 Research Article
Research Article
Next-Generation Healthcare: Exploring GAI, Computational Medicine & Tissue Engineering, within Biomedical Engineering
Zarif Bin Akhtar*
Department of Computing, Institute of Electrical and Electronics Engineers (IEEE), USA
Zarif Bin Akhtar, Department of Computing, Institute of Electrical and Electronics Engineers (IEEE), USA
Received Date:June 26, 2025; Published Date:July 07, 2025
Abstract
Recent advancements in molecular engineering have significantly reshaped the landscape of modern medicine, catalyzing innovations across diagnostics, therapeutics, and regenerative healthcare. The COVID-19 pandemic underscored the urgent demand for continued breakthroughs in this interdisciplinary space. This study investigates the convergence of biomedical and molecular engineering, emphasizing its influence on regenerative medicine, biomaterials, tissue engineering, and the deployment of cutting-edge biotechnologies. Central to this work is an in-depth examination of biomaterials in regenerative applications, organ-on-a-chip systems, and the transformative capabilities of bioprinting in fabricating functional tissues and organs. Furthermore, a detailed case study explores current innovations in drug development, immune system modulation, gene editing, and precision medicine—highlighting the evolving design, screening, and processing pipelines for biologics. The research also assesses the expanding role of AI-driven computing technologies, including artificial intelligence, deep learning, machine learning, and computer vision, in medical diagnostics and anatomical analysis. These tools are revolutionizing biomedical engineering by enabling data-driven solutions for complex clinical challenges. Finally, the study explores the integration of bioinformatics, accelerated computing, and functional genomics, illuminating their potential to predict and address future health issues and contributing to the advancement of personalized and predictive healthcare.
Keywords:Artificial Intelligence (AI), Biomedical Engineering (BME), Biomedical Applications & Devices, Deep Learning (DL), Machine Learning (ML), Healthcare Informatics, Medical Informatics
‘Introduction
In recent years, the field of medical science has experienced transformative advancements, leading to pioneering innovations that have redefined healthcare practices across diagnostic, therapeutic, and patient care domains. These developments have not only improved disease detection and treatment strategies but have also significantly enhanced the overall quality of patient care, offering renewed hope for managing complex health conditions [1,2,3]. Among these advancements, regenerative medicine stands out as a dynamic area of innovation focused on the repair, replacement, or regeneration of damaged tissues and organs. This is achieved through advanced techniques such as stem cell therapy, gene editing, and tissue engineering, each of which holds the potential to revolutionize treatments for previously incurable diseases.
The fabrication of implantable artificial organs—including synthetic hearts, kidneys, and skin grafts—using combinations of polymers and biological materials, marks a major milestone in modern medicine. These innovations aim to expand the availability of life-saving solutions for patients awaiting organ transplants. In parallel, nanotechnology has emerged as a critical enabler of targeted drug delivery systems, reducing off-target effects and opening new pathways for treating diseases like cancer. Noteworthy breakthroughs involve the use of nanoparticles to create imaging agents that selectively identify and visualize pathological cells, offering more precise and less invasive diagnostic tools.
Gene editing technologies, particularly CRISPR-Cas9, have further accelerated progress by enabling precise genetic modifications with potential applications in treating genetic disorders and complex diseases such as Alzheimer’s, cancer, and HIV. While the promise of these techniques is considerable, ongoing ethical and safety considerations remain central to their broader clinical adoption [4,5,6]. Concurrently, the integration of artificial intelligence (AI) and machine learning (ML) into healthcare systems is reshaping clinical workflows. These technologies facilitate the analysis of large-scale medical datasets, enhancing diagnostic accuracy, optimizing personalized treatment plans, and enabling proactive patient monitoring [7,8,9]. Prominent applications include AI-driven models capable of identifying early signs of conditions like psychosis, predicting patient outcomes, and assisting in cancer detection. In oncology, CAR T-cell therapy represents a breakthrough in immunotherapy by employing genetically engineered T cells to target malignant cells. This approach has shown considerable success in treating certain lymphomas and is being investigated for wider oncological use. The rapid development and success of mRNA-based vaccines, most notably those deployed during the COVID-19 pandemic, demonstrate a shift toward adaptable and efficient vaccine platforms with implications for future infectious disease management [10,11,12].
Technological advancements such as 3D printing have facilitated the creation of customized prosthetics, surgical models, and implants, streamlining medical procedures and improving patient-specific care. Telemedicine, especially during the global health crisis, has expanded access to remote healthcare services, enhancing convenience and reducing systemic costs. Moreover, immersive tools like virtual reality (VR) are transforming medical training by simulating complex procedures in safe, controlled environments. Wearable health technologies, including fitness trackers and smartwatches, are empowering users to monitor key health indicators such as heart rate, sleep, and physical activity— fostering preventive healthcare and enabling clinicians to track patient health remotely. This study is situated at the intersection of these advancements within the broad scope of biomedical engineering, an evolving discipline that integrates engineering principles with medical and biological sciences to address challenges in healthcare innovation. Biomedical engineering, also known as medical engineering, plays a pivotal role in the development of technologies for diagnostics, therapy, and hospitalbased healthcare management.
Originally an interdisciplinary specialization, biomedical engineering has matured into a distinct and essential field. Biomedical engineers contribute to innovations ranging from biocompatible prosthetics and therapeutic devices to advanced imaging technologies such as MRI and ECG/EEG, as well as regenerative tissue models and pharmaceutical development [13,14,15]. Complementing this field is biological engineering or bioengineering, which applies biology-inspired design principles to practical applications across medicine, agriculture, and environmental systems [16,17,18]. Bioengineering draws from a diverse scientific foundation—including biocatalysis, biomechanics, thermodynamics, and bioinformatics—and supports innovations in medical diagnostics, disease modeling, renewable systems, and engineered microorganisms. Cuttingedge developments include portable disease detection devices, prosthetic limbs, biosynthetic pharmaceuticals, and lab-grown tissues. The synergy between bioengineering and biomedical sciences facilitates collaboration with clinicians and researchers to replicate or modify biological processes using engineering methods. Biomedical engineers work closely with multidisciplinary teams to understand biological systems and translate that knowledge into devices and techniques that improve patient health and quality of life. Notable accomplishments stemming from this collaboration include the creation of dialysis machines, joint replacements, vascular surgical technologies, and even artificial hearts—each contributing meaningfully to extending human life and enhancing patient outcomes.
Methods and Experimental Analysis
This research adopted a comprehensive and systematic methodology to investigate the intersection of Artificial Intelligence (AI), Accelerated Computing, Biomedical Peripherals, Molecular Engineering, and Regenerative Medicine in the context of biomedical application systems. The approach was iterative and structured, ensuring that each phase contributed meaningfully to the overarching objective of advancing technical computing functionality within Biomedical Engineering. The study commenced with an in-depth background research investigative explorations aimed at gathering extensive background knowledge. This step facilitated the identification of current trends, emerging technologies, and potential research gaps within the interdisciplinary fields of AI, bioinformatics, and regenerative medicine. Sources included peer-reviewed journals, technical reports, and validated scientific repositories, ensuring the credibility of the foundational information. Following this, relevant datasets and knowledge components were systematically collected and integrated into the KNIME Analytics Platform, an open-source tool widely used for data-driven scientific research. Within KNIME, various data mining techniques and analytical workflows were developed and deployed. The dataset underwent preprocessing and post-processing stages to ensure accuracy, consistency, and relevance, with emphasis on eliminating redundancy and noise.
To assess the impact of AI and computational tools within biomedical frameworks, performance analytics were carried out. Data visualizations were generated to represent trends, patterns, and anomalies, enabling intuitive understanding of the findings. Furthermore, design prototypes of intelligent biomedical systems and regenerative medicine models were simulated using customdeveloped modules and plugins, facilitating visual analysis and interactive exploration. The analytical outcomes were evaluated using a range of performance metrics such as accuracy, sensitivity, specificity, and precision. These metrics provided comparative insights into the effectiveness of the proposed methods against traditional computing approaches. Particular attention was paid to the adaptability, scalability, and real-time efficiency of the solutions within clinical and research-oriented biomedical scenarios. Subsequently, the results were interpreted in light of the initial research objectives, focusing on the integration of AI with medical informatics, the role of molecular engineering in regenerative therapies, and the influence of computational peripherals on biomedical workflows. The discussion highlighted practical implications, theoretical advancements, and potential clinical applications derived from the experimental observations.
Lastly, the research findings were consolidated into a comprehensive conclusion, acknowledging the inherent limitations of the study, such as data availability and system complexity. Recommendations for future research directions were proposed, particularly in enhancing AI-driven regenerative medicine techniques and optimizing computing frameworks for nextgeneration biomedical applications. This methodology enabled a holistic exploration of the research domain, demonstrating how AI-enhanced tools, smart peripheral integration, and molecular innovations can collectively reshape the future of Biomedical Engineering—paving the way for improved diagnostic, therapeutic, and prognostic solutions.
Background Research and Investigative Explorations towards Available Knowledge
The field of regenerative medicine holds immense promise within biomedical engineering, focusing on enhancing cell activity to promote tissue regeneration. Often, damaged or injured tissues exhibit limited natural healing potential, impeding crucial processes like cell migration, proliferation, and differentiation. Scientific advancements have been made to augment these natural healing abilities, potentially leading to patient-friendly tissue regeneration methods. However, conventional cell culture conditions, primarily reliant on polystyrene dishes, fail to replicate the complex cellular interactions found in native tissues. This discrepancy in cell conditions results in diminished cell activity in vitro compared to in vivo settings, impacting essential functions such as differentiation, proliferation, metabolism, and cytokine secretion. Consequently, discrepancies arise between in vitro drug screening outcomes and preclinical or clinical studies due to variations in cell condition and activity.
To advance regenerative medicine, it is imperative to enhance cell function and activity both in vitro and in vivo. Biomaterials play a pivotal role in augmenting cell activity for regenerative medicine, facilitating advancements in tissue regeneration potential. Collagen, a natural biomaterial abundantly present in the body and a crucial component of the extracellular matrix (ECM), has been extensively utilized to promote cell activity in various tissues, including bone, cartilage, muscle, and cancer. For instance, collagen scaffolds, coupled with controlled drug release systems, have facilitated bone regeneration, while collagen-fibrin hydrogels have supported osteogenic differentiation of cells. Moreover, anisotropic collagen scaffolds have stimulated muscle bundle assembly and invasive cancer cell migration, showcasing collagen’s versatility in tissue engineering and drug research. Gelatin, derived from collagen, presents another valuable biomaterial. Gelatin-based hydrogels offer high biocompatibility and support cell sheet viability, growth, and function. These hydrogels can release growth factors like basic fibroblast growth factor (bFGF), which enhance cardiac contractile function and promote the expression of specific proteins like β-casein in epithelial cells [19-21].
Gelatin sheets, when combined with ovarian tissues and bFGF, significantly enhance stromal and endothelial cell proliferation. In wound healing applications, gelatin sheets impregnated with platelet-rich plasma accelerate capillary and tissue formation. Furthermore, gelatin-based hydrogel systems with drug release capabilities are employed to mimic cancer cell invasion, enabling the evaluation of cancer cell behavior in response to various stimuli like transforming growth factor-β1. Alginate, a natural polysaccharide derived from seaweed, finds widespread use in cell encapsulation systems for tissue engineering and drug research [22-33]. Alginate-based hydrogels support embryonic stem cell differentiation into primordial germ cells and promote osteogenesis and mineralization when encapsulating mesenchymal stem cells. Injectable alginate-based hydrogels have been developed for cell delivery to damaged tissues, offering advantages such as oxygen permeability and biocompatibility [34-44]. These injectable gels eventually disappear after transplantation, avoiding long-term interference with tissue regeneration.
Moreover, alginate-based hydrogels have been utilized to create cancer tissue models that mimic cancer invasion and metastasis, facilitating anti-cancer drug screening. Chitosan, a biopolymer derived from chitin, is renowned for its biocompatibility and versatility, finding applications in various regenerative medicine approaches such as blood vessel regeneration, cartilage formation, bone regeneration, intervertebral disc therapy, and skin tissue engineering [45-55]. Chitosan-based scaffolds and membranes mimic native tissue properties, promoting cell adhesion, proliferation, and expression of tissue-specific markers. Chitosan nanohybrids and composites exhibit enhanced bioactivity and osteoconductivity for bone regeneration. Additionally, chitosan hydrogels support nerve regeneration and serve as drug delivery systems, fostering stem cell and neuron culture and migration. Silk fibroin, derived from silkworms, boasts unique properties for tissue engineering applications. Silk fibroin scaffolds support bone and cartilage regeneration, creating a microenvironment conducive to osteogenic and chondrogenic differentiation. These scaffolds can be optimized by incorporating other materials like gelatin to enhance cell growth and tissue-specific gene expression. Additionally, silk fibroin membranes have been investigated for their effects on acoustic energy transfer and tensile strength in cartilage and tympanic membrane regeneration. Agarose stands as a widely utilized biomaterial in regenerative medicine due to its distinctive properties. Composed of D-galactose and 3.6-anhydro L-galactopyranose units, agarose can absorb water and facilitate the permeation of oxygen and nutrients to encapsulated living cells. It forms gels through hydrogen bonding and electronic interactions, eliminating the need for harmful crosslinking agents. Importantly, agarose exhibits low immunogenicity [11-33]. Researchers leverage its tunable properties to create gels of varying stiffness for tissue engineering applications. Combining agarose with polydopamine enhances water content, cell adhesion, collagen deposition, and angiogenesis, making it a valuable tool for regenerative medicine, including nerve and cornea regeneration. Matrigel, derived from a complex protein mixture found in mouse Engelbreth-Holm- Swarm tumor, serves as an alternative basement membrane for cell culture when replicating human basement membrane integrity is challenging.
Matrigel is particularly valuable in cancer research, aiding in invasion assays, morphology evaluation, and gene expression studies [18-38]. When combined with other biomaterials like alginate, Matrigel maintains high malignancy, spreading, migration, and invasion activities of cancer cells, crucial for studying cancer cell behavior in a biomimetic matrix. Matrigel-assisted tissue engineering holds promise in cancer tissue engineering and anti-cancer drug validation, enhancing the accuracy of in vitro experiments. Poly (lactic acid) (PLA) and poly (lactic-co-glycolic acid) (PLGA) stand out as biomaterials with significant applications in regenerative medicine. PLA, boasting an elastic modulus similar to bone, finds suitability in bone tissue engineering, especially when combined with hydroxyapatite (HA), crucial for ECM remodeling and homeostasis. Porous PLA-HA scaffolds support efficient culture of osteoblast cells, with HA distribution enhancing cell adhesion and wettability. PLGA, known for its biodegradability and biocompatibility, supports nerve cell culture, promoting axonal growth and nerve regeneration, with PLGA conduits showing promise in peripheral nerve regeneration when combined with substances like salidroside [25-35]. Biomaterials play a vital role in various aspects of regenerative medicine, from supporting cell culture to aiding tissue engineering and drug delivery. Natural biomaterials like collagen, gelatin, alginate, chitosan, and silk fibroin offer diverse advantages, including biocompatibility, controlled drug release, and support for tissue-specific differentiation. These biomaterials have been instrumental in advancing regenerative medicine’s potential for tissue regeneration and disease treatment, contributing to the development of in vitro models to study disease mechanisms and drug responses accurately. Bioengineering is an interdisciplinary field that applies engineering principles to solve problems in the life sciences. Throughout history, the design and production of medical devices, including prosthetic devices, have been prevalent. Examples of ancient prosthetics, such as wooden digits found in Egyptian tombs, highlight early attempts to replace missing body parts. In the late 1700s, Luigi Galvani’s experiments explored the relationship between electricity and animal physiology.
This paved the way for using electrical impulses within the body for diagnostic purposes, as seen in the field of electro cardiology. Galvani’s student, Alessandro Volta, later invented the first battery in the early 18th century, leading to the application of electricity for therapeutic uses. Additionally, Wilhelm Roentgen’s discovery of X-rays in the 19th century revolutionized diagnostic procedures by utilizing electromagnetic radiation. In the 20th century, the world witnessed remarkable discoveries and breakthroughs in bioengineering. Mechanical, electrical, and chemical engineering principles converged to create complex medical systems. These advancements included the development of dialysis, pacemakers, artificial hearts, responsive prosthetic devices, and DNA testing that underlies within various genetic technologies [22-44]. As we enter the 21st century, bioengineering remains a dynamic field for technological breakthroughs and exciting new developments that hold the potential to significantly enhance the quality of life. Biological engineering is a discipline rooted in the biological sciences, similar to how other engineering fields are based on specific scientific principles. The term “bioengineering” was coined in 1954 by scientist Heinz Wolff, marking the recognition of this field as a distinct branch of engineering. Initially, bioengineering focused on electrical engineering due to its application in medical devices and machinery. As engineers and life scientists collaborated, they realized the need for a deeper understanding of biology in engineering work. This led engineers interested in biological engineering to devote more time to studying biology, psychology, and medicine to enhance their knowledge in these areas. In recent years, the term biological engineering has also been applied to environmental modifications aimed at soil protection, slope stabilization, watercourse management, and ecological enhancement [33-44]. Agricultural engineering has also been encompassed within biological engineering due to their shared focus on living organisms. The first biological engineering program in the United States was established at the University of California, San Diego in 1966, followed by similar programs at MIT and Utah State University. Many agricultural engineering departments worldwide have also rebranded themselves as agricultural and biological engineering or agricultural and biosystems engineering. Biological engineering spans a wide range of scales and complexities, applying engineering principles to systems ranging from the molecular level (such as molecular biology and biochemistry) to cellular and tissuebased systems (including devices and sensors) to whole organisms and even entire ecosystems [22-44]. It encompasses various fields, including microbiology, pharmacology, immunology, neurobiology, and neuroscience, and offers a broad base of knowledge to address diverse biological challenges. Bioinformatics is an interdisciplinary field that focuses on developing methods and software tools to analyze and interpret biological data.
It combines computer science, statistics, mathematics, and engineering to unravel the complexities of biological information [25-35]. As an umbrella term, bioinformatics encompasses a broad range of biological studies that utilize computer programming as a crucial part of their methodology. It also refers to specific analysis “pipelines” that are commonly used, particularly in the field of genomics. Bioinformatics plays a vital role in identifying candidate genes and nucleotides, such as single nucleotide polymorphisms (SNPs) [22-33]. The identification of these genetic variations helps in understanding the genetic basis of diseases, unique adaptations, desirable traits in agricultural species, and differences between populations. Beyond its practical applications, bioinformatics also seeks to uncover the underlying organizational principles within nucleic acid and protein sequences [33-44]. By studying the patterns and structures of these biological molecules, researchers aim to gain insights into their functions and evolutionary relationships. In other words, bioinformatics serves as a powerful tool in biological research, enabling scientists to analyze large-scale biological datasets, identify genetic variations, and explore the organization and function of biological molecules. It contributes to advancing our understanding of various biological phenomena and has significant implications in fields such as medicine, agriculture, and evolutionary biology [28-38]. Biomedical engineering encompasses various subfields that contribute to advancing healthcare and improving human well-being.
Biomechanics focuses on studying the mechanical aspects of biological systems at different levels, from whole organisms to cell organelles, using principles of mechanics. Biomaterial’s science explores the interaction of materials with living systems and has applications in medicine, biology, tissue engineering, and materials science [26-36]. Biomedical optics combines physics, engineering, and biology to study the interaction of biological tissue and light for sensing, imaging, and treatment purposes. Tissue engineering aims to create artificial organs and tissues for transplantation, utilizing biological materials and engineering techniques [25- 35]. Genetic engineering involves the direct manipulation of an organism’s genes, finding applications in various fields such as crop improvement and the production of pharmaceuticals. Neural engineering focuses on understanding, repairing, replacing, or enhancing neural systems using engineering approaches [35-45]. Pharmaceutical engineering is an interdisciplinary science that combines drug engineering, drug delivery systems, pharmaceutical technology, chemical engineering operations, and pharmaceutical analysis to improve medicinal treatment. Each of these subfields contributes to the advancement of biomedical engineering and has the potential to significantly impact healthcare and quality of life.
Functional genomics is a field within molecular biology that aims to understand the functions and interactions of genes and proteins. It utilizes the vast amount of data generated by genomic and transcriptomic projects, focusing on dynamic aspects such as gene transcription, translation, regulation of gene expression, and protein-protein interactions. Unlike the static aspects of genomic information, such as DNA sequence, functional genomics takes a genome-wide approach to study these questions using high-throughput methods [36-46]. The definition of function in functional genomics is often based on the “causal role” of a gene or protein, referring to its sufficiency and necessity for a particular function. The goal of functional genomics is to comprehensively understand the functions of genes and proteins, aiming to eventually encompass all components of a genome. This includes studying the biochemical, cellular, physiological properties, and natural genetic variations of genes and proteins. Functional genomics encompasses various techniques and applications. It involves studying aspects of the genome itself, such as mutations and polymorphisms, as well as measuring molecular activities using techniques like transcriptomics, proteomics, and metabolomics. Multiplex techniques are commonly used to measure the abundance of gene products or analyze the effects of gene variants and mutants. By integrating these measurements, functional genomics seeks to provide a more comprehensive understanding of how the genome specifies function and contributes to systems biology approaches. Functional genomics plays a crucial role in unraveling the complex functions and interactions of genes and proteins, providing insights into biological processes and improving our understanding of various diseases and biological systems.
Biomedical Applications, Peripheral Devices: Medical Informatics & Regenerative Medicine
The field of Biomedical Engineering has witnessed remarkable growth in recent years, emerging as a powerful interdisciplinary domain that blends engineering principles, advanced technology, and medical sciences to address complex healthcare challenges. This dynamic integration has cultivated an environment of innovation, enabling biomedical engineers to develop revolutionary solutions that have transformed healthcare delivery and significantly improved global health outcomes. One of the most impactful areas within biomedical engineering is the development of innovative healthcare technologies. Engineers in this field have contributed to groundbreaking inventions such as prosthetic limbs, artificial hearts, bionic contact lenses, and the camera pill—a capsule equipped with a camera, battery, light source, and transmitter that captures real-time internal imagery of the gastrointestinal tract. These innovations have revolutionized patient care, making diagnosis and treatment more precise, less invasive, and highly personalized.
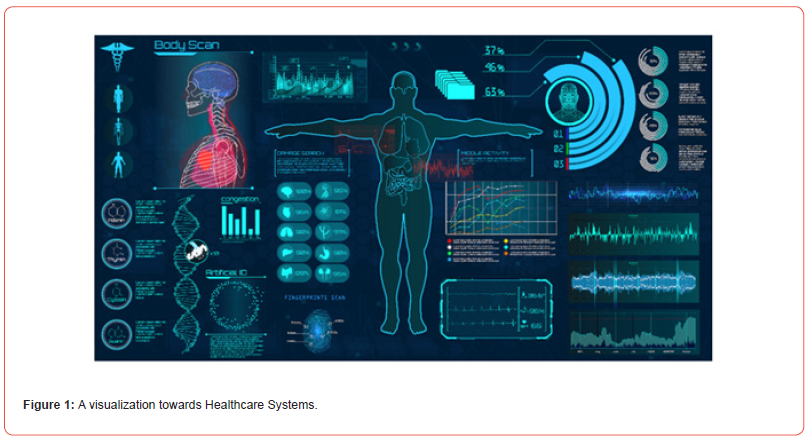
Beyond these inventions, biomedical engineers contribute to the advancement of medical research and pharmaceutical development, including the formulation of novel drugs and treatment strategies for critical illnesses like cancer. Their contributions extend into procedural innovations such as laser-assisted surgeries, which offer precise, minimally invasive, and long-term therapeutic outcomes. To further elaborate, Figure 1 illustrates the interconnected nature of physical and cyber healthcare systems, alongside a broad overview of the healthcare data spectrum. This visual representation demonstrates how medical informatics integrates with real-world applications to enhance system-wide diagnostics, monitoring, and treatment planning. Collaboration lies at the heart of biomedical innovation.
Biomedical engineers work closely with healthcare professionals—doctors, surgeons, nurses, and technicians—to design and refine diagnostic and therapeutic devices, including MRI machines, dialysis units, ultrasound scanners, and realtime monitoring tools. These technologies play a vital role in enhancing diagnostic accuracy, streamlining treatment pathways, and improving patient monitoring. The evolution of wearable biomedical devices such as pacemakers, health trackers, and biosensors represents another transformative shift. These devices offer continuous, non-invasive monitoring, empowering patients and clinicians with actionable insights. Biomedical engineers investigate complex biological processes to better understand system functionality, enabling the development of tailored, realtime solutions for chronic and acute health conditions.
Role of Biomaterials in Regenerative Medicine
The role of biomaterials in regenerative medicine and advanced
biomedical applications is of critical importance. These materials—
engineered to replace, restore, or enhance damaged tissues and
organs—are central to addressing chronic diseases and traumarelated
conditions. Recent advancements in biochemistry, molecular
biology, material science, and engineering have significantly
expanded the scope of biomaterials in clinical practice. Biomaterials
often serve as scaffolds, closely mimicking the extracellular matrix
(ECM), which provides structural and biochemical support to
tissues. These scaffolds play a vital role in promoting cell adhesion,
proliferation, and differentiation, contributing to effective tissue
regeneration and organ development.
• Natural hydrogels (e.g., chitosan, collagen, and
decellularized tissues) exhibit high biocompatibility and
biodegradability, making them ideal for tissue engineering.
• Synthetic hydrogels, such as polyethylene glycol (PEG),
offer scalable production and customizable mechanical
properties. They are widely used in 3D cell culturing, enabling
better simulation of in vivo environments.
Tuning hydrogel properties, such as porosity, stiffness, and degradation rate, facilitates enhanced control over cellmaterial interactions and optimizes regenerative outcomes. However, challenges persist—especially regarding the dynamic and heterogeneous nature of native tissue microenvironments. Synthetic hydrogels often require harsh chemical synthesis conditions, necessitating rigorous purification to prevent cytotoxic effects.
Emerging techniques, such as photochemical crosslinking, now allow spatiotemporal control of hydrogel architecture, offering new possibilities in 3D bioprinting and tissue modeling. This precise manipulation is crucial for mimicking the complex structurefunction relationships found in human tissues. Furthermore, a deeper understanding of molecular signaling pathways involved in cell–material interactions is vital. Integrating bioactive molecules and cues into scaffolds can drive specific cellular responses, enhance tissue integration, and improve healing efficiency. Monitoring these cellular responses through biosensing systems provides valuable feedback to optimize scaffold designs and delivery systems. The design of multifunctional biomaterials— capable of delivering drugs, signaling molecules, and providing mechanical support—represents the next frontier in regenerative medicine. Microfabrication technologies now enable the creation of intricate architectures that resemble native tissue structures, opening avenues for engineered organs and customized implants.
Industry Progress and Commercialization
The intersection of biomedical innovation and industry continues to grow, with numerous companies actively engaged in developing organ and tissue products that show significant clinical potential. Ongoing research and development (R&D) efforts are accelerating the translation of lab-scale discoveries into scalable medical solutions. Table 1 provides a summary of key companies, products, and their specific contributions to tissue regeneration, biomaterial design, and personalized therapeutic solutions. These include commercialized synthetic scaffolds, 3D printed tissues, and advanced wound-healing systems. Biomedical engineering continues to transform healthcare through innovations in medical informatics, wearable technologies, diagnostic devices, and regenerative medicine. The synergy between engineering and life sciences offers unprecedented opportunities to improve patient care, enhance clinical procedures, and advance personalized medicine. As biomaterials and intelligent medical systems become increasingly sophisticated, the future of healthcare will be defined by precision, adaptability, and bio-integration, ultimately contributing to improved patient outcomes and a healthier society.
Table 1:Selection for the various Commercially Available Biomaterials within Regenerative Medicine.

Advancements: Health Informatics, Medical Science, Regenerative Medicine
Biomedical engineering continues to lead groundbreaking innovations in medical science, health informatics, and regenerative medicine, transforming healthcare delivery and patient outcomes. At the core of this transformation is the integration of advanced technologies into clinical applications—redefining diagnosis, treatment, and medical education. One of the most significant developments in this field is the emergence of robotic-assisted surgery, which offers unparalleled precision and control. These robotic systems minimize hand tremors, enable minimally invasive procedures, and result in reduced incision sizes, faster recovery times, and a lower risk of post-operative infections. In addition, telesurgery—where surgeons operate remotely via robotic systems—is gaining traction, extending specialized surgical care to remote and underserved regions. Another area witnessing remarkable progress is tissue engineering, particularly with the use of 3D printing technologies.
Biomedical engineers are now capable of printing functional biological tissues and organs that may one day be used for transplantation. Additionally, 3D-printed models of human tissues, such as blood vessels and cardiac structures, are being utilized for disease modeling and evaluating therapeutic responses. These innovations not only enhance our understanding of complex physiological systems but also contribute to personalized medicine. The convergence of artificial intelligence (AI) and virtual reality (VR) with biomedical engineering is also paving the way for novel healthcare applications. AI-driven algorithms are revolutionizing medical image analysis, enabling faster and more accurate diagnosis of diseases such as cancer, cardiovascular conditions, and neurological disorders. Meanwhile, VR is transforming medical training by offering immersive simulations that allow clinicians to practice surgical procedures and improve interpersonal skills such as empathy and patient communication. Figure 2 illustrates the diversity of big data types in healthcare, visualizing how data science plays a pivotal role in modern medical informatics.
These technological innovations are no longer confined to research laboratories. They are actively being integrated into hospitals and clinics, enhancing imaging technologies, optimizing surgical outcomes, and improving the educational tools used by healthcare professionals. From robotic surgery systems to AIassisted diagnostics and VR-based medical education, the impact of biomedical engineering on contemporary healthcare is both widespread and profound. In parallel, regenerative medicine stands as a rapidly evolving domain focused on repairing, replacing, or regenerating damaged tissues and organs. By leveraging strategies such as tissue engineering, stem cell therapy, and artificial organ development, regenerative medicine aims to restore the functionality of biological systems compromised by trauma, disease, or aging.
While traditional approaches—like autografting—have been effective, they are often limited by complications such as tissue rejection. In response, biomedical engineers and researchers are increasingly focusing on immunomodulation to guide tissue regeneration. A deeper understanding of the immune system’s role in healing has revealed its critical influence on graft acceptance, tissue repair, and biomaterial integration. Key immune components such as macrophages, neutrophils, and cytokines are involved in inflammation, tissue remodeling, and the synthesis of essential signaling molecules. Two emerging strategies in immunomodulatory regenerative medicine are the use of biomaterials and scaffoldbased systems. Traditional biomaterials—metals, ceramics, and polymers—carry the risk of immune rejection. However, recent innovations involve engineering these materials to elicit targeted immune responses, either promoting or suppressing inflammation based on therapeutic needs. Factors such as crosslinking density, surface hydrophobicity, and composition determine the immunological compatibility of the material.
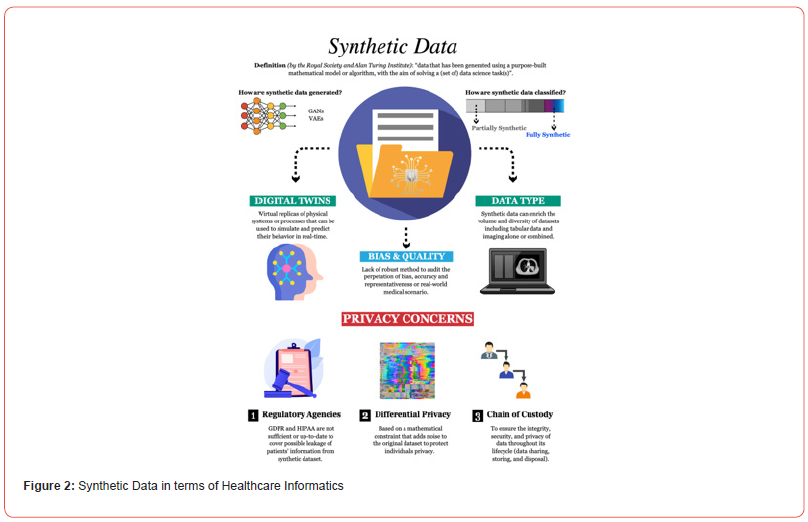
An especially promising avenue is the use of decellularized extracellular matrix (ECM) as a biocompatible scaffold. By removing cellular elements from biological tissues, researchers preserve a natural scaffold that supports cell adhesion, migration, and differentiation. This method has shown potential in various applications, including liver bioengineering, where ECM scaffolds crosslinked with nanomaterials like graphene oxide have demonstrated improved functionality, potentially offering alternatives to conventional organ transplantation. These innovations in biomaterial science, immune system regulation, and regenerative scaffolding are expanding the possibilities for personalized, effective, and durable medical treatments. As biomedical engineers continue to explore the dynamic interplay between biological systems and synthetic environments, regenerative medicine stands poised to redefine the future of therapeutic healing.
Innovations: Tissue Engineering
Tissue engineering and regenerative medicine are among the most innovative and rapidly advancing fields in biomedical engineering, aimed at repairing, replacing, or regenerating damaged or diseased tissues and organs. These disciplines merge principles of biology, materials science, and engineering to address complex medical challenges that traditional treatments cannot adequately solve. Tissue engineering integrates scaffolds, biologically active molecules, and living cells to create functional tissue constructs. This multidisciplinary approach strives to restore, maintain, or enhance tissue function and is at the core of numerous groundbreaking biomedical applications. While several tissue-engineered products—such as FDA-approved artificial skin and cartilage—have reached clinical use, their adoption remains limited due to challenges in scalability, vascularization, and integration within host systems. Regenerative medicine, closely aligned with tissue engineering, extends the scope further by harnessing the body’s innate capacity for self-repair, often aided by stem cells, growth factors, or bioengineered materials. These fields are increasingly seen as complementary, and their terminologies are often used interchangeably, reflecting a shared goal: to move beyond managing diseases to fully curing them through biological reconstruction.
The innovation in this domain goes beyond therapeutic applications. Tissue-engineered systems are now used in nonclinical areas such as biosensors for detecting toxins and tissue chips for preclinical drug testing. These models offer accurate insights into human biological responses and are instrumental in reducing animal testing while accelerating personalized medicine.
The tissue engineering process typically begins with the design of biocompatible scaffolds, which may be synthetic (polymers, ceramics) or natural (decellularized tissues). These scaffolds serve as the foundation for cell adhesion, proliferation, and tissue development. In some advanced models, cells, scaffolds, and growth factors are combined simultaneously, enabling the formation of self-assembling tissue constructs. An emerging technique involves decellularizing donor organs to retain structural integrity, followed by repopulating them with a patient’s own cells—offering a promising path toward personalized organ regeneration.
One prominent application is the development of bioengineered liver tissue using decellularized scaffolds, which can serve as drug testing platforms or, potentially, as transplantable units. Similarly, advancements in vascularized tissue constructs hold promise for improving outcomes in bone healing, cartilage repair, and treatment of spinal cord injuries or peripheral nerve damage. Despite notable achievements, widespread clinical translation remains a challenge. While procedures like bladder implants, engineered skin grafts, and joint cartilage replacements have seen success, replicating complex, fully functional organs like the heart, lungs, or kidneys continues to be a formidable task.
The lack of adequate vascular networks, immune rejection, and functional integration are critical barriers researchers are actively working to overcome. Recent breakthroughs supported by organizations such as the National Institute of Biomedical Imaging and Bioengineering (NIBIB) include the creation of biological gels and adhesives for cartilage regeneration, stem cell-derived bone tissues, and angiogenic factors to stimulate vascular growth within engineered constructs. Additionally, research into kidney regeneration using patient-derived cells is showing potential for addressing chronic kidney disease and mitigating donor organ shortages. In disciplines such as orthopedics, cardiology, neurology, and dermatology, tissue engineering is providing transformative solutions—from healing complex bone fractures and restoring spinal cord function to regenerating vascular tissues. The development of immunomodulatory biomaterials further enhances tissue acceptance and integration by modulating host immune responses, thus improving the longevity and effectiveness of implants. Ultimately, the vision of producing fully functional, transplantable organs is within reach, though technical and biological hurdles remain. As research continues to address vascularization, scaffold biocompatibility, and immune response management, tissue engineering is poised to redefine the future of regenerative therapies—offering hope for millions worldwide and significantly advancing the frontiers of modern medicine.
Advances: Organs-On-A-Chip
Organ-on-a-chip (OOC) technology represents a cutting-edge advancement in biomedical engineering, particularly within the field of bio-MEMS (Micro-Electro-Mechanical Systems). These sophisticated, multi-channel 3D microfluidic devices integrate living cell cultures within a biomimetic chip environment, precisely engineered to emulate the mechanical, physiological, and biochemical functions of entire human organs or organ systems. OOCs bridge the longstanding gap between conventional 2D cell cultures and in vivo studies, offering a more accurate and dynamic in vitro model of human tissue behavior. This technology holds transformative potential for drug development, disease modeling, and toxicology testing by offering an alternative to traditional animal models. Numerous studies have successfully demonstrated the replication of specific organ functions—such as those of the heart, brain, liver, lung, kidney, and gut—using OOC platforms. Despite these advancements, current iterations of OOC devices may still oversimplify the human body’s complex organ interactions and cellular microenvironments. To overcome these limitations, research is focused on advancing microphysiometry— an integration of microfabrication, microelectronics, and microfluidics—to achieve more intricate physiological modeling. A landmark example includes liver-on-a-chip platforms used to study the pathophysiology of viral infections, such as hepatitis, providing detailed insight into organ-specific responses to viral agents.
Complementary to OOC technologies, lab-on-a-chip (LOC)
devices have also seen significant progress. LOC systems
miniaturize entire laboratory processes onto a single chip, allowing
for the manipulation of particles and fluids within microfluidic
channels. These devices offer advantages such as reduced reagent
usage, greater portability, improved process control, and costeffective
fabrication. LOC platforms have been widely applied in
cellular biology, enabling high-resolution studies of cell motility,
stem cell differentiation, signal transduction, and embryogenesis.
The evolution from 2D to 3D cell cultures marked a significant leap
in cell biology, yet these models often lack the ability to replicate
crucial aspects such as tissue-to-tissue interfaces and mechanical
stimuli. Organs-on-chips address these limitations by incorporating
dynamic fluid flow, nutrient gradients, mechanical forces, and
real-time responsiveness—closely mimicking the physiological
environment of human organs. OOCs now serve as next-generation
3D tissue models that effectively recreate complex biological
behaviors, including cellular signaling, tissue morphogenesis,
and organ-specific responses. These devices not only simulate
biochemical activities but also reproduce dynamic mechanical
conditions—such as the rhythmic contraction of cardiac tissue or
the breathing motions of lung cells. Recent developments in OOC
technology have led to the creation of a wide range of organ models
including:
• Brain-on-a-chip
• Gut-on-a-chip
• Lung-on-a-chip
• Heart-on-a-chip
• Kidney-on-a-chip
• Liver-on-a-chip
• Skin-on-a-chip
• Blood vessel-on-a-chip
• Prostate-on-a-chip
• Endometrium-on-a-chip
Each of these platforms provides invaluable tools for understanding disease mechanisms, testing pharmaceuticals, and assessing toxicological effects in a controlled and human-relevant setting.
The concept of human-on-a-chip or multi-organ-on-a-chip represents the next frontier, where interconnected organ chips work synergistically to replicate human systemic physiology. This innovation opens the door for more comprehensive modeling of complex disease states, individualized drug responses, and predictive toxicology—thus significantly reducing the dependency on animal testing.
Organ-on-a-chip technology is revolutionizing biomedical research and healthcare innovation. By providing a more accurate, ethically responsible, and mechanistically informative platform for studying human biology, OOCs are poised to play a central role in the future of precision medicine, drug discovery, and disease prevention.
Biomedical Applications: The AI Perspectives
Artificial Intelligence (AI), particularly through the implementation of machine learning (ML) techniques, has become an indispensable tool in biomedical research. Its strength lies in the ability to process and analyze complex, high-dimensional datasets, providing predictive capabilities and uncovering intricate patterns that would otherwise remain hidden through traditional analytical methods. One of the core applications of machine learning in biomedicine is predictive modeling using measurable patient or environmental data. For instance, in psychiatric medicine, ML algorithms have been leveraged to predict mood fluctuations based on passive data collected from smartphones, such as behavioral patterns and speech features. In neuroscience, machine learning has enabled the decoding of neural activity to infer cognitive states and motor intentions, which has significantly advanced brain-computer interfaces (BCIs) and neuroprosthetics. Moreover, machine learning functions as a benchmark against which humangenerated models can be evaluated. This not only helps identify gaps in existing theories but also fosters the discovery of novel biological mechanisms. ML excels in capturing nonlinear relationships and handling temporal dynamics, both of which are prominent in complex biological systems. As biomedical datasets continue to grow in volume, velocity, and variety, the limitations of human cognition in interpreting such data are increasingly mitigated by the scalability and adaptability of AI-driven approaches.
In the field of neural decoding, ML methods—especially deep neural networks and ensemble learning techniques—have consistently outperformed traditional linear models. These algorithms have proven effective in capturing the rich, nonlinear interactions between neural signals and behavioral outputs. Similarly, in neural encoding, ML provides a more sophisticated understanding of how external stimuli are represented within the brain, setting new standards for computational neuroscience. Importantly, the growing accessibility of AI tools—ranging from user-friendly software platforms to automated machine learning (AutoML) systems—has empowered biomedical researchers without extensive computational backgrounds. These tools allow scientists to focus on formulating hypotheses and interpreting outputs, rather than on the underlying algorithmic details.
Advances in 3D Bioprinting: Bridging AI with Tissue Engineering
The advent of 3D printing, and specifically 3D bioprinting, has emerged as a transformative innovation within biomedical engineering. By integrating living cells with biomaterials during the printing process, 3D bioprinting enables the fabrication of biologically functional structures that mimic native tissues. Projections suggest the global 3D bioprinting market will exceed $4.7 billion by 2025, highlighting its rapidly expanding influence in healthcare. One of the primary objectives of 3D bioprinting is to address the global shortage of transplantable organs. While fully functional bioengineered organs are still under development, simpler constructs such as bioprinted skin, cartilage, and cardiac patches have already shown clinical and research utility. These tissues are instrumental for drug screening, toxicity assessment, and regenerative medicine. The strengths of 3D bioprinting include its ability to produce anatomically precise structures, incorporate heterogeneous cell populations, and enable controlled delivery of biomolecules and growth factors. Nonetheless, the challenge of vascularization—the integration of functional blood vessels within bioprinted tissues—remains a critical barrier to the successful creation of large, complex organs.
Innovations such as laser direct-write (LDW) printing have achieved significant milestones in achieving single-cell spatial resolution. Researchers at Tulane University have used LDW technology to create sophisticated cellular architectures, including neural circuits and muscle fibers, opening avenues for disease modeling and functional tissue analysis. Furthermore, companies like Cellink have advanced the development of standardized bioinks tailored for various tissue types, making bioprinting more scalable and reproducible. In parallel, researchers at the University of Southern California have leveraged 3D printing to design modular microfluidic systems—cost-effective tools that simplify diagnostic assays and improve process control in lab-on-a-chip applications. The field is now progressing towards 4D bioprinting, where printed constructs are engineered to dynamically respond to environmental stimuli, such as temperature, pH, or mechanical forces. Industry leaders like GE Healthcare are investing in advanced bioprinting platforms that integrate digital modeling, high-resolution imaging, and real-time control for enhanced precision and reliability. Notable breakthroughs include Organovo’s Novo Tissues, therapeutic liver tissues designed for patients with rare liver diseases. Additionally, the development of hybrid additive manufacturing systems that combine 3D printing with plasma-based surface treatments has enabled the production of scaffolds with gradient properties— ideal for tissue-specific regeneration.
The Convergence of AI and Bioprinting
The synergy between artificial intelligence and 3D bioprinting holds immense promise. AI algorithms are being integrated to optimize print parameters, monitor bioprinting processes in real time, and model biological behaviors post-printing. Through data-driven feedback loops, AI can enhance structural integrity, cell viability, and functional outcomes in printed tissues. As AI and bioprinting technologies mature, their combined capabilities are expected to revolutionize personalized medicine, enabling patient-specific tissue fabrication and predictive modeling for therapy selection. This convergence marks a new era in biomedical innovation—one where complex biological systems can be engineered, understood, and enhanced through computational intelligence.
Case Studies Analysis: Drug Delivery Systems, Functional Genomics, Immune Engineering
Machine learning (ML) has significantly impacted biology and bioinformatics, particularly in functional genomics and systems biology, by enabling the modeling, prediction, and interpretation of complex biological phenomena. In genomics, ML algorithms have facilitated breakthroughs in regulatory, structural, and functional genomics by improving predictions of gene expression, identifying gene functions, and classifying protein structures. Advanced methods such as convolutional neural networks (CNNs) and recurrent neural networks (RNNs) have been deployed to model gene regulatory mechanisms and decode epigenetic patterns. ML has also been integrated with natural language processing (NLP) to mine large-scale genomic databases and biomedical literature, enhancing tasks such as relation extraction, gene-disease association discovery, and named entity recognition.
One of the most transformative applications of ML in genomics is in next-generation sequencing (NGS). Algorithms powered by supervised and unsupervised learning have significantly reduced the computational time and cost of sequencing genomes. Similarly, ML plays a key role in optimizing gene-editing tools like CRISPR-Cas9 by improving target site selection and predicting off-target effects with high accuracy. In proteomics, ML algorithms are essential for interpreting complex datasets generated by mass spectrometry. Techniques such as support vector machines (SVMs) and deep learning have enabled accurate protein identification, classification, and interaction mapping. These insights aid in biomarker discovery and disease diagnostics. ML has also been applied in microarray data analysis, improving gene expression profiling through advanced clustering, dimensionality reduction, and predictive modeling techniques. These applications enable researchers to uncover gene-disease relationships, forecast gene behavior, and design targeted therapies. Text mining, another powerful ML application, has streamlined the extraction of biological knowledge from vast scientific publications. Coupled with NLP, ML allows for automated annotation of gene functions, drug-target predictions, and the identification of molecular interactions. In systems biology, ML supports the development of computational models to simulate and predict biological network behaviors, including genetic, protein-protein, and metabolic networks. Algorithms such as Bayesian networks and genetic algorithms are commonly used to infer gene regulatory networks and explore phenotype-genotype relationships.
In parallel, the case study of drug delivery systems (DDS), particularly in the realm of immunotherapeutics, highlights the convergence of bioengineering, biomaterials, and computational approaches. Oral delivery of immunotherapies offers substantial advantages in terms of ease of administration and patient compliance, yet it presents formidable challenges such as enzymatic degradation, pH variability, and mucosal barriers in the gastrointestinal (GI) tract. The GI tract, rich in immune cells, provides a unique opportunity for site-specific immune modulation. Oral administration can induce systemic tolerance, potentially minimizing antidrug antibody responses and improving therapeutic efficacy. Immunotherapeutics including interleukins, monoclonal antibodies, nucleic acids (e.g., mRNA, DNA), and smallmolecule modulators benefit from biomaterial-based DDS that target specific intestinal regions.
Biomaterials with mucoadhesive properties, such as thiolated polymers, enhance the residence time of drugs by forming covalent bonds with mucosal surfaces, thereby improving local delivery for diseases like inflammatory bowel disease (IBD) and ulcerative colitis. Innovative DDSs like self-nanoemulsifying drug delivery systems (SNEDDS), in conjunction with mucolytic agents, enhance mucosal penetration and bioavailability. These systems allow precise delivery of anti-inflammatory agents, growth factors, and vaccines, thereby enabling targeted immune modulation. To traverse the epithelial barrier, advanced strategies utilize passive and active transport mechanisms including transcytosis, carriermediated diffusion, and the incorporation of cell-penetrating peptides (CPPs). Technologies such as molecular motors and transient permeabilizers facilitate systemic drug absorption, leading to more effective immune responses against mucosal infections. Emerging biomaterials—both natural (e.g., chitosanbased nanoparticles) and synthetic (e.g., PEGylated systems)— demonstrate promising capabilities for oral immunotherapeutic delivery. Devices like MucoJet, which injects immunotherapeutics through mucosal surfaces using pressurized microjets, exemplify innovation in this space. These technologies are instrumental in enhancing the pharmacokinetics and therapeutic index of orally administered biologics. Furthermore, next-generation systems are progressing towards 4D bioprinting—dynamic drug delivery platforms that respond to environmental stimuli (e.g., pH, temperature, enzymes). These adaptive materials offer enhanced spatiotemporal control of drug release, furthering the goals of personalized and precision medicine. Despite remarkable progress, challenges remain. Key obstacles include maintaining drug stability, ensuring reproducible targeting, controlling manufacturing costs, and navigating regulatory hurdles for clinical translation. Nevertheless, the integration of biomaterials, AI-driven modeling, and advanced DDS presents a transformative approach to immune engineering and therapeutic development.
Advances: Gene Editing, Precision Medicine
Precision medicine is at the forefront of transformative healthcare, offering the promise of personalized treatments tailored to an individual’s unique genetic makeup, lifestyle, and environment. Despite its immense potential, several challenges must be overcome to ensure its widespread adoption and clinical success. This section explores the recent advancements in gene editing and precision medicine, while also addressing the hurdles and opportunities that lie ahead. One of the most revolutionary technologies driving this transformation is genome editing, particularly tools like CRISPR-Cas9. These techniques enable targeted modifications to the genome, allowing for the correction of disease-causing mutations and offering new avenues for the treatment of previously intractable genetic disorders. Gene editing not only improves therapeutic precision but also opens doors to curative treatments for rare and complex diseases. However, implementing gene editing in clinical settings presents challenges such as the assessment of benefit-risk ratios, especially in cases involving small patient populations and rare diseases. Traditional clinical trial methodologies often fall short in these scenarios. As a result, novel statistical frameworks, such as pairwise comparisons of patient outcomes, are emerging as essential tools for evaluating treatment efficacy and safety in precision medicine.
Another major driver of precision healthcare is populationscale genomics. Initiatives like Estonia’s 100,000 Genomes Project exemplify the growing trend of utilizing genomic data at a national level to identify disease susceptibilities and optimize therapeutic interventions well before disease onset. This proactive approach shifts the healthcare paradigm from reactive treatment to preventive and predictive care. While the advancements are substantial, numerous challenges persist. These include issues related to data privacy, ethical implications, regional disparities in regulation, and accessibility of advanced therapies. Ensuring the secure storage, sharing, and analysis of vast genomic datasets is critical, as is developing ethical standards that can keep pace with rapidly evolving biotechnologies. Moreover, addressing the off-target effects of gene editing technologies remains an ongoing priority to ensure patient safety and therapeutic accuracy. The growing momentum in gene therapy and editing was underscored in recent forums such as the DIAmond session on “Precision Medicine, Gene Editing, and Gene Therapy”, which emphasized the need for cross-sector collaboration to overcome current limitations. Although societal acceptance is increasing and research continues to expand, concerns regarding treatment affordability, regulatory oversight, and equitable access must be carefully addressed.
Looking forward, the future of precision medicine lies in its integration into preventive healthcare, with the potential to significantly reduce long-term healthcare costs and improve population health outcomes. Key to this future will be international collaboration among stakeholders—including researchers, industry leaders, regulators, and patient advocacy groups—to harmonize regulations, foster innovation, and uphold ethical standards. Furthermore, public education and patient engagement are essential for enhancing societal understanding and acceptance of personalized medicine approaches. Gene editing and precision medicine are redefining modern healthcare by enabling individualized treatment strategies, early disease detection, and targeted therapeutic interventions. While challenges remain, the continued advancement of technologies, combined with thoughtful regulation and global cooperation, will pave the way for a more effective, equitable, and preventive healthcare ecosystem.
Healthcare and Medical Informatics: Drug Design, Discovery, Screening
Artificial Intelligence (AI) has emerged as a transformative force in the healthcare industry, significantly improving patient care, diagnosis accuracy, treatment personalization, and operational efficiency. Its integration across various healthcare domains has paved the way for smarter clinical decision-making, enhanced disease prevention strategies, and accelerated drug development. Machine Learning (ML) and Natural Language Processing (NLP)—two prominent AI technologies—have been instrumental in redefining how clinicians interact with medical data. ML enables predictive modeling by analyzing vast clinical datasets to identify patterns, detect anomalies, and provide accurate forecasts. This facilitates precision medicine, allowing clinicians to tailor treatments based on a patient’s genetic makeup, lifestyle, and health history. Deep Learning, a sophisticated subset of ML, has further enhanced capabilities in speech recognition, image classification, and the analysis of unstructured data like medical notes, radiology images, and pathology slides. Natural Language Processing plays a crucial role in interpreting electronic health records (EHRs), streamlining administrative workflows, and generating actionable insights from complex health data. It enhances the efficiency of medical documentation, enables realtime clinical alerts, and supports improved diagnosis through data-driven recommendations. Though rule-based expert systems have historically supported clinical decision-making, they are increasingly being replaced by AI-powered systems due to the latter’s superior adaptability, learning capabilities, and accuracy. AI is now being utilized across a broad spectrum of medical domains— from diagnostics and administrative automation to population health and telemedicine.
Clinical Applications of AI
• Cardiology: AI algorithms are aiding in the diagnosis
and risk stratification of cardiovascular conditions such as
coronary artery disease. Wearable devices and IoT-based
technologies enable real-time cardiac monitoring, enabling
early intervention.
• Dermatology: AI models outperform dermatologists
in certain diagnostic tasks, such as skin cancer detection, by
leveraging large annotated image datasets.
• Gastroenterology: AI enhances endoscopic image
interpretation, identifying lesions and abnormalities more
efficiently.
• Infectious Diseases: AI is used to predict treatment
outcomes, track antimicrobial resistance, and diagnose diseases
like malaria, tuberculosis, and meningitis.
• Musculoskeletal Disorders: AI aids in diagnosing jointrelated
disorders such as knee pain, improving access to care in
underserved populations.
• Oncology: AI facilitates tumor detection, cancer risk
assessment, molecular analysis, and drug development. It has
shown promise in diagnosing breast and prostate cancers with
high accuracy.
• Ophthalmology: FDA-approved AI systems are already in
use for screening diabetic retinopathy and other eye diseases.
• Pathology & Radiology: AI supports image interpretation
and enhances quality control by reducing noise and improving
resolution in CT and MRI scans.
• Psychiatry: Predictive models and chatbot-based
therapies are being explored to support the treatment of
anxiety, depression, and other mental health conditions.
• Primary Care: AI enhances decision support, predictive
modeling, and patient engagement through intelligent
platforms.
• Telemedicine: Real-time AI systems enable remote patient
monitoring, early warning systems, and EHR interpretation,
increasing access to healthcare in remote regions.
Drug Discovery and Design:
Despite advances in biotechnology and computational biology, drug discovery remains a complex, time-consuming, and resourceintensive endeavor. AI and computational modeling are now at the forefront of revolutionizing this process. Drug discovery involves identifying and designing therapeutic compounds that interact with specific biological targets. Computational methods, such as bioinformatics, molecular modeling, and machine learning, are utilized to analyze biological interactions, predict drug efficacy, and optimize molecular structures. Biopharmaceuticals and therapeutic antibodies are gaining traction, with AI enhancing their design for improved efficacy, stability, and safety. The process of drug development typically follows a structured pathway, including target identification, lead optimization, preclinical testing, clinical trials, and regulatory approval.
A notable innovation in this field is the Computational Analysis of Novel Drug Opportunities (CANDO) platform, which accelerates drug repurposing. For instance, during the Ebola outbreak, CANDO integrated computational predictions with in vitro validation to identify existing drugs with potential antiviral properties, reducing the cost and time associated with new drug development.
Recent breakthroughs also include
• Hybrid Compounds: Novel molecules combining Nitric
Oxide (NO) with diterpenoids are being synthesized for
antitumor properties, showcasing potential as next-generation
anticancer agents.
• Protein-Protein Interactions: Tools like iPPBS-Opt apply
pseudo amino acid composition and wavelet transforms to
predict protein-binding sites, offering deeper insights into
cellular mechanisms and aiding drug target identification.
• Drug Safety and Toxicity: Studies examining the
nephrotoxicity of antibiotics like vancomycin provide molecular
insights that can lead to safer therapeutic protocols.
• Targeted Therapeutics: Research continues into
antimalarial, antiviral, antimicrobial, antiepileptic, and
anti-inflammatory drugs, with AI models aiding compound
screening and efficacy prediction.
Industry Collaborations and Technological Integration
Major tech companies are significantly contributing to AI
integration in healthcare:
• IBM Watson collaborates with cancer centers for
personalized oncology.
• Microsoft Hanover analyzes medical literature to optimize
cancer drug therapies.
• Google DeepMind partners with the UK’s NHS for AIdriven
diagnostic support.
• Tencent and Intel are developing intelligent healthcare
platforms and predictive systems.
• Neuralink, spearheaded by Elon Musk, is pushing
boundaries with brain-machine interface technologies.
These collaborations enable large-scale data access and innovation, especially beneficial in developing countries where AI is bridging gaps in healthcare access, diagnostics, and treatment delivery.
Ethical and Regulatory Considerations
With AI’s growing influence, ethical considerations and regulatory frameworks are evolving to ensure responsible and fair usage. Regulations such as HIPAA (USA) and GDPR (Europe) emphasize patient data privacy and consent. The U.S. FDA’s Action Plan guides the regulation of AI-enabled medical devices, while additional guidelines address ethical concerns related to autonomy, justice, and non-maleficence.
AI is redefining healthcare by enhancing diagnosis, treatment personalization, administrative efficiency, and drug discovery (Figure 3). Its potential to revolutionize medical informatics lies in continued innovation, ethical deployment, and strong collaboration between technology providers, healthcare professionals, and regulatory bodies. As AI matures, it is set to deliver transformative solutions, especially in underserved and resource-limited settings, ultimately leading to improved patient outcomes and global health equity.
The New Future: Biologics
The pharmaceutical landscape is undergoing a transformative shift, with biologics rapidly emerging as the dominant force in innovative drug development and sales. According to Global Data’s report “Future of Pharma—Looking Ahead to 2022”, biologics are set to outpace small molecules in terms of revenue, with projections indicating a $120 billion sales advantage by 2027. These complex therapies, which include monoclonal antibodies, gene therapies, and cell-based treatments, are increasingly seen as the “primary engines of value creation,” as noted by Quentin Horgan, Managing Analyst at Global Data.
The surge in biologics reflects a broader industry trend driven by their superior efficacy in treating complex and chronic conditions, particularly in fields such as oncology and immunology. Monoclonal antibodies like Keytruda (Merck), Opdivo (Ono Pharmaceuticals), and Dupixent (Regeneron Pharmaceuticals) currently dominate the market and are expected to account for 46% of all biologics sales by 2027. Notably, Keytruda alone is projected to represent 4% of total biologics revenue, emphasizing its pivotal role in cancer treatment.
However, the most striking growth is anticipated in emerging biologic categories, such as gene therapies and gene-modified cell therapies. These innovative modalities are forecasted to experience over 1,000% growth between 2022 and 2027, fueled by promising pipeline candidates like RPA-501 from Rocket Pharmaceuticals, which is currently in early clinical development. This trend underscores the industry’s pivot towards next-generation treatments with transformative therapeutic potential.
In contrast, traditional small-molecule drugs—despite their long-standing role in medicine due to low molecular weight, predictable pharmacokinetics, and cost efficiency—face growing challenges. Generic competition post-patent expiry and limited effectiveness against certain complex diseases are contributing to their relative decline in strategic focus.
Biologics, although associated with higher production costs, intricate manufacturing processes, and patient-specific immune responses, offer significant advantages in treating previously untreatable or hard-to-manage diseases. As advancements in bioprocessing technologies continue and economies of scale improve, the accessibility and affordability of biologics are expected to increase, further reinforcing their dominance.
The pharmaceutical industry’s future appears firmly aligned with biologics, not only as revenue leaders but also as innovation front-runners in a rapidly evolving therapeutic environment.
Results and Findings
The research exploration underscores the transformative potential of Artificial Intelligence (AI), regenerative medicine, tissue engineering, and organ-on-a-chip (OoC) technologies in redefining the future of healthcare.
Artificial Intelligence in Healthcare
AI continues to revolutionize healthcare through its capability to process and analyze large datasets rapidly and with high precision. Machine learning algorithms are increasingly used to identify diagnostic patterns and predict clinical outcomes, significantly enhancing the accuracy and efficiency of medical decision-making. A particularly impactful domain is medical imaging, where AI models can detect abnormalities in X-rays, CT scans, and MRIs with greater speed and fewer errors than traditional methods. This facilitates faster diagnoses and early interventions. Additionally, personalized medicine benefits greatly from AI. By integrating a patient’s genetic profile, medical history, and lifestyle data, AI systems can deliver customized treatment strategies that cater to individual health needs. Remote patient monitoring powered by AI is also showing strong promise. Continuous health tracking enables the early detection of complications, allowing timely medical intervention and reducing hospital readmission rates. Figures 4 and 5 offer an experimental visualization design, illustrating key findings and providing an intuitive depiction of AI’s potential in future healthcare applications.
Regenerative Medicine and Immune-Responsive Biomaterials
Regenerative medicine spans four primary domains—cell transplantation, tissue engineering, drug development, and gene therapy—all of which depend on highly active and functional cell systems. Recent investigations emphasize not only the importance of stimulating cell activity but also the need to understand the interaction between biomaterials and immune cells in the microenvironment. Immune cells, such as neutrophils and macrophages, play a vital role in the success of regenerative therapies. Their phenotypic states—M1 (pro-inflammatory) and M2 (anti-inflammatory)—respond dynamically to local environmental cues and material stimuli. Unintended activation of M1 macrophages by biomaterials may compromise tissue regeneration efforts. Moreover, the integration of nanomaterials in regenerative therapies has led to new findings around bio-corona formation, immune sensing, immune evasion, and biodegradation. These insights highlight the necessity for biomaterial designs that account for immune responses to improve therapy efficacy and safety.
Tissue Engineering: Future Prospects and Ethical Considerations
Tissue engineering holds immense potential for restoring, replacing, and rejuvenating damaged tissues by combining biological principles with engineering practices. Advancements in biomaterials, scaffolding techniques, and cell culture technologies are enabling the development of functional, patient-specific tissues with increasingly clinical relevance. Despite ongoing technical challenges, continued research is moving the field closer to widespread medical adoption. However, ethical concerns— particularly regarding the sourcing and use of human cells and embryonic materials—must be carefully navigated. Ensuring compliance with stringent ethical guidelines is critical to preserving donor autonomy and scientific integrity in this rapidly advancing domain.
Organ-on-a-Chip (OoC) Technology
OoC platforms have become a cornerstone in biomedical modeling and drug discovery. These microengineered systems mimic the structure and function of human organs at a microscale, providing more physiologically relevant data compared to traditional models. Progress in this field has led to the development of multi-organ chips, or interconnected OoCs, with the vision of constructing a complete “human-body-on-a-chip”. Such integrated systems could revolutionize pharmaceutical testing by offering a viable alternative to animal models and facilitating faster drug development cycles. Key enabling technologies include advanced biomaterials, microfabrication, and 3D bioprinting, which collectively enhance device functionality, reduce costs, and improve reproducibility. The inclusion of embedded sensors further streamlines real-time monitoring of physiological parameters, enabling automation and predictive diagnostics. Table 2 presents a graphical summary of the contextual developments across these domains, emphasizing the converging trends in AI, regenerative science, and biomedical engineering.
Table 2:Selection of Biomaterials within Regenerative Medicine Applications.

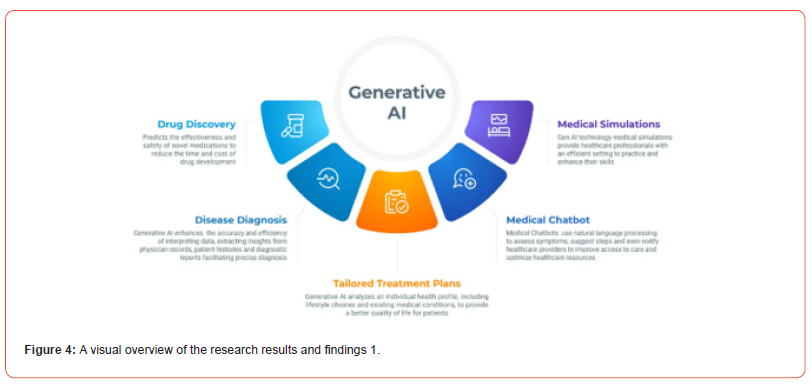
Discussions and Future Directions
The integration of Artificial Intelligence (AI) into healthcare systems has catalyzed significant advancements across numerous domains, including medical imaging, drug development, and personalized medicine. AI-driven algorithms are increasingly capable of processing and interpreting vast and complex datasets—such as patient records, genetic profiles, and clinical trial results—to identify patterns that may elude conventional analytical techniques. These capabilities enable earlier and more accurate diagnoses, as well as optimized treatment plans tailored to individual patient needs. Moreover, natural language processing (NLP) has emerged as a transformative tool within the AI-healthcare interface. By extracting actionable insights from unstructured data sources—such as clinician notes, discharge summaries, and electronic health records—NLP significantly enhances clinical decision-making and streamlines administrative workflows. The proliferation of AI-powered wearable devices represents another pivotal development, enabling the real-time monitoring of vital signs and the early detection of anomalies. These technologies not only empower patients with continuous self-monitoring but also support healthcare providers in proactive intervention, thereby reducing the risk of acute episodes and hospital readmissions. In parallel, regenerative medicine and tissue engineering are undergoing rapid evolution. The convergence of additive manufacturing, medical imaging, advanced biomaterials, and cellular engineering has enabled the fabrication of increasingly sophisticated, patient-specific tissue constructs. Despite these advancements, critical challenges remain—particularly in ensuring functional maturation, precise vascularization, innervation, and immune compatibility of engineered tissues. The immune response to biomaterials, for instance, remains a major hurdle, as the activation of pro-inflammatory cells can impair tissue regeneration and integration. Oral immunotherapeutics offer an attractive drug delivery route due to their non-invasive nature and patient compliance. However, achieving therapeutic efficacy is complicated by biological barriers like mucosal membranes and enzymatic degradation in the gastrointestinal tract. Recent innovations in mucoadhesive and permeation-enhancing delivery systems show promise in overcoming these limitations, thus broadening the applicability of oral formulations in immunotherapy and other systemic treatments.
Future Directions
Looking ahead, several emerging trends and research priorities
are likely to shape the future of AI, regenerative medicine, and drug
delivery systems in healthcare:
1. Advanced AI Models and Ethical Integration The
development of more sophisticated and ethically aligned AI
models will further enhance predictive analytics, diagnostic
precision, and patient monitoring. Emphasis on explainable
AI (XAI) and data privacy will be crucial for fostering trust and
ensuring responsible deployment in clinical settings.
2. Multimodal AI Systems Combining imaging, genetic,
behavioral, and environmental data will lead to the creation of
multimodal AI systems capable of holistic patient assessment.
This integration will enhance the accuracy of personalized
treatment plans and predictive diagnostics.
3. Scalable Regenerative Solutions Future research must
address scalability in tissue engineering, focusing on the
development of standardized, reproducible, and cost-effective
biomanufacturing protocols. Emphasis should also be placed
on immune-compatible biomaterials and strategies for realtime
monitoring of tissue development in vivo.
4. Organoid and Organ-on-a-Chip Technologies The
progression from organ-on-a-chip systems to human-body-ona-
chip models will revolutionize disease modeling and drug
testing, reducing reliance on animal studies and accelerating
the pace of therapeutic discovery.
5. Next-Generation Drug Delivery Continued innovation
in nanocarriers, mucoadhesive systems, and smart delivery
platforms will enhance the bioavailability and targeting
efficiency of orally administered drugs, including vaccines and
immunotherapeutics.
6. Collaborative and Interdisciplinary Research The
convergence of AI, biomedical engineering, materials science,
and clinical research will be essential to overcoming existing
challenges and translating experimental innovations into realworld
medical applications.
The synergy between AI, regenerative medicine, and advanced drug delivery platforms is poised to redefine the landscape of modern healthcare. With sustained investment in multidisciplinary research and ethical implementation, these technologies hold the promise of not only improving patient outcomes but also creating a more efficient, personalized, and accessible healthcare system for future generations.
Conclusions
The rise of accelerated computing and artificial intelligence (AI) has ushered in an era of unprecedented possibilities, fundamentally reshaping how we perceive, process, and interact with data, particularly within healthcare and life sciences. Despite the extraordinary advancements and the rapid integration of intelligent systems and data devices, significant ethical and practical concerns persist—especially when these technologies intersect with human health and well-being. The more powerful and complex the systems become, the greater the potential consequences of unintended outcomes. As a society, our continual evolution in computational technologies urges us to explore deeper into the unknown, pushing the boundaries of what machines and human intellect can achieve together. However, in this relentless pursuit of innovation, we must never lose sight of our core responsibility: to safeguard human life with unwavering ethical and moral integrity. When technological decisions carry the weight of life and death—as highlighted during the COVID-19 pandemic—the need for responsible deployment, transparent governance, and regulatory oversight becomes not just important, but imperative.Looking toward the future, we are on the brink of transformative shifts across both engineering and biomedical sciences. These changes will redefine human interaction with technology and medicine, enabling remarkable innovations—from regenerative therapies to intelligent drug delivery systems. Yet, in the excitement of progress, it is essential to remain vigilant and grounded in the principles of safety, inclusivity, and long-term sustainability. Emerging questions around human mortality, transhumanism, and post-biological futures must be addressed with a multidisciplinary, ethically aware lens.In the specific context of immunotherapy and pharmaceutical science, maintaining the natural function and integrity of biological systems is a critical priority. Routes such as sublingual and buccal delivery present promising alternatives to traditional methods, offering rapid therapeutic action while bypassing first-pass metabolism. However, widespread adoption is still limited due to regulatory and formulation challenges. Future research will likely emphasize immunoengineering and biomaterial design to enhance specificity and efficiency without compromising the delicate balance of the immune system.
Additionally, while biologics represent the forefront of therapeutic innovation—particularly for complex diseases such as cancer and autoimmune disorders—small molecules continue to play a vital role in modern medicine. Their ability to penetrate cell membranes and target intracellular pathways, coupled with scalable and cost-effective production, makes them indispensable, especially in treating chronic diseases requiring long-term, affordable care. The future of healthcare thus lies in a balanced integration of biologics and small molecules, creating a diversified and synergistic therapeutic landscape.The advancement of AI, immunoengineering, and pharmaceutical technologies must be harmonized with ethical foresight, human-centered design, and respect for biological complexity. We must remember, above all, that no system is infallible, and every human being is inherently liable to error. As we move forward into a new frontier of medicine and computation, let us do so with humility, responsibility, and an unwavering commitment to the betterment of humanity. Supplementary information. The various original data sources some of which are not all publicly available, because they contain various types of private information. The available platform provided data sources that support the findings and information of the research investigations are referenced where appropriate.
Acknowledgments. The author would like to acknowledge and enthusiastically thank the GOOGLE Deep Mind Research with its associated pre-prints access platforms. This research was deployed and utilized under the various platforms and provided by GOOGLE Deep Mind which is under the support of the GOOGLE Research and the GOOGLE Research Publications under GOOGLE Gemini platform. Using their provided platform of datasets and database files with digital software layouts consisting of free web access to a large collection of recorded models that are found in research access and its related open-source software distributions which is the implementation and simulation of analytics for the proposed research which was undergone and set in motion. There are many datasets, data models which are resourced and retrieved from a wide variety of GOOGLE service domains. All the data sources and various domains from which data has been included and retrieved for this research are identified, mentioned and referenced where appropriate. However, various original data sources some of which are not all publicly available, because they contain various types of private information. The available platform provided data sources that support the findings and information of the research investigations are referenced where appropriate.
Declarations
• Funding No Funding was provided for the conduction of this research.
• Conflict of interest/Competing interests
There are no Conflict of Interest or any type of Competing
Interests for this research.
• Ethics approval
The author declares no competing interests for this research.
• Consent to participate
The author has read and approved the manuscript and have
agreed to its publication.
• Consent for publication
The author has read and approved the manuscript and have
agreed to its publication.
• Availability of data and materials
The various original data sources some of which are not all
publicly available, because they contain various types of private
information. The available platform provided data sources that
support the findings and information of the research investigations
are referenced where appropriate.
• Code availability
Mentioned in details within the Acknowledgements section.
• Authors’ contributions
Mentioned in details within the Acknowledgements section.
References
- Zarif Bin Akhtar Artificial intelligence within medical diagnostics: A multi-disease perspective. Artificial Intelligence in Health 5173.
- Akhtar ZB (2025) Exploring AI for pain research management: A deep dive investigative exploration. J Pain Res Manag 1(1): 28-42.
- Akhtar Z B, Rozario V S (2025) AI Perspectives within Computational Neuroscience: EEG Integrations and the Human Brain. Artificial Intelligence and Applications.
- Akhtar Z B (2024) Generative artificial intelligence (GAI): From large language models (LLMs) to multimodal applications towards fine tuning of models, implications, investigations. Computing and Artificial Intelligence 3(1): 1498.
- Zarif Bin A (2024) Computer Vision and Beyond: A Deep Dive Exploration and Investigation. Trends Tech Sci Res 7(3): 555711.
- Akhtar ZB (2024) Unveiling the evolution of generative AI (GAI): a comprehensive and investigative analysis toward LLM models (2021–2024) and beyond. Journal of Electrical Systems and Inf Technol 11,22.
- Akhtar ZB (2024) The design approach of an artificial intelligent (AI) medical system based on electronical health records (EHR) and priority segmentations. J Eng.
- Zarif Bin Akhtar (2023) Accelerated Computing a Biomedical Engineering and Medical Science Perspective. Annals of the Academy of Romanian Scientists Series on Biological Sciences 12(2): 138-164.
- Z B Akhtar, V Stany Rozario (2020) "The Design Approach of an Artificial Human Brain in Digitized Formulation based on Machine Learning and Neural Mapping," 2020 International Conference for Emerging Technology (INCET).
- (2025) Exploring the Role of Molecular Engineering in Regenerative Medicine.
- (2025) Elivabooks.com.
- Akhtar Z B, Rawol A T (2024) Economy and empirical research perspectives towards Artificial Intelligence: A deep dive investigative exploration analysis. Economy 11(1): 1–18.
- Akhtar Z, Rawol A (2025) Harnessing artificial intelligence (AI) towards the landscape of big earth data: Methods, challenges, opportunities, future directions. Journal of Geography and Cartography 8(1): 10224.
- Zarif Bin Akhtar (2024) Securing the Future of Mobility: Understanding the Security Perspectives of Cybersecurity, Operating Systems (OS) Security, Mobile Computing. Open Access Journal of Computer Science and Engineering 1(1): 51-66.
- Akhtar Z B, Rawol A T (2024) Harnessing artificial intelligence (AI) for cybersecurity: Challenges, opportunities, risks, future directions. Computing and Artificial Intelligence 2(2): 1485.
- Z Bin Akhtar (2024) “Artificial Intelligence (AI) within the Realm of Cyber Security,” Insight. Electr. Electron Eng pp. 1-11.
- Abdul Rahman R, Mohamad Sukri N, Md Nazir N, Ahmad Radzi MA, Zulkifly AH, et al. (2015) The potential of 3-dimensional construct engineered from poly(lactic-co-glycolic acid)/fibrin hybrid scaffold seeded with bone marrow mesenchymal stem cells for in vitro cartilage tissue engineering. Tissue Cell 47(4): 420-430.
- Xu W, Wang Z, Liu Y, Wang L, Jiang Z, et al. (2018) Carboxymethyl chitosan/gelatin/hyaluronic acid blended-membranes as epithelia transplanting scaffold for corneal wound healing. Carbohydr Polym 192: 240-250.
- Bencherif S, Gsib O, Egles C (2017) Fibrin: An underrated biopolymer for skin tissue engineering. J Mol Biol Biotech 2: 1.
- Monteiro IP, Shukla A, Marques AP, Reis RL, Hammond PT (2015) Spray-assisted layer-by-layer assembly on hyaluronic acid scaffolds for skin tissue engineering. J Biomed Mater Res A 103(1): 330-340.
- Tresoldi C, Pacheco P, Patricia D, Elisa F, Roberta G, et al. (2017) Alginate/Gelatin Hydrogels to Coat Porous Tubular Scaffolds for Vascular Tissue Engineering. Eur Cell Mater: 33.
- Rouwkema J, Khademhosseini A (2016) Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol 34(9): 733-745.
- Ewart Lorna, Apostolou Athanasia, Briggs Skyler A, Carman Christopher V, Chaff Jake T, etal. (2022) "Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology". Communications Medicine 2(1): 154.
- G Orive, E Anitua, J L Pedraz, D F Emerich (2009) “Biomaterials for promoting brain protection, repair and regeneration,”. Nature Reviews Neuroscience 10(9): 682-692.
- M Singelyn, J A DeQuach, S B Seif-Naraghi, RB Littlefield, P J Schup-Magoffin, et al. (2009) “Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering,”. Biomaterials 30(29): 5409-5416.
- M J Hernandez, R Gaetani, V M Pieters, Nathan W Ng, Audrey E Chang, et al. (2018) “Decellularized extracellular matrix hydrogels as a delivery platform for MicroRNA and extracellular vesicle therapeutics,”. Adv Ther (Weinh) 1(3): 1800032.
- F López-Gallego, A I Benítez-Mateos (2020) “Manufacturing of protein-based biomaterials coupling cell-free protein synthesis with protein immobilization,”. Methods in Molecular Biology 2100: 335-343.
- A Ounkaew, P Kasemsiri, K Jetsrisuparb, Hiroshi Uyama, Yu-I Hsu, et al. (2020) “Synthesis of nanocomposite hydrogel based carboxymethyl starch/polyvinyl alcohol/nanosilver for biomedical materials,”. Carbohydrate Polymers 248: 116767.
- H Cao, L Duan, Y Zhang, J Cao, K Zhang, et al. (2021) “Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity,”. Signal Transduction and Targeted Therapy 6(1).
- H P S Abdul Khalil, E B Yahya, H A Tajarudin, V Balakrishnan, H Nasution (2022) “Insights into the role of biopolymer-based xerogels in biomedical applications,” Gels 8(6): 334.
- P Vinchhi, S U Rawal, M M Patel (2021) Drug Delivery Devices and Therapeutic Systems. pp. 395-419.
- C O Crosby, B Stern, N Kalkunte, S Pedahzur, S Ramesh, et al. (2022) “Interpenetrating polymer network hydrogels as bioactive scaffolds for tissue engineering,”. Reviews in Chemical Engineering 38(3): 347-361.
- John Denis Enderle, Joseph D Bronzino (2012) Introduction to Biomedical Engineering. Academic Press. pp. 16–. ISBN 978-0-12-374979-6.
- Hatze Herbert (1974) "The meaning of the term biomechanics". Journal of Biomechanics 7(2): 189-190.
- Couvreur Patrick, Vauthier Christine (2006) "Nanotechnology: Intelligent Design to Treat Complex Disease". Pharmaceutical Research 23 (7): 1417-1450.
- Curtis Adam SG, Dalby Matthew, Gadegaard Nikolaj (2006) "Cell signaling arising from nanotopography: implications for nanomedical devices". Nanomedicine 1(1): 67-72.
- Jacobson Lewis E, Olayan May, Williams Jamie M, Schultz Jacqueline F, Wise Hannah M, et al. (2019). "Feasibility and safety of a novel electromagnetic device for small-bore feeding tube placement". Trauma Surgery Acute Care Open 4(1): e000330.
- (2003) "Medical Device Regulations: Global overview and guiding principles" (PDF). World Health Organization (WHO).
- Goyal Megh R (2018). Scientific and Technical Terms in Bioengineering and Biological Engineering. CRC Press ISBN 978-1-351-36035-7.
- (2011) "Biomedical Engineering - Electrical and Computer Eng. Ryerson". Ee.ryerson.ca. 2011-08-04. Archived from the original on September 27, 2011. Retrieved.
- (2011)"Ryerson Biomedical Engineering Students Invent Brain-Controlled Prosthetic Arm". STUDY Magazine. 2011-04-01. Retrieved.
- Fagette Jr Paul H, Horner atricia I, eds. (2004). Celebrating 35 years of Biomedical Engineering: An Historical Perspective (PDF). Landover, MD: Biomedical Engineering Society p. 4.
- Kassab Ghassan S (2004) "YC "Bert" Fung: The Father of Modern Biomechanics". Mechanics & Chemistry of Biosystems: MCB 1(1): 5-22.
- Gallegos Emma (2010) "Alfred E. Mann Foundation for Scientific Research (AMF)". Aemf.org. Retrieved 2011-09-24.
- Pasotti Lorenzo, Zucca Susanna (2014) "Advances and Computational Tools towards Predictable Design in Biological Engineering". Computational and Mathematical Methods in Medicine. 2014: 369681.
- Cuello JC (2006) Engineering to biology and biology to engineering, The bi-directional connection between engineering and biology in biological engineering design. International Journal of Engineering Education 22(1).
- Johnson AT, Phillips WM (1995) "Philosophical foundations of biological engineering". Journal of Engineering Education (84): 311-318.
- (2018) "Convention on Biological Diversity". 13 May 2016. Retrieved.
- Vincent Julian FV, Bogatyreva Olga A, Bogatyrev Nikolaj R, Bowyer Adrian, Pahl Anja-Karina (2006) "Biomimetics: its practice and theory". J R Soc Interface 3(9): 471-482.
- "Systems biology | Britannica". www.britannica.com. Retrieved 2023-02-15.
- Graur D, Zheng Y, Price N, Azevedo RB, Zufall RA, et al. (2013) "On the immortality of television sets: "function" in the human genome according to the evolution-free gospel of ENCODE". Genome Biology and Evolution 5(3).
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, et al. (2005) "Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles". Proc Natl Acad Sci U S A 102(43): 15545-15550.
- Wang H, Zu Q, Chen J, Yang Z, Ahmed MA (2021) "Application of Artificial Intelligence in Acute Coronary Syndrome: A Brief Literature Review". Adv Ther 38(10): 5078-5086.
- Sotirakos S, Fouda B, Mohamed Razif NA, Cribben N, Mulhall C, et al. (2022) Harnessing artificial intelligence in cardiac rehabilitation, a systematic review". Future Cardiology 18(2): 154-164.
- Chen W, Sun Q, Chen X, Xie G, Wu H, et al. (2021) Deep Learning Methods for Heart Sounds Classification: A Systematic Review". Entropy 23(6): 667.
-
Zarif Bin Akhtar*. Next-Generation Healthcare: Exploring GAI, Computational Medicine & Tissue Engineering, within Biomedical Engineering. Iris On Journ of Sci. 1(5): 2025. IOJS.MS.ID.000523.
-
Tsumoite, Chemical formula, Micro-pressure hardness, Reflectance, Lattice parameters based on the X-ray diffractogram analyses
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






