 Mini Review
Mini Review
The Enthalpy of Formation Of L- α -Amino Acids
Stefan Perisanu*1, Daniela Gheorghe2 and Ana Neacsu2
1Department of General Chemistry, Polytechnic University of Bucharest, Romania
2Institute of Physical Chemistry “Ilie Murgulescu” of the Romanian Academy, Romania
Stefan Perisanu, Department of General Chemistry, Polytechnic University of Bucharest, str. Polizu nr. 1, Bucureşti, Romania.
Received Date: October 27, 2020; Published Date: November 17, 2020
Abstract
The reported values for the enthalpies of formation of α-amino acids in crystalline and ideal gas state are critically examined. Experimental values obtained by means of combustion calorimetry of the solid are compared with values calculated by means of group additivity. The ideal gas enthalpies of formation obtained by combining the solidstate values with the standard sublimation enthalpies are compared with ones calculated both additively and by means of quantum chemistry methods.
Keywords: Enthalpy of Formation; Amino acid; crystalline; Ideal gas; Calculated
Introduction
Numerous attempts were made during the last 90 years (a few were made even before) in order to determine the value of the parameter characterizing the stability of a molecule i.e. the enthalpy of formation, for an extremely biochemically important class of compounds, the amino acids. The proteinogenic L-α-amino acids were most investigated but the DL-stereoisomer was used in many cases, as well. The existence of differences between the enantiomers was also of interest. The aim of this paper is to present a state of the art of the existence of reliable values of standard enthalpies of formation in crystalline and ideal gas state for 27 α-amino acids.
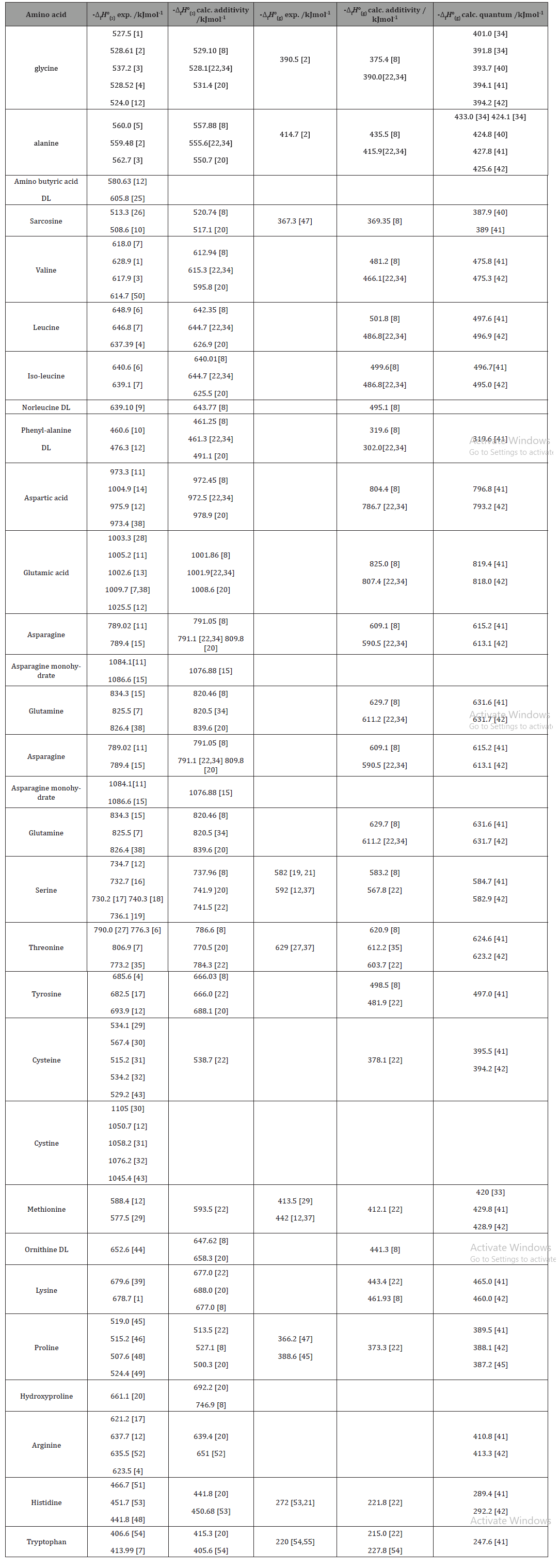
Table 1:Experimental and calculated standard enthalpies of formation of L-α-amino acids*
Discussion
Experimental values of enthalpies of formation in crystalline state obtained by means of heats of combustion in bomb calorimeters are shown in the second column of Table 1. The next column contains values of the same quantity calculated additively by means of contributions of groups of atoms. The fourth column contains values of the gas phase standard enthalpy of formation obtained from its value in solid state and the standard state heat of sublimation. Two kinds of calculated values of the gas phase enthalpy of formation are available. The first one is based on group additivity and the second on quantum chemical calculations (last two columns respectively) (Table 1).
*All data refer to the L- enantiomers if not specified otherwise.
For about 17 of the investigated amino acids at least two values of experimental solidstate enthalpies of formation agree fairly well (within a range of 4 kJ/mol). Divergent values of different authors are met for 8 molecules. A single reported value is available in the case of 2 amino acids. Calculated solid state enthalpies of formation of mono and dicarboxylic acids reported in references [8,22,34] are in good agreement with most experimental data. The calculated values with group contributions recommended by [20] are frequently less negative than experimental ones for monocarboxylic acids but not for dicarboxylic. The behaviors of asparagine and glutamine are particular. Asparagine has the tendency to build a monohydrate, while glutamine unlike most amino acids melts before decomposing. Some discrepancies between experimental and calculated values may be due to the inability of group additivity schemes to take into account all hydrogen bonds existing inside the crystalline lattice of a certain amino acid. A reasonable agreement between experimental and calculated values is observed also for hydroxy-amino acids, but not for all heterocyclic and sulfur containing ones. The experimental values for the ideal gas state standard enthalpy of formation are much scarcer than for the crystalline state. This is because the vapor pressure of amino acids at 298 K is very low so that most measurements were done at higher temperatures. Consequently most reported standard state heats of sublimation are extrapolation values. With the very limited number of reliable experimental values it is impossible to evaluate the performance of different calculation methods for the standard enthalpy of formation in gaseous state.
Conclusions
Reliable data for the standard enthalpy of formation in crystalline state of α-amino acids are available. Group additivity calculations are used as a tool for checking the quality of experimental data and in order to correlate the thermochemical quantities with material’s structure. A considerable amount of research remains to be done in order to have a comprehensive image about the ideal gas state enthalpy of formation of most compounds of this class. A special effort has to be done in order to valorize the existing data about heats of sublimation by using performing methods of obtaining its value at the standard temperature.
Conflicts of Interest
The authors declare no conflict of interest regarding the publication of this paper.
References
- Vasil'ev VP, Borodin VA, Kopnyshev SB (1991) Calculation of the standard enthalpies of combustion and of formation of crystalline organic acids and complexones from the energy contributions of atomic groups. Russ J Phys Chem (Engl Transl) 65: 29-32.
- Ngauv SN, Sabbah R, Laffitte M (1977) Thermodynamique de composes azotes. III. Etude thermochimique de la glycine et de la l-α-alanine. Thermochim Acta 20: 371-380.
- Hutchens JO, Cole AG, Stout JW (1963) Heat capacities from 11 to 305°K., entropies, and free energies of formation of L-valine, L-isoleucine, and L-leucine. J Phys Chem 67: 1128-1130.
- Huffman HM, Fox SW, Ellis EL (1937) Thermal data. VII. The heats of combustion of seven amino acids. J Am Chem Soc 59: 2144-21.
- Contineanu I, Marchidan DI (1984) The enthalpies of combustion and formation of D-alanine, L-alanine, DL-alanine, and β-alanine. Rev Roum Chim 29: 43-48.
- Wu D, Zhu Y, Gao Z, Qu S (1993) Determination of combustion heat of some amino acids. J Wuhan Univ (Natl Sci Ed) 78-82.
- Tsuzuki T, Harper DO, Hunt H (1958) Heats of combustion. VII. The heats of combustion of some amino acids. J Phys Chem 62: 1594-1595.
- Domalski ES, Hearing ED (1993) Estimation of the thermodynamic properties of C–H–N–O–S–Halogen compounds at 298.15 K. J Phys Chem Ref Data 22: 805-1160.
- Strepikheev AA (1955) Mutual interaction of amino and carboxyl groups in amino acids. Doklady Acad Nauk SSSR 102: 543-545.
- Breitenbach JW, Derkosch J, Wessely F (1952) Energetik der Bildung hochmolekularer Polypeptide. Monatsh Chem 83: 591-598.
- Huffman HM, Ellis EL, Fox SW (1936) Thermal data. VI. The heats of combustion and free energies of seven organic compounds containing nitrogen. J Am Chem Soc 58: 1728-1733.
- Yang XW, Liu JR, Gao SL, Hou YD, Shi QZ (1999) Determination of combustion energies of thirteen amino acids. Thermochim Acta 329: 109-115.
- Contineanu I, Chivu L, Perisanu St (2005) The Enthalpies of Combustion and Formation of L-α-Glutamic and 6-Amino-Hexanoic Acids. J Therm Anal Calorim 82: 3-6.
- Contineanu I, Marchidan DI (1997) The enthalpies of combustion and formation of L-, D-and DL aspartic acids. Rev Roum Chim 42: 605-608.
- Contineanu I, Neacsu A, Perisanu StT (2010) The standard enthalpies of formation of L-asparagine and L-α-glutamine. Thermochim Acta 497: 96-100.
- Sabbah R, Laffitte M (1978) Enthalpy of formation of L-serine in the solid state. Thermochim Acta 23: 192-195.
- Lukýanova VA, Papina TS, Gimadeev AA, Sagadeev EV, Barabanov VP (2011) Standard formation enthalpies for α-amino acids: L-serine, L-arginine, and L-tyrosine. Moscow Univ Chem Bull 66: 88-91; Вестник Московского университета. Серия 2: Химия 52: 108-112.
- Kochergina LA, Volkov AV, Khokhlova EA, Krutova ON (2011) Thermodynamic characteristics of protolytic equilibria of L-serine in aqueous solutions. Russ J Phys Chem A 85: 881-889.
- Neacşu A, Gheorghe D, Contineanu I, Tănăsescu S, Perişanu Şt (2014) A thermochemical study of serine stereoisomers. Thermochim Acta 595: 1-5.
- Salmon A, Dalmazzone DJ (2007) Prediction of enthalpy of formation in the solid state at 298.15 K using second-order group contributions-part 2: carbon– hydrogen, carbon–hydrogen–oxygen, and carbon–hydrogen–nitrogen– oxygen compounds. J Phys Chem Ref Data 36: 19-58.
- Badelin VG, Tyunina EYu, Girichev GV, Giricheva NI, Pelipets OV (2007) Relationship between the molecular structure of amino acids and dipeptides and thermal sublimation effects. J Struct Chem 48: 647-653.
- Sagadeev EV, Gimadeev AA, Barabanov VP (2010) The enthalpies of formation and sublimation of amino acids and peptides. Russ J Phys Chem A 84: 209-214.
- Contineanu I, Perisanu St, Neacşu A (2006) The enthalpies of combustion and formation of the isomers of amino-benzoic acid. Rev Roum Chim 51: 323-327.
- Nabavian PM, Sabbah R, Chastel R, Laffitte M (1977) Thermodynamique de composés azotés. J Chim Phys 74: 115-126.
- Contineanu I, Marchidan DI (1994) The enthalpies of combustion and formation of some isomers of aminobutyric (aminobutanoic) acid. Rev Roum Chim 39: 1391-1395.
- Sabbah R, Laffitte M (1977) The enthalpy of formation of sarcosine in the solid state, J Chem Thermodyn 9: 1107-1108.
- Contineanu I, Neacşu A, Gheorghe D, Tănăsescu S, Perişanu Şt (2013) The thermochemistry of threonine stereoisomers. Thermochim Acta 563: 1-5.
- Sakiyama M, Seki S (1975) Enthalpies of combustion of organic compounds. II. L- and D-glutamic acid. Bull Chem Soc Jpn 48: 2203-2204.
- Sabbah R, Minadakis C (1981) Thermodynamique de substances soufrees. II. Etude thermochimique de la l-cysteine et de la l-methionine. Thermochim Acta 43: 269-277.
- Becker G, Roth WA (1934) Zur Bestimmung der Verbrennungswarme von organischen Schwefelverbindungen. Z Phys Chem 169: 287-296.
- Sunner S (1946) Determination of combustion heats of organo-sulphur compounds. Svensk Kim Tidr 58: 71-81.
- Huffman HM, Ellis EL (1935) Thermal Data. II. The heats of combustion of l-cysteine, of l-cystine, β-thiolactic acid and β, β'-dithiodilactic acid. J Am Chem Soc 57: 41-46.
- Roux MV, Notario R, Segura M, Chickos JS, JF Liebman (2012) The enthalpy of formation of methionine revisited. J Phys Org Chem 25: 916-924.
- Sagadeev EV, Gimadeev AA, Chachkov DV, Barabanov VP (2009) Empirical and ab initio Computation of the Thermochemical Parameters of Amino Acids: I. Monoamino Carbonic Acids and Monoamino Dicarbonic Acids and Their Amides. Russ J Gen Chem 79(3): 453-457.
- Lukyanova VA, Druzhinina AI, Pimenova SM, Ioutsi VA, Buyanovskaya AG, Takazova RU, Sagadeev EV, Gimadeev AA (2018) Thermodynamic properties of L-threonine. J Chem Thermodyn 116: 248-252.
- Tyunina VV, Krasnov AV, Badelin VG, Girichev GV (2016) Enthalpy of sublimation of hydroxyl-containing amino acids: Knudsen’s effusion mass spectrometric study. J Chem Thermodyn 98: 62-70.
- Tyunina VV, Krasnov AV, Tyunina EY, Badelin VG, Rybkin VV (2019) Enthalpies of sublimation of L-methionine and DL-methionine: Knudsen’s effusion mass spectrometric study. J Chem Thermodyn 135: 287-295.
- Hutchens JO, Cole AG, Robie RA, Stout JW (1963) Heat Capacities from 11 to 305 K, Entropies and Free Energies of Formation of L-Asparagine Monohydrate, L-Aspartic Acid, L-Glutamic Acid, and L-Glutamine. J Biol Chem 238(7): 2407-2412.
- Lutkin AI, Chernikov VV, Krutova ON, Skvortsov IA (2017) Standard enthalpies of formation of L-lysine and the products of its dissociation in aqueous solutions. J Therm Anal Calorim 130: 457-460.
- Dorofeeva OV, Ryzhova ON (2009) Revision of standard molar enthalpies of formation of glycine and L-alanine in the gaseous phase on the basis of theoretical calculations. J Chem Thermodyn 41: 433-438.
- Dorofeeva OV, Ryzhova ON (2014) Gas-Phase Enthalpies of Formation and Enthalpies of Sublimation of Amino Acids Based on Isodesmic Reaction Calculations. J Phys Chem A 118: 3490-3502.
- Karton A, Yu LJ, Kesharwani MK, Martin JML (2014) Heats of formation of the amino acids re-examined by means of W1-F12 and W2-F12 theories. Theor Chem Acc 133: 1-15.
- Roux MV, Foces-Foces C, Notario R, da Silva MAVR, da Silva MDMC, Santos AFLOM, Juaristi E (2010) Experimental and computational thermochemical study of sulfur-containing amino acids: L-cysteine, L-cystine, and L-cysteine-derived radicals. S-S, S-H, and C-S bond dissociation enthalpies. J Phys Chem B 114: 10530-10540.
- Ponomarev VV, Migarskaya LB (1960) Heats of combustion of some amino-acids. Russ J Phys Chem 34: 1182-1183.
- Santos AFLOM, Notario R, Ribeiro da Silva MAV (2014) Thermodynamic and Conformational Study of Proline Stereoisomers. J Phys Chem B 118: 10130-10141.
- Sabbah R, Laffitte M (1978) Enthalpy of formation of solid l-proline. J Chem Thermodyn 10: 101-102.
- Sabbah R, Laffitte M (1978) Thermodynamique de composes azotes. IV. Etude thermochimique de la sarcosine et de la l-proline. Bull Soc Chim Fr 1: 50-52.
- Vasilev VP, Borodin VA, Kopnyshev SB (1989) Standard enthalpies of formation of l-histidine and l-proline. Russ J Phys Chem 63: 891-892.
- Contineanu I, Neacşu A, Zgîrian R, Tănăsescu S, Perişanu Şt. (2012) The Standard Enthalpies of Formation of Proline Stereoisomers. Thermochim Acta 537: 31-35.
- Ribeiro da Silva MAV, Ribeiro da Silva MDMC, Santos LMNBF (2000) Standard molar enthalpies of formation of crystalline L-, D- and DL-valine. J Chem Thermodyn 32: 1037-1043.
- Wilson SR, Watson ID, Malcolm GN (1979) Enthalpies of formation of solid cytosine, L-histidine and uracil. J Chem Thermodyn 11: 911-912.
- Perişanu St, Contineanu I, Neacsu A, Tanasescu S (2010) The calorimetric study of some guanidine derivatives involved in living bodies nitrogen metabolism. J Therm Anal Calorim 101: 1127-1133.
- Neacsu A, Gheorghe D, Contineanu I, Sofronia AM, Teodorescu F, Perişanu St (2018) Enthalpies of Combustion and Formation of Histidine Stereoisomers. Hindawi J Chem, Article ID 7801381, 6 pages https://doi.org/10.1155/2018/7801381
- Gheorghe D, Neacşu A, Contineanu I, Tănăsescu S, Perişanu St (2017) A calorimetric study of L-, D- and DL-isomers of tryptophan. J Therm Anal Calorim 130(2): 1145-1152.
- Tyunina VV, Krasnov AV, Tyunina EY, Badelin VG, Girichev GV (2014) Enthalpy of sublimation of natural aromatic amino acids determined by Knudsen’s effusion mass spectrometric method. J Chem Thermodyn 74: 221-226.
-
Stefan Perisanu, Daniela Gheorghe, Ana Neacsu. The Enthalpy of Formation Of L- α -Amino Acids. Insi in Chem & Biochem. 1(3): 2020. ICBC. MS.ID.000515.
-
Antitumor Activity, Novel Azole compound, Carcinoma, Swiss Albino Mice.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






