 Research Article
Research Article
The Study of Effective Methods for Cleaning of Contaminated Water and Soil, Participation of Natural Radionuclides in the Processes Occurring in the Plant Mass
Khagani Mammadov*
Institute of Radiation Problems of the National Academy of Sciences of Azerbaijan, Azerbaijan Republic
Khagani Mammadov, Institute of Radiation Problems of the National Academy of Sciences of Azerbaijan, Azerbaijan Republic, AZ1143, Baku sity, H.Javid avenue, 121, Azerbaijan.
Received Date: September 23, 2021; Published Date: October 05, 2021
Abstract
A comprehensive physical, chemical and microbiological analysis of water, vegetation and soil samples taken from regions of the country was carried out. The distributions of heavy metals, trace amounts of natural radionuclides and other inorganic components in environmental objects were studied. By systematic studies have established that the degree of assimilation of the K40 isotope by green plant mass from water and soil is about 7-10 times higher than the degree of assimilation of radioactive isotopes of other elements. The K40 radioisotope is identified in all environmental objects. Initiation of the process by ionizing radiation and further elementary reactions of active particles creates the real condition for the highly endothermic processes of dissociation of water and carbon dioxide molecules, synthesis of new organic molecules and molecular oxygen. The role of gamma radiation of K40 radioisotope should be taken into account at comprehensive analysis of initial stage of photosynthesis. This conclusion is consistent with the revealed facts of increased plant yields on soils with relatively high concentrations of natural radioisotopes, observation of photosynthesis under thick layers of water in the presence of only long-wave infrared rays or in the absence of chlorophyll and oxygen. The cleaning method of soil contaminated with heavy metals and radionuclides have been studied, and the method for the physical adsorption and chemical sorption of organic pollutants (oil products) on the surfaces of wood cheeps floating in open water tank irradiated with ionizing rays has been developed.
Keywords: Physical adsorption; Chemical sorption; Natural radionuclides; Plant mass; Photosynthesis
Introduction
In order to study changes in environmental objects and determine the degree of pollution of plant, soil and water reservoirs in the country, by the staff of the Radiochemistry laboratory of the Institute of Radiation Problems of the Azerbaijan National Academy of Sciences has systematically taken numerous samples of water, soil and vegetation since 2015 and carried out comprehensive analytical-chemical, physico-chemical, radiometric and microbiological examination of these samples in stationary laboratory conditions. Studying the distribution of toxic elements in the soil is an urgent task to solve problems related to environmental safety [1,2]. The accumulation of large amounts of harmful substances in the soil causes the risk of their entry into living organisms by translocation and migration paths along the soil-water-vegetation chain [3,4]. Soil is the upper layer of the lithosphere exposed to living organisms and the atmosphere. The main part of the soil is formed by chemical compounds in the form of various minerals. To eliminate side complications in the analysis, soil samples with humus, a layer of oxidized plant debris from trees and contaminated with random organic debris and emissions were not taken. Studying the various forms of the presence of chemical elements in minerals, organic residues and emissions, soil colloids, determining the amount of oxides, hydroxides, carbonates, bicarbonates, nitrates, nitrites, sulfates, phosphates in soil samples allow us to estimate the ecological state of the soil. Systematic pollution of the soil with small amounts of anthropogenic emissions leads to an increase in the concentration of xenobiotics in other environmental objects (water reservoirs and vegetation). The increase in technogenic pressure on the environment, the processing of minerals by outdated technological processes and the consequent pollution of environmental objects with small amounts of xenobiotics can cause the formation of ecological crisis zones. Therefore, there is a need for systematic measurements and studies to obtain results on the distribution of radionuclides, heavy metals and other xenobiotics in the soil, vegetation, water reservoirs of the country, trends in the direction of the emergence of zones of environmental crisis, information for predicting changes and the rate of change in the environment. The ability to clean by various methods local areas of the earth contaminated with radionuclides and heavy metals and to study the options for implementing these processes are the most important tasks of chemistry and are important for solving many pressing environmental problems [5- 10].
Material and Methods
The soil samples taken were treated with distilled water, weak solutions of acid and alkali with periodic mixing and filtration, isolation of sparingly soluble particles in a centrifuge with further evaporation to obtain minerals, heavy metals and radionuclides after a written description in the journal of taken samples, visual inspection, weighing, drying, cleaning from random foreign objects or pebbles by passing through sieves [6-11]. Radiometric measurements were carried out using the İnSpector-1000 and Radiagem-2000 radiometers (manufactured by Canberra and equipped with alpha, beta and gamma detectors) and the İdentiFİNDER radiometer identifier (manufactured by Thermo Scientific). Gamma spectrometer with HPGe detector (manufactured by “Canberra”), atomic absorption AA-6800 spectrometer (manufactured by ”Shimadzu”), Expert-3L and XRF X-ray fluorescence spectrometers were used in the process of physical-chemical analysis of minerals obtained by evaporation of water samples, weakly acid and weakly alkaline extracts of soil samples, by heating and treatment by nitric acid solution of green grass samples [7-9]. Pre-sterilized glassware was used to take water samples and samples were taken, stored and transported in accordance with the requirements of standards (GOST) 24481-80 and 18968-73. When water samples were taken in parallel with radiometric measurements the sensory, chemical and microbiological rapid test analysis were carried out. To determine the compliance of the quality of the water sample with the requirements of the standards for drinking water, stationary laboratory sensory, analytical, physical-chemical and microbiological analysis were carried out in compliance with the conditions and requirements of standards (GOST) 2761-84 , 3351- 74, 2874-82. We used express test napkins - certified ISO 9001 and 13485 quality control systems - manufactured by R-Biopharm (Germany) for conducting microbiological rapid tests and selective nutrient media produced by Hi-Media (India) and Condalab (Spain), incubators with an automated thermostat and colony counters to determine the number and types of microorganisms in stationary laboratory conditions [7-9].
Discussions of the Results
About 30-35% (300-350 g / kg) of the total mass of soil samples consists of water. The amount of oxygen in composition of dried soil samples is 240-260 g (in wet soil this indicator is approximately 50% or 500 g), carbon 18-20 g., silicon 310-330 g., aluminum 62-80 g. The total amount of moisture, oxygen, carbon, silicon, aluminum is 980 - 990 g / kg (350 + 240 + 18 + 310 + 62 = 980 g. or 300 + 260 + 20 + 330 + 80 = 990 g). The quantity of Mg compounds in water reservoirs is observed in the range of 6-25 mg / l, in plants 50-60 mg / kg. In some samples of plant P found in trace amounts. Concentrations of Mg compounds equals 80-90 mg / kg and the trace amounts of “microelements” As, Sn, Cu, Cr, Zr, Mo, Br, Ba below 1 mg / kg are observed in soil samples.
Radiometric measurements and analysis
The analyses performed show that the mineral balance of the samples of drinking water varies in the interval 0.3-3.5 g / l. The mineral composition of water (taken from water reservoirs, rivers, springs, water pipes), plant (green grass samples of pastures and flat areas, minor additives from young leaves of trees and shrubs in the bulk of green grass, taken from the plain edges of forests) and soil samples were determined. The results of these analyses are shown in tables 1, 3 and 5. The results of the analysis of water samples are shown in tables 1 and 2. The results of the analysis of the minerals of vegetation samples are given in tables 3 and 4. The results of the analysis of soil samples are shown in Tables 5 and 6.
As can be seen from Table 5, the total amount of inorganic components that determine the soil fertility is only 10-20 g / kg. Only elements with concentrations that vary by region were shown in the tables for clarity and readability. Having relatively stable concentrations of light elements / Si, C, Al, P, Mg / and trace concentrations of heavy / Ti, Zr, Ba, Sn, Cu, Mo , Co, Eu, Ra, Th / elements are not considered in these tables. Unlike distilled water, dilute acid and alkaline solutions emit relatively large amounts of heavy metals and radionuclides from soil samples (5-10 times more depending on the concentration of acid and alkali).
As a rule, the comprehensive sensory, physicochemical and microbiological (Ec=0-3/liter) indicators of water samples taken at all times of the year from the water supply lines (water ways) of the regions of the country meet the requirements of standards 2874- 82, AZS 216-2006, AZS 282-2007 for “Drinking Water” [7-9]. The analysis showed that in underground and spring waters the number of detected (in one liter) colonies of Escherichia coli (Ec) varies in the range of 3-15 and this number remains practically constant
during the year. The use of water from these sources us drinking water is permissible only after filtration and boiling. In the water of wells dug in the artisanal way and in the water of the Kur River, the number of Ec in one liter of water varies in the range of 15-30. In rainy weather, microscopic fungi, Ec, Coliform, pathogenic Nag-vibrios, Salmonella, Staphylococcus, Streptococcus are detected in the water of the Araz and Kur Rivers and in coastal water of the Caspian Sea [7-9].
The concentration (radiation activity) of the Na22 and K40 isotopes in minerals of drinking water taken from water pipes for population and organizations of the all regions of the country is 0.3-1.4 Bq/l and 0.1-0.4 Bq/l. These values are characteristic low values for natural spring waters. The dose rate of gamma radiation from the background of these regions is 0.02-0.15 μZv/h (Tables 1-6).
Table 1:Concentrations of the chemical components in samples taken from water sources of the Azerbaijan regions.
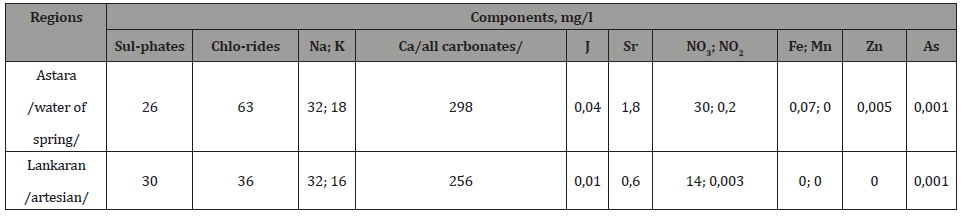
Table 2:Activity of radionuclides in water samplest Taken from the water sources of region of Azerbaijan.
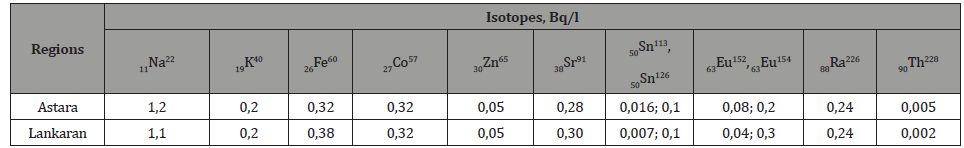
Table 3:Concentrations of chemical components in the minerals of vegetation samples taken in regions of the country.
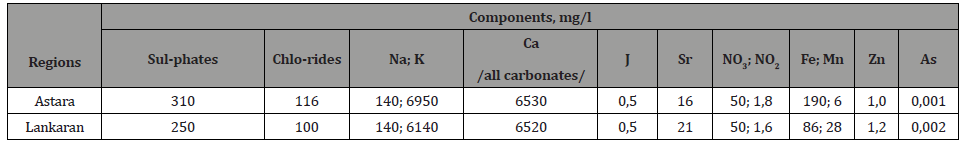
Table 4:Activity of radionuclides in plant samples taken from green plains and pastures of regions of the country.
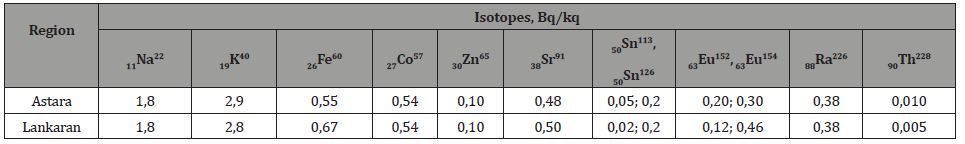
Table 5:Concentrations of components of soil samples taken from regions of the country.
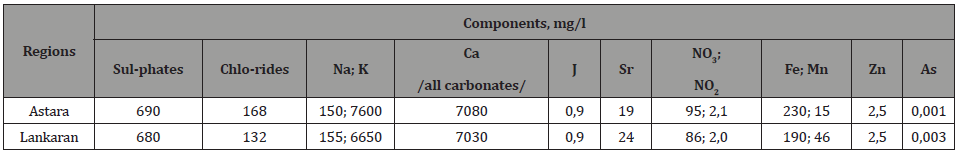
Table 6:Results of radiometric measurements and activity of radionuclides in soil of regions of the country.

Table 7:Comparison of the rates of elementary reactions for radiolysis of oxygen-containing water contaminated with organic substances, oil products (RH, ROH) in volume to which was added the mass of cut wood chips.

According to the «Law of the Republic of Azerbaijan on Radiation Safety of the Population», the permissible value (PV) of the average annual dose for the population is 1 mZv, which is equivalent to the absorbed dose rate 0.12 μZv/h. The measurement’s result show that the values of the total ionization radiation (absorbed dose rate) in many areas of the country (0.03-0.12 mZv/h) do not exceed the maximum permissible value. The local areas of the country where the absorbed dose rate of the total radiation (0.15-0.85 μZv/h) is much higher than the PV were defined as a result of radiometric measurements. Such sources that create high dose rates include granite-marble coating of interior details of underground transport communications, radionuclides in building stone materials, gray, brownish-red and black granite-marble coatings of entrance steps and front walls of several architectural buildings of national importance, plaques, monuments and radioactive sources used in industrial enterprises.
The comparison of the indices of tables 2, 4 and 6, as well as the spectra obtained by gamma spectroscopy of minerals of water, vegetation and soil samples, shows identical values for the activity of the K40 isotope in 1 kg soil and in 1 kg vegetation samples. These values are 7-10 times exceeding the corresponding activity value of the K40 isotope for 1 liter of water taken from the same site. This ratio for other elements varies in range 1,4-1,7. These values show that the process of assimilation by plants the K40 isotope from water and soil samples is more efficient than the process of assimilation by plants other natural radioactive elements.
Discussion of participation of natural radionuclides in the photosynthesis
As is known, photosynthesis is the largest biochemical process on Earth and it is a complex chemical process of converting light energy and infrared radiation into the energy of chemical bonds of organic substances with the participation of photosynthetic pigments (plant chlorophylls, bacteriochlorophyll bacteria, archaea bacteriorhodopsin). The established low values of the energy of light quanta ( much less than 5 eV ) allow us to conclude that the nature of the course of photosynthesis is complex and the complex biochemical mechanisms of the process of dissociation of water molecules in plants are proposed.
Photosynthesis can be represented in the following equation: 6СО2 + 6Н2О → С6Н12О6 + 6О2.
In one year, green algae release 3.6*1011 tons of oxygen into the Earth’s atmosphere, which is about 90% of all oxygen produced during photosynthesis on Earth. The binding energy of the hydrogen atom with the hydroxyl group of the water molecule is 5 eV / molecule [7-9]. Two types of pigments were found in living organisms (the retinal vitamin A derivative is less common, and chlorophylls are involved in photosynthesis in most organisms). In accordance with this biochemical mechanism, chlorine-free and chlorophyll photosynthesis are isolated. The efficiency of chlorophyll-free photosynthesis is relatively low (one H+ is transferred to one absorbed quantum of light). This process is found in the mating membrane of halobacteria. As a result of the operation of the light-dependent proton pump (bacteriorhodopsin) of the membrane, the energy of sunlight transforms into the energy of the electrochemical gradient of protons on the membrane. Chlorophyll photosynthesis is more energy efficient. At least one H+ is transferred to each absorbed light quantum against the gradient, and energy is stored in the form of reduced compounds (ferrodoxin, etc.). Oxygen-free chlorophyll photosynthesis (purple, green bacteria and heliobacteria) proceeds without oxygen evolution. Oxygen chlorophyll photosynthesis (higher plants, algae, cyanobacteria, etc.) is accompanied by the release of oxygen. In the initial (photophysical) stage of photosynthesis, light quanta are absorbed by pigments, they transition to an excited state and energy is transferred to other molecules of the photosystem (plastoquinone). In the photochemical stage, charge separation occurs in the reaction center. The chlorophyll molecule transferring its electron to plastoquinone turns into a radical-cation and under its influence the water molecule loses its electron (Н2О - е- → Н+ + .ОН). Hydroxyl radicals formed under the influence of positively charged manganese ions are converted into oxygen and water (4.OH → O2 + 2H2O). In the chemical stage, light quantum is absorbed by another chlorophyll molecule and it transfers its electron to ferrodoxin. Next, biochemical reactions of the synthesis of organic substances using energy accumulated at the already described light-dependent stages take place.
However, the proposed biochemical mechanism does not unambiguously explain the inconsistencies of some thermodynamic parameters. The total reaction describing photosynthesis (6CO2 + 6H2O → C6H12O6 + 6O2) is characterized by high endothermicity (3080 kJ / mol), the energy of Н-О bond dissociation in Н2О molecules is 485-498 kJ / mol (5.0-5.2 eV / molecule ) and the energy of С = О bonds in the СО2 molecule is equal to 799 kJ / mol (8.3 eV / molecule). These data indicate excessively high values of the potential energy barrier of these processes and the great difficulty in initiating them (the practical impossibility of carbon dioxide dissociation) by the energy of visible light quanta having energies much less than 5 eV. The energy of gamma rays (1.45 MeV) emitted by the K40 isotope is many times higher than the value of the energy of Н-О bond dissociation in Н2О molecules (5 eV) and the energy of С = О bond dissociation in the СО2 molecules (8,3 eV / molecule). In addition, K40 isotopes were found without exception in all the samples taken from the environment, and the geometric dimensions of the studied vegetation samples were directly proportional to the activity (concentration) of K40 detected in them.
We concluded that for a complete description of the multistage processes photosynthesis, in addition to the biochemical mechanism shown in the scientific literature, the radiochemical mechanism of water and carbon dioxide molecules dissociation under the influence of gamma rays of natural isotopes (in mostly K40, having the greatest activity in vegetation), that we have proposed should also be taken into account. A comparative analysis of the geometric, qualitative, organoleptic characteristics of the vegetation cover of different areas is in good agreement, proportional to the concentration of K40 in these plants. The high energy of gamma rays of K40 and the relatively high activity (relatively high concentration) are the reason for the increase in the current concentration of radicals in the mass of the plant, which is equivalent to the acceleration of high-barrier endothermic process of dissociation of water and CO2 molecules. The analysis of numerous samples of water, soil, vegetation, livestock products showed the presence of Na22 and K40 radioisotopes in all samples, without exception.
As is known, after completion of reactions in spurs, the values of the primary radiation-chemical yield of water gamma radiolysis products at pH = 4–9 are: G(H+aq) = 3.4; G(eaq) = 2.9; G(H) = 0.6;G(.OH) = 2.9; G(.O) = 0.0067; G(H2) = 0.45; G(H2O2) = 0.75; G (OHaq) =0.6 ion / 100 eV. The value of G(H+aq) is 4.25 H+/100 eV [11]. Taking into account the average value of K40 activity in 1 kg of plant mass(1.2-3.0 Bq/kg), exposure to this isotope during the year leads to the formation of 4.7*1012 H+ aq/ year. kg ( 2.5 (quanta/(sec.kg))*(4.25H+aq/100 eV)*(1.46*106 eV/quanta) = 15*104 H+aq/s.kg = 4.7* 1012H+ aq/year. kg).
As can be seen from Table 2 in addition to K40 radioactive isotopes of other elements also detected in plant mass. Thus, the total value for 1 kg of plant mass formed during one year under the influence of natural gamma rays of all isotopes and cosmic radiation will be 1015 H+ aq. This is lower than the value of the resulting H+ ions which were participated in photosynthesis. In the 1980s, the radiation- chemical chain process of hydrogen conversion into carbon monoxide was studied [12-14]. After the radiation initiation of the process Н2 + СО2 → СО + Н2О (Н2 → Н. + .Н or Н2* + СО2 → СО + .ОН + .Н) begins stages of the chain reaction (Н. + СО2 → СО + .ОН; .ОН + Н2 → Н2О + .Н). Similar to the radiation-chemical process Н2 + СО2 → СО + Н2О closed cycles of elementary reactions (Н. + CO2 → СО + .ОН; .ОН + RH → Н2О + .R or Н+ + СО2 → СО+ + .ОН, R+ + СО2 → СО+ + RО.; Н+ + RR → R+ + RН, СО+ + RR → RСО + R+) in plant mass also take place in the presence of K40 and Na22.
After reactions of radiation initiation of decomposition of molecules of water, carbon dioxide and organic matrix (Н2О → H. + .OH, СО2 → СО + О, RН → R. + H.) other elementary stages of the continuation of the process and the death of active particles proceed (H.+ O2 → HO.2, RН + .OH → R. + H2O, CH.3+ O2 → HCO. + H2O, R(Н2). + O2→ RН + HO.2, R. + R. → RR, R. + HCO. → RHCO, 2HO.2 → H2O2 + O2, R. +.OH → ROH). Therefore, we presented the process of photosynthesis with the following image: (Figure 1).

Initiation of the process by ionizing radiation (the formation of radicals, atoms and ions) and further elementary reactions creates the real condition for the highly endothermic processes, dissociation of water and carbon dioxide molecules and synthesis of new organic molecules and molecular oxygen in addition to the synthesis processes, proceeding as provided by the biochemical mechanism of photosynthesis [7-9,12-14].
The role of gamma radiation of the natural radionuclides should be taking into account in a comprehensive analysis of photosynthesis. This conclusion is supported by the multiplicity of intermediate products of water radiolysis (the radiation-chemical yields of which are given above), water and possibility of multiple participation of active particles (H., .OH, R., H+, H+aq, eaq, R+, CO+) of radiolysis of water and organic matrix in the cycles of elementary reactions. Little exothermicity (2.8 kJ / mol) of the total reaction of the radiolytic conversion of a mixture of hydrogen and carbon dioxide into carbon monoxide and water, the formation of new radicals and ions during many elementary reactions occuring in the plant mass also testifies in favor of this conclusion.
The study of effective methods for cleaning of contaminated water and soil
Initiation of the process of decomposition of organic contaminants occurs in the entire volume of contaminated water, because of the high penetrating possibility property of the ionizing rays of 60Co. This process can also take place in the mass of cut wood chips added to contaminated water. Hence, the process of radiation treatment of water contaminated with organic contaminants or petroleum products will be more efficient. It is necessary to compare the rates of elementary reactions from the scheme given in Table 7 for the radiation conversion of organic contaminants in oxygen-containing water, for more understandably presentation of the main directions of the process [11-19].
The value of the absorbed dose rate of ionizing radiation from our installation with a 60 isotope is 0.33 Gy/s or 2.06⋅1015 eV/g⋅s. The conversion rate of these pollutants according to reaction (1) is 3. (10-13-10-15) M/s. For comparison the rate of reaction (3) is equal to 5.(10-6-10-7) M/s, taking into account concentrations of organic pollutants (10-1000 μg/kg) of wastewater of the country’s manufacturing plants. The ratio of the rates of each elementary reaction to the rate of reaction 3 (Wn/W3) are estimated taking into account the rate constants, the values of the primary concentrations of the initial and intermediate products. Reactions (1) and (2) are reaction initiation reactions, reactions (4), (5), (8), (9), (11), (12), (17), (22), (23), (24), (28), (29), (33), (34) are the chain termination reactions (Table 7).
The elementary reactions (7), (8), (9), (11), (17), (20, 21, 22, 23, 24) are reactions, which are necessary for the formation of new products of the process. The ratio of the rates of these elementary reactions to the rate of elementary reaction (3) for irradiation of water contaminated with organic compounds shows that the rates of reactions (11) and (24) forming new organic compounds have comparable values and these values far exceed the rates of other product-forming reaction. Consequently, the main channels for the radiation conversion of organic compounds in water are the formation of hydroxyl-substituted derivatives, organic peroxides and the products of their mutual recombination, mainly connected by an oxygen bridge.
As can be seen from Table 7, the macromolecules of the organic matrix of the wood chips are actively involved in the reaction process by means of the reactions (10), (11) and (14), which are characterized by high velocities ( W10 / W3 = 100, W11 / W3 = 10- 4-10-5 and W14 / W3 = 10-5-10-6 ). The hydroxyl-substituted and peroxide radicals of organic compounds (petroleum products) ultimately in the presence of wood chips are joined not only to other hydroxyl-substituted radicals, but also to macro radicals of the organic matrix of wood chips. This fact is confirmed by higher values of the weight of organic compounds (petroleum products) detected on the surface of wood chips when they are removed from irradiated water. These values at an absorbed dose of 10 kGy are 1.5-2.2 times higher than the weight of organic compounds (petroleum products) adsorbed on the surface of wood chips in non-irradiated water, held under identical conditions. When airflow is injected into the experimental tank containing 10 m3 of contaminated water, wooden chips, with a total weight of 10 kg, float around the entire volume of the reservoir, which favors the adsorption of oil products from their entire volume of water.
Weak solutions of acids and alkalis were used for the separation of heavy metals from soil samples. After filtration and evaporation of soil extracts in the obtained minerals the total activity of radioactive elements was found to be identical to the activity of radionuclides in the studied initial soil samples. Weak acid and alkaline solutions effectively release heavy metals and radionuclides from the soil samples. Extraction of 0.2 kg soil samples (the analysis results of which are shown in tables 1-6) with 1 liter acid and alkaline solutions 0.1 M, 0.2 M, 0.5 M, 1.0 M respectively led to the release of 17%, 30%, 45%, 68% of heavy metals and radionuclides from analyzed soil samples.
A comparative analysis of the results of these experiments with the data of previous experiments using traditional adsorbents concluded that the cleaning of soil contaminated with radioisotopes is effective by sequentially treating it with solutions of weak acids and alkalis.
Conclusions
The process of assimilation by green plants the K40 isotope from water and soil samples is more efficient than the process of assimilation by plants other natural radioactive elements. The energy of gamma rays (1.45 MeV) emitted by the K40 isotope is many times higher than the value of the binding energy of hydrogen with a hydroxyl group in water molecules (5 eV). In addition, K40 isotopes were found without exception in all the samples taken from the environment, and the geometric dimensions of the studied vegetation samples were proportional to the activity (concentration) of K40 detected in them. The established low values (lower than 5 eV) of the energy of light quanta allow us to conclude that the nature of the course of photosynthesis is complex. Initiation of the process by ionizing radiation and further elementary reactions creates the real condition for the highly endothermic processes, dissociation of water and carbon dioxide molecules, synthesis of new organic molecules and molecular oxygen in addition to the biochemical synthesis processes.
The participation of natural radionuclides in photosynthesis are confirmed with the presence of Na22 and K40 in all samples taken from environmental objects, with increasing plant yields proportional to concentrations of natural radionuclides in soil, with observation of photosynthesis in the mating membrane of extreme halobacteria. This conclusion is confirmed also with observation of photosynthesis under thick layers of water, in the presence of only long-wave infrared rays or in the absence of chlorophyll and oxygen.
Relatively high values of the weight of removed oil components during irradiation of the reservoir indicate to chemical sorption in addition to the physical adsorption on the surface of wood chips. This important effect can be take into account in radiation purification of water contaminated with various organic compounds, crude oil and phenol.
The method of cleaning the soil from heavy metals and radionuclides by extraction with weak acidic and alkaline solutions is more effective than cleaning methods using adsorbents. The correct application of this method allows us to restore soil fertility.
Conflict of Interest
Authors declare no competing financial interest.
Acknowledgments
The work was done outside of any funding program.
References
- (2008) Neutralization of contaminated soils: monograph // Under the general. ed.Y.A. Mozhaiskogo. Ryazan: Meshchersky branch of the GNU VNIIGiM of the Russian Agricultural Academy.
- Matskevich AV, Pronev VV (2017) Technologies for the rehabilitation of radioactively contaminated territories. Proceedings of the scientific conference, Sankt-Peterburq: 226.
- Orlov DS Sadovnikova LK, Sukhanova NI (2005) Chemistry of soils / Moscow, “Higher. School”.
- Mukha VD, Kartamyshev NI, Mukha DV (2003) Agricultural soil science / Ed.V.D. Flies. Moscow, “Kolos”.
- IA Samofalova (2009) The chemical composition of soils and soil forming components. Study guide. Perm: Publishing house of the Federal State-Funded Educational Institution of Higher Professional Education Perm State Agricultural Academy.
- AS Piskunov (2004) Methods of agrochemical research / Moscow, “Kolos”.
- KhF Mamedov (2014) Radiolysis and Photolysis of Water Solutions of Phenol / European researcher. Series A, No. 7-1: 1216-1236.
- Kh F Mamedov (2020) Research of distribution of heavy metals and radioactive elements in sources of drinking water, soil and grass cover of Azerbaijan regions / Petrozavodsk, “Tsifra”.
- F. Mamedov (2012) Radiolytic decomposition of Zearalenone in wheat / Immunopathology, allergology, infectology /. All-Russian public organization Public National Academy of Mycology V 1 74-77.
- VP Kovrigo, IS Kaurichev, LM Burlakova (2008) Soil science with the basics of geology / Moscow, “Kolos”.
- AK Pikaev (1986) Modern radiation chemistry. Radiolysis of gases and liquids. M., Nauka, V2.
- MA Kurbanov, KhF Mamedov, VR Rustamov (1988) The chain formation of hydrogen during thermoradiolysis of gas mixtures of H2S-CO. J Chemistry of High Energies 22(3): 218-220.
- MA Kurbanov, Kh F Mamedov (1989) Photochemical oxidation of hydrogen sulfide in a gas mixture of CH4-H2S- O2. All-Union Conference on Photochemistry, Novosibirsk: p.222.
- MA Kurbanov, VR Rustamov, Kh F Mamedov, ZI Iskenderova, BG Dzantiev (1986) Chain transformation during radiolysis of gas-phase mixtures CO2-H2. J. Khimicheskaya Fizika 5(1): 135-136.
- MM Mogilevich, EM Pliss (1990) Oxidation and oxidative polymerization of unsaturated compounds. M.: Khimiya.
- AK Pikaev (1987) Modern radiation chemistry. Solid and polymers. Applied asperts. M. Nauka 3.
- IM Piskarev (2004) Purification water of open reservoirs by chain reactions of hydroxyl radicals. MGU.
- VA Roginskiy (1988) Phenolic antioxidants. Reactivity and Efficiency. M: Nauka.
- P Neta, J Grodkowski (2005) Rate constants for reactions of phenoxyl radicals in solution. J Phys Chem 34(1): 109-199.
-
Khagani Mammadov. The Study of Effective Methods for Cleaning of Contaminated Water and Soil, Participation of Natural Radionuclides in the Processes Occurring in the Plant Mass. Insi in Chem & Biochem. 2(1): 2021. ICBC. MS.ID.000526.
-
Inorganic components, Physical adsorption, Chemical sorption, Natural radionuclides, Plant mass, Photosynthesis, Radiochemistry
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






