their existence. The first dyes that were used were natural, from minerals, plants, insects and animals [1,2]. The use of natural dyes flourished until the middle of the 19th century. People used a limited number of dyes, in order to make fabrics whose color is not affected by air, sun and water. The progress in Organic Chemistry and the discovery of the structures of chemical compounds of dyes opened the way for the industrial production of synthetic dyes. The advantages of synthetic paints are their simple and economical application in large quantities, the variety of colors they provide as well as their resistance to various tests [3,4]. Today, due to environmental awareness (such as waste management problems, high environmental footprint) and the negative effects of chemical dyes on both human health and sustainable development [5,6], there is a tendency to reuse plant dyes. The prerequisites for finding the application of the use of natural dyes are the low production price and the durability of the paint in various tests similar to synthetic dyes. A major disadvantage of natural dyes is the low resistance of the paint to sunlight.
On this basis, the pigment properties of various plant species were investigated. For the finding and selection of species, the parameters were the conservation of biodiversity, the possibility of using some plant species (as) in alternative cultivation and the use of by-products of various crops. Plant tissues were collected from various parts of Greece. This was followed by identification of plant species, purification and aqueous extraction of plant tissues. A ratio of plant tissue to water was observed, depending on the plant material. The antioxidant potential of the aqueous extracts was evaluated by the method of interaction with the substance 2,2-dienol-1-picrylhydrazyl (DPPH). Dyeing of cotton and wool samples by the use of plant extracts followed. The color factors of the painted samples were measured and the results are in the CIELab color model. The samples were subjected to the test of color resistance to solar radiation according to the ISO 105-B02 standard (Color fastness to artificial light Xenon arc fading lamp test). Finally, the results were evaluated by visual observation in observation chamber VeriVide Leslie Huble Ltd., with standard lighting D65 (daylight). The conclusions of the research followed. The scope of the current study was to investigate whether use of plant as a dye in the dyeing of fabrics (cotton and wool), can provide color resistance to light and correlation between resistance and antioxidant potential of plant extracts. Furthermore, the selection of plants was made with the aim of saving natural resources, thus contributing to sustainable development and the protection of the natural environment in general, taking into account that plants can be considered, in a degree, as renewable natural resources.
Materials and Methods
Materials
Whole plants or part of the plants (collected from the environment of Greece) were used for aqueous extraction. The above aqueous extracted liquors were used as the main dye bath for the dyeing. This method is not suitable for heat-sensitive and water-insoluble coloring substances [7]. Two types of fabrics were used in the present study, white neat knitted cotton fabric (185 g/ m2) No 20 English and natural off-white neat woven wool fabric (155 g/m2). The samples used for the dyeing were 10g each.
The chemical reagents used in the study were: alum KAl(SO4)2*12H2O (Fluka GmbH Switzerland), stable radical 2,2-Dienol-1-picrylhydrazyl (DPPH) (Merck Darmstadt, Germany), anionic detergent (Standard Soap Without Optical Brightening Agent, ISO 105: C06 B2S, SDL International Ltd., England), NaCl (Merck KGaA, Germany). For the light fastness, standard Blue Wool Refer ence Standards No.1-No.8 (conforming to requirements of BS EN ISO 105 B08) and Grayscale for Assessing Change in Color (ISO 105-A02: 1993, BS EN 20105-A02: 1995, BS 1006-A02: 1990, SDC Standard Methods, 5th Edition A02 and BS1006-A03: 1990, SDC Standard Methods, 5th Edition A03) were used [8]. The visual observations were executed by the same experienced researcher in all cases, under the appropriate light spot.
Apparatus
A thermostatic 16-position open-type dye bath (Raypa, Spain) was used for all the dyeings. Color measurements were performed using a Microflash datacolor international spectrophotometer. A Xenon Q-Sun test chamber (Xe-1-B) (Q-LAB, USA) was used for the light fastness measurements. The evaluation of the color change after the color fastness tests was performed in a VeriVide observation chamber with standard lighting D65 (Leslie Hubble Ltd, UK).
Methods Collection and identification of plants
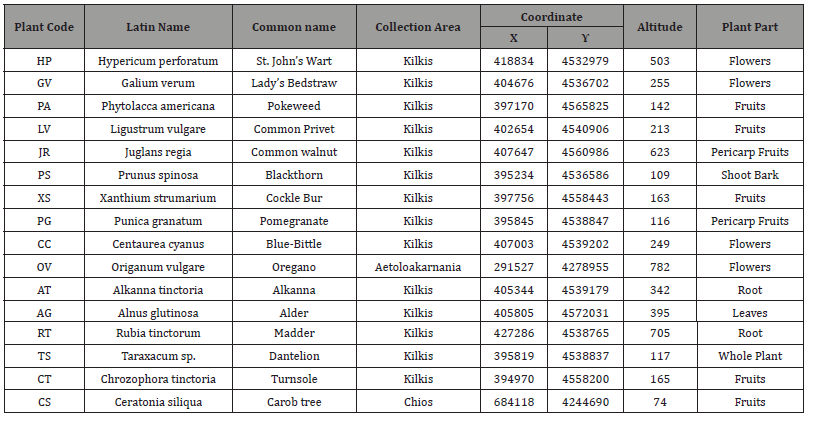
The collection of plant material was accomplished in selected areas of Greece, listed in Table 1. are the details of the exact collection location of each species. For the identification of plant samples, were mainly used the entries of “Flora Europaea” [9] and “Flora Hellenica” [10]. The book “Vascular Plants of Greece - An annotated checklist” was used to update the nomenclature of the identified taxa [11].
Table 1:Τaxa data used in the current study.
Dyeing Procedure
Depending on the part of the plant species and the vegetative
state in which the plant was, the following ratio was created and
observed for the extraction:
a. Fresh Flowers, 6% fresh weight by weight of water.
b. Dried Flowers 6% dry weight by weight of water.
c. Dried Fruits, 20% dry weight by weight of water.
d. Fresh Fruits, 35% fresh weight by weight of water.
e. Shoot Bark 6% dry weight by weight of water.
f. Plant Roots 7% dry weight by weight of water.
g. Pericarp Fruits, 30% fresh weight by weight of water.
h. Fresh Plants30% fresh weight by weight of water.
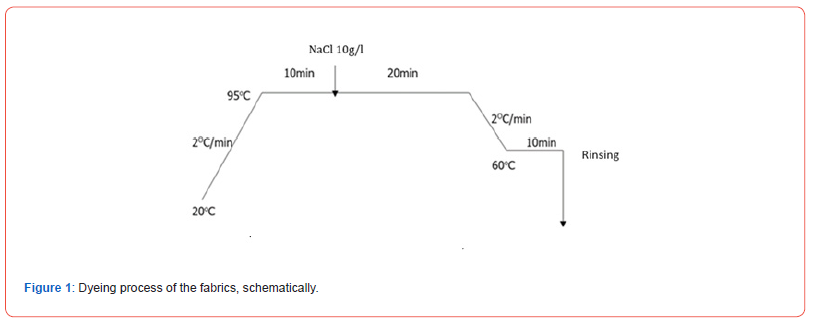
The extraction of all plant tissues was done at 95 °C for 1 h. The mordanting process was done with an alum solution of 15 g/L at a bath ratio of 1:30 at a temperature of 80 °C for 20 min. All dyeings were performed at a 1:20 liquor ratio. The samples and extracts were placed in the dyeing machine, and the temperature was increased from 20 to 95 °C, at a constant rate of 2 °C/min. The dyeing process remained at this temperature for 10 min. Then NaCl was added at a ratio of 10 g/L, and the dyeing continued for another 20 min. The machine was then cooled at a constant rate of 2 °C/ min up to 60 °C, where it remained for 10 min at this temperature. Then the fabric was rinsed with water only for dye excess removal (Figure 1).
Color Measurements
The color of the dyed samples was measured using a Microfash datacolor international spectrophotometer. Reproducibility was checked by taking four measurements focusing on different spots, and the average reflectance values over the range 400–800 nm were taken [12]. The results are in the CIELAB color system, where each color is described by three coordinates or factors called L*, a* and b*. The K/S values were calculated using the Kubelka–Munk equation which gives a numerical value of the amount of color in the substrate [13]: K/S = A X C = (1-R)2 /2R
where K is the absorption (%), depending on the amount of dye, S is the scattering (%), depending only on the substrate, A is a constant, depending on color, wavelength and substrate, C is the color concentration and R is the reflectance of a monochromatic light beam (of variable wavelength) in the region of the vis spectrum, with respect to the standard white surface of the instrument [13].
Determination of Antioxidant Activity
To evaluate the antioxidant activity of the extracts, interaction with 2,2-dienol-1-picrylhydrazyl (Sigma Aldrich, DPPH) in solution was performed and the absorbance was measured at λmax=517 nm relative to the corresponding samples without DPPH at two times intervals (20 and 60 min) [14]. Reduction in the reaction rate upon the addition of DPPH is used as an indicator of the radical behavior of the reaction. Three measurements were made for each extract and the average was calculated (the deviation does not exceed 10%). Results are expressed as a percentage of free radical scavenging capacity (% radical scavenging capacity, RSC) [15]: RSC (%) = (Ac – As) / Ac, where As and Ac represent the absorbance of each sample and of the control sample, respectively.
Color fastness to artificial light
The ISO 105-B02 (color fastness to artificial light xenon arc fading lamp test) standard was used to measure the light fastness of the dyed samples [16,17]. An initial exposure of 72 h was executed. followed by three additional exposures of 72 h each. Samples whose had high fastness to artificial light, underwent three additional exposures of 72 h each. Each of the above tests lasted 72h a total of 9 twenty-four hours.
Results and Discussion
Color measurement
Table 2:Colorimetric coordinates a*, b*, L*, C, ho, R% and K/S of the dyed cotton samples.
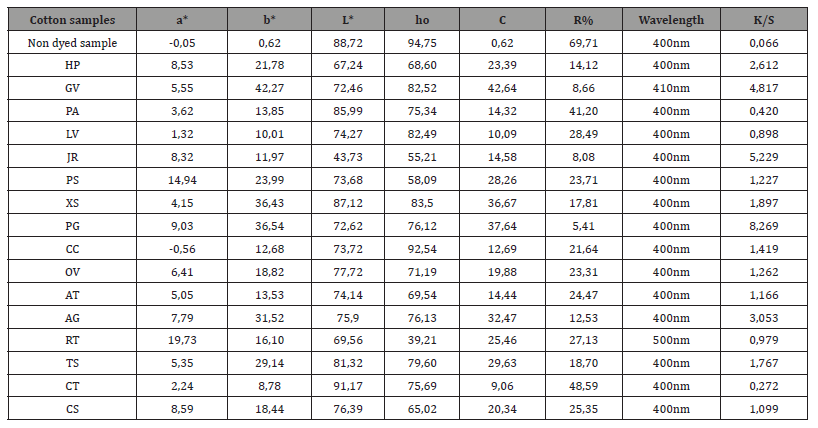
Table Abbreviations: a*, b*, L*, ho = colorimetric coordinates, C = color concentration, R% = percentage refection, K/S = relation of the R% with C of the color in the substrate.
Table 3:Colorimetric coordinates a*, b*, L*, C, ho, %R and K/S of the dyed wool samples.
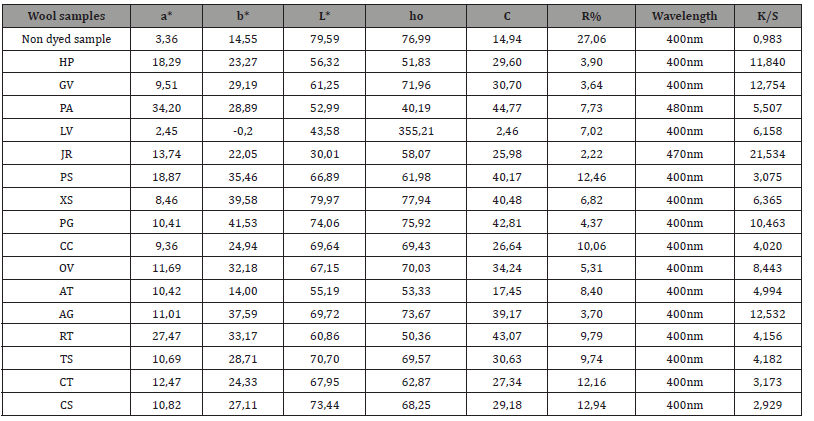
Table Abbreviations:a*, b*, L*, ho = colorimetric coordinates, C = color concentration, R% = percentage refection, K/S = relation of the R% with C of the color in the substrate.
The colorimetric coordinates a*, b*, L*, C*, h°, the percentage refection %R and the K/S values of the dyed and non-dyed cotton samples are given in Table 2 and the dyed and non-dyed wool samples are given in Table 3. All dyed samples (cotton and wool) have K/S values higher than undyed samples indicating that aqueous plant extracts have coloristic properties. As observed, the K/S values of the dyed wool samples are significantly higher compared to the corresponding ones of the cotton samples. The difference in dye adsorption is due to the different bonds with which the dyestuff binds to the fibers of cotton (cellulose) and wool (proteins). The minimum reflectance % R in most samples is at the same wavelength of 400 nm. Exceptions are the GV and RT cotton samples and the PA and JR in wool samples. The colorimetric coordinates and the value who are in satisfactory accordance to the visual evaluation and the color acquired by the dyed samples.
During the dyeing process, the wool fiber, which is positively charged due to the protonation of its amino groups, absorbs the negatively charged dye extract. In this respect, the dyestuff resembles an acid dye in the dyeing of wool [5,18]. The adsorption on wool indicates a monolayer of molecules on infinite wool dye sites taking place mainly through electrostatic interactions between the cationically charged wool fiber and the anionically charged molecules of dyestuff [5,18].
However, cotton fiber is negatively charged due to the ionization of cellulose hydroxyl groups in the aqueous environment of the dye and repels the negatively charged dye extract molecules. The adsorption of the dye on cotton takes place mainly through Van der Waals interactions, meaning that cellulosic molecules and dye molecules, upon being planar and non-branched molecules, facilitate each other’s approach enough to allow the development of Van der Waals interactions which operate at short distances [5,18]. Therefore, there is much less and weaker adsorption of the dye from the cotton fiber compared to the impressively higher absorption by the wool fiber. Dyed cotton samples had higher lightness (L*) value compared to dyed wool samples which correspond to less absorbed by the cotton (lower K/S value). An exception is the dyed cotton sample with aqueous extract of PG, which has L* value higher than the dyed wool sample and high value K/S.
Color fastness to Artificial light
After the interaction of the aqueous extracts of plants with the substance DPPH for two different time periods (20 and 60 min), a high antioxidant activity was observed (Table 4). Specifically, during the first measurement at 20 min all values were greater than 70. Higher values (greater than 89) were calculated for the extracts TS, RT, CS, HP, PG, JR and XS. The PA value was lower at 71. During the second measurement at 60 min, an increase in the antioxidant activity was observed in most extracts. Exceptions are HP, GV, XS, AG and TS in which there was a decrease in the % RSC price.
Color fastness to Artificial light
The color fastness of the dyed samples to the artificial light was assessed visually by using the Blue Wool Standards Scale (rating 1 is the lowest endurance and rating 8 is the highest). The results are given in Table 5. The non dyed samples are graded with excellent 8. The color fastness of natural dyes to artificial light is generally quite low due to the photochemical oxidation caused by the dyes (organic chromophore substituents). Wool fiber creates stronger bonds with dyes, due to its greater affinity with dyes and its polar free groups.
Table 4:Antioxidant activity of the extracts (%RSC) 20 min and 60 min.
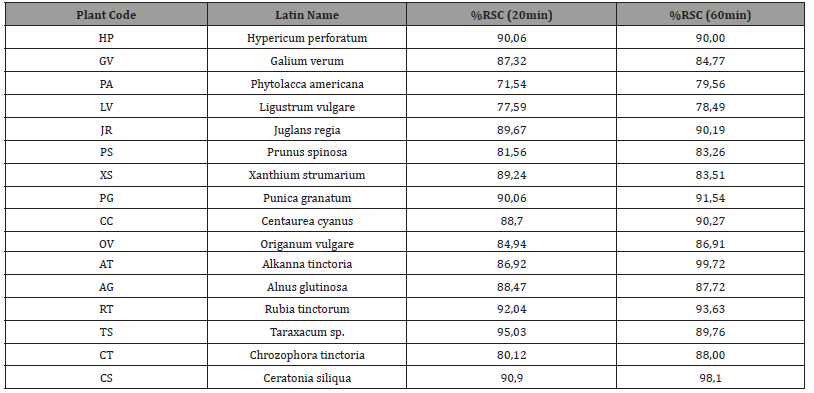
Table Abbreviations:%RSC = percentage of free radical scavenging capacity.
Table 5:Color Fastness Light Tests of cotton and wool samples.
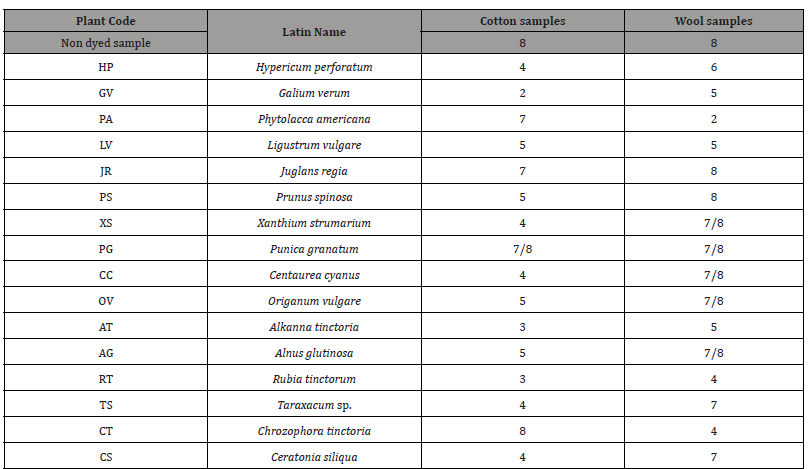
From the results in the current study, it was observed that the color of the wool samples shows higher color fastness to artificial light compared to the color of the respective cotton when dyed with the extract of the same plant species. Exceptions are two cotton samples CT and PA which have excellent color fastness to artificial light but have the lowest K/S values, therefore they had absorbed minimal color and had a light hue which was slightly affected by artificial light.
Two samples stood out among the dyed cotton samples, PG and JR, which had excellent color fastness to artificial light and high values of 7/8 and 7 respectively. It was observed that these samples had the highest K/S values among the cotton samples (8,27 and 5,23). In addition, the value of the antioxidant capacity of PG and JR extracts was one of the highest measured among the extracts and increased over time from 20 min to 60 min.
The color fastness to artificial light of the dyed cotton samples LV, PS, OV and AG was quite good and was rated with 5 on the blue wool scale. The dyed cotton samples TS, CS, HP and XS had a moderate strength equal to the 4 on the blue wool scale. The antioxidant capacity of the above extracts was quite good but the K/S value was moderate. Two dyed cotton samples AT, RT was low color fastness to artificial light of 3 and the K/S value was very low. Exception was the dyed cotton sample GV, which had the lowest color fastness to artificial light and was rated with 2 but had a good value K/S (4,82) and the extract had a high antioxidant activity (87,32). From the results of the color fastness to artificial light of the dyed wool samples, it was observed that most of them had from good to excellent durability. Exceptions are the RT and CT which had medium values 4 and the dyed wool sample PA which had low value 2 on the blue wool scale.
It was observed that there was dyed wool samples which had excellent color fastness to artificial light. Two wool samples JR and PS was rated with 8, five wool samples, XS, PG, CC, OV and AG was rated with 7/8 and two wool samples TS and CS was rated with 7 on the blue wool scale. Of the above samples, CC, TS and CS had low K/S value were light in color and their color fastness to artificial light was high, as expected. The remaining samples with excellent light resistance had high K/S values and therefore they absorbed enough color. In addition, the values of the antioxidant activity of these extracts were very high (greater than 81 in 20 min and greater than 83 in 60 min).
The excellent light fastness of the wool dyed samples JR, PS, XS, PG, OV, AG and the cotton samples JR, PS and PG obtained under the conditions specified by the ISO B02 was further confirmed by overexposing the dyed samples for another 144h making the total exposure time 216 h, which overpasses the conditions specified by the ISO B02. The results are given in Table 6. The light fastness results obtained the wool samples PS, XS, PG, OV, AG and the cotton sample PG were surprisingly excellent and remained unchanged between 7 to 8 on the blue wool scale. The dyed wool sample JR was rated with 4 and the cotton sample JR was rated with 4/5. While the cotton sample PS was rated with 2.
Table 6:Color Fastness Light Tests of cotton and wool samples (216 hours).
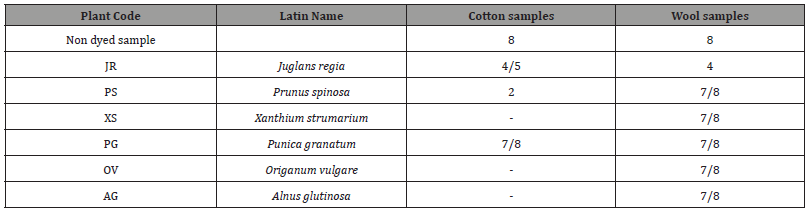
The extremely high fastness to artificial light of the above samples, matching the light fastness of some synthetic dyes (Vat dyes are recommended to specialized application where high light fastness is required such as automotive interiors). In general, the color fastness of plant dyes to solar radiation is quite low, due to the photochemical oxidation caused by the dyes (chromophore substituents). The aqueous extracts from the above plants, although they are plant pigment, have excellent resistance to solar radiation.
Conclusion
Cotton and wool samples were dyed successfully with the aqueous extract of plants. High coloristic values were obtained on wool samples indicating the possible formation of electrostatic bonds between the protonated amino groups of wool and negatively charged aqueous extract molecules. The light fastness of the wool samples was excellent, being equal to the most light-fast synthetic dyes. The light fastness of the cotton dyed samples was very good. Although natural dyes have low color light fastness, the results of the present study showed that there are plant species that give quite high to excellent color light fastness at the same level of synthetic colors..
The excellent color fastness of dyed wool and cotton samples was proportional to the combination of high antioxidant activity % RSC of the extracts and the high K/S value of the samples. An exception was the color in the cotton sample GV which, although it had high K/S values and antioxidant activity, it had minimal light fastness. It is possible the color resistance of dyed cotton and wool samples to light depends on factors other than the combination of K/S and antioxidant capacity of the aqueous plant extract. This present work shows that the above specific plants can be successfully used for the dyeing of cotton and wool fabrics with good overall dyeing characteristics and exceptionally high light fastness. These plant species could possibly be new alternative cultivation for the production of natural dyes, thus contributing to environmental protection and sustainable economic growth.
References
- MA Rahman Bhuiyan, A Islam, A Ali, MN Islam (2017) Color and chemical constitution of natural dye henna (Lawsonia inermis L.) and its application in the coloration of textiles. J Clean Prod 167: 14-22.
- ET Güzel, R Karadag, (2021) Sustainability of Organic Cotton Fabric Dyeing with a Natural Dye (Gallnut) and Analysis by Multitechnique Approach J Nat Fibers 18(8): 1107-1118.
- H Schweppe (1992) Handbuck der Naturfarbstoffe. Vorkommen, Verwendung, Nachweis, Ecomed, Landsberg/Lech, Germany, pp. 800.
- B Glover (1998) Doing what comes naturally in the dyehouse. J Soc Dyers Colour 114(1): 4-7.
- P Naidis, S Lykidou, M Mischopoulou, E Vouvoudi, NF Nikolaidis (2023) Study of the dyeing properties of saffron and ultrafiltrated saffron powders, as colourants for natural and synthetic fibers. Color Technol 139(5): 565-577.
- OEKO-TEX® Standard 100, Edition 4/2010, OEKO-TEX, Zurich.
- R Rana, K Dhiman, MS Ashawat (2022) Natural Coloring Agents for Fibers and Their Medicinal Values: A Review. J Nat Fibers 19(16): 14755-14770.
- (1991) BS1006, Methods of test for color fastness of textiles and leather, 5th ed. J Soc Dyers Colour 107: 84.
- TG Tutin, VH Heywood, NA Burges, DM.Moore, DH Valentine, et al. (1968, 1972, 1976, 1980) Flora Europaea 2, 3, 4, 5. Cambridge University Press, Cambridge, USA.
- TG Tutin, NA Burges, AO Chater, JR Edmonson, VH Heywood, et al. (1993) Flora Europaea 1, 2nd edition. Cambridge University Press, Cambridge, USA.
- A Strid and K Tan (eds). (1997) Flora Hellenica. Vol: 1, Koeltz Scientific Books, Kö
- B Rigg, Colorimetry and the CIE system in colour physics for industry. The Society of Dyers and Colourists, ed. by R McDonald (Bradford, 1997 ISBN:0901956708), pp. 83.
- I Eleftheriadis, N Nikolaidis, E Tsatsaroni (2016) Chemistry and Color Technology. Greek Academic Electronic Textbooks and Aids, Athens, Greece, pp. 176.
- S Kedare, RP Singh (2011) Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol 48(4): 412-422.
- L Chaires Martinez, M Perez Vargas, A Cantordel Angel, F Cruz Bermudez, H Jimenez Avalos (2013) Total Phenolic Content and Antioxidant Capacity of Germinated, Popped, and Cooked Huauzontle (Chenopodium berlandieri ssp. nuttalliae) Seeds. Cereal Chem 90(3): 263-268.
- Methods of Test for Colour Fastness of Textiles and Leather. The Society of Dyers and Colourists. BS 1006:1990. Bradford UK.
- A Hall (2008) Colour Fastness standards for Textiles and Leather. Grom BS 1006 to BS EN ISO 105. Journal of The Society of Dyers and Colourists 112(5-6): 144-145.
- P Naidis, S Lykidou, M Mischopoulou, E Vouvoudi, N Nikolaidis (2022) Study of the Dyeing Properties of Annato and Ultrafiltrated Annato as Colourants for Natural Fibres. J Nat Fibers 19(14): 7885-7895.
-
Tsouka Niki*, Nikolaidis Nikolaos, Lazari Diamanto, Dimitriadis Kyriakos and Theodoropoulos Konstantinos. Light Fastness Testing of Cotton and Wool Samples Dyed with Natural Dyes and Correlation the Results with the Antioxidant Activity of the Plants. J Textile Sci & Fashion Tech 10(4): 2024. JTSFT.MS.ID.000748.
-
Light fastness; natural dyes; %RSC; cotton, wool; Iris Publishers; Iris Publishers Group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






