 Research Article
Research Article
Sorbents for Fine Treatment of Wastewater from Organic Pollutants
Gagik Torosyan* and Dezi Hovhannisyan
National polytechnic university of Armenia, Armenia
Gagik Torosyan, National polytechnic university of Armenia, 105, Teryan, 0009 Yerevan, Republic of Armenia, Armenia.
Received Date: January 21, 2020; Published Date: January 29, 2020
Abstract
The removal of organic pollutants from wastewater is an urgent problem as these compounds contain in many industrial drains. Phenol, aniline, nitrobenzene are strong poisonous substance. The results of studies on finding sorbents for wastewater treatment are presented from these organic molecules. Results of researches here are submitted in the field of application natural and synthetic zeolites in quality of sorbents of mentioned organics. It has investigated the adsorptive properties of zeolites at removal organic molecules from a them solutions in tetrachloromethane / or hexane/ and water.
Keywords:Sorption; Sorbents; Natural and synthetic zeolites; Wastewater Treatment; Organic pollutants; Phenol; Aniline; Nitrobenzene
Introduction
Organic pollutants are retained by the highly developed surface of sorption material based on aluminosilicates under the influence of molecular and electrostatic forces, by chemical affinity and adsorption. As aluminosilicates, natural and modified zeolites are used. Major deposits of clinoptilolite-rich tuff are spread all over the world, e.g. Armenia, in Noemberyan region. Mordenite rich tuff exists in Shirak region in Armenia.
This article presents the results of a study of the sorption abilities of Armenian zeolites for the absorption of organic molecules from water and organic solvents. It should be noted that earlier we found that adsorption in the studied concentration limits for phenol has a linear dependence on the refractive index [1]. In these studies, we used this simple method for determining the concentrations of the studied organic compounds in solutions, which was subsequently corrected by liquid chromatography and ultraviolet spectroscopy.
Experimental part
UV spectra were recorded on a Specord-50 spectrophotometer. Compounds were analyzed by GLC using a LChM-80 devise, with thermal conductivity detector, a column temperature of 200-250°C, a column length of 2000x3 mm, 10% Apiezon L on an Inerton- AW carrier (0.20-0.25 mm), gas velocity the carrier (helium) 60 ml / min [1]. Preparation of aluminosilicates. Natural zeolite (clinoptilolite and mordenite) were dried for several hours to remove the remaining water at a temperature of 350-400°C. H-mordenite, clinoptilolite and ZSM-5 with an ammonium salt were prepared according to the procedure [2].
Removal of phenol on zeolites. Accurately weighed portions of sorbents were added to specific volumes of phenol in water, the initial concentrations of which varied. The mixture was thoroughly shaken for 6 hours. Then the sample was left to stand for 24-60 hours. Adsorption was almost complete in the first 48 hours. The amount of phenol precipitated was determined refractometric methods and also UV spectrophotometric. The results of the absorption of phenol by various sorbents are shown in Tables 1 & 2.
Table 1: Phenol sorption from solution of CCL4*.
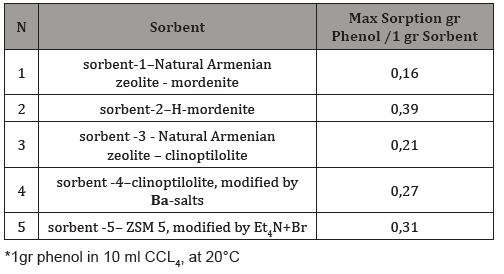
Table 2: Phenol sorption from water solution*.
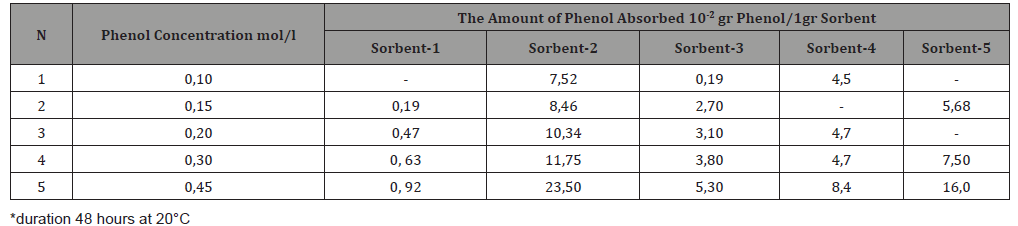
Removal of nitrobenzene and aniline from the organic solution. 5 ml of a solution of nitrobenzene in hexane in various concentrations from 0.5 to 1.5 N and aniline 1.5 N solution in tetrachloromethane were mixed with 0.5 g of zeolite. The resulting mixture was centrifuged, and then settled for 3 hours to one week. Next, the organic mixture was subjected to UV spectrometric analysis, liquid chromatography, and molar refraction was also measured (Table 3 &4). The molar refraction of the organic mixture was measured daily (Table 3 &4).
Table 3: ND20 values for various nitrobenzene/hexane system.
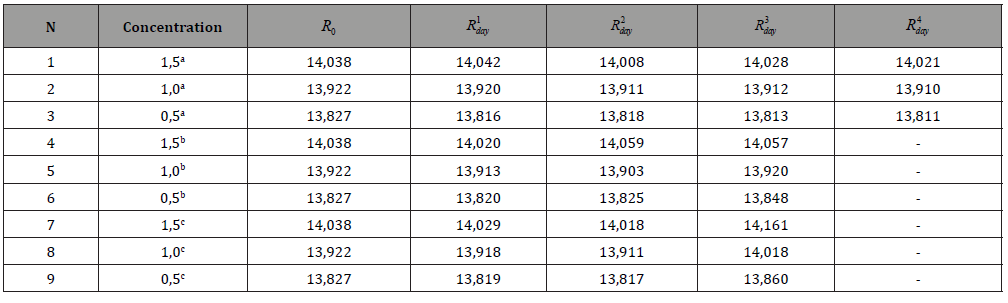
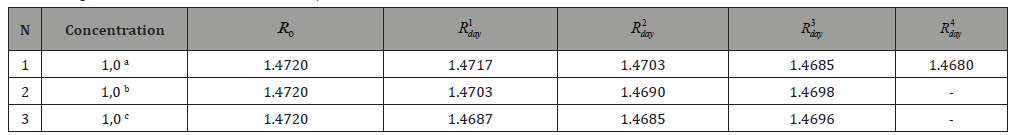
Table 4: ND20 values for various aniline / CCl4 systems.
Discussion
The adsorption properties of zeolites by removing organic pollutants from an aqueous solution or an organic (tetrachloromethane hexane) solvent have been investigated. Measurements were also carried out for aqueous phenol solutions in concentrations of 0.1-0.45 mol / L (Table 2). It was previously found that adsorption in these limits increases and has a linear dependence on the refractive index [2]. The amount of phenol was determined on the basis of a calibration graph and corrected using data from UV spectral analysis, as well as liquid chromatography for a solution of phenol in CCI4.
The amount of phenol absorbed increases with increasing concentration of solutions. The most active adsorbent is Sorbent-2. The amount of adsorbed pollutant was calculated as the difference between the total amount of contaminant added to the stock solution and its residue in the final solution. As can be seen from the data in Table 2, the H-form of mordenite turned out to be the best adsorbent of the studied organic molecules from aqueous solutions. Molecules cannot penetrate the pores of natural zeolites having sizes less than 5 Ao. In all likelihood, adsorption occurs on the surface of the zeolite, where hydrogen bonds are formed between the hydroxyl group of phenol, as well as the amine group of aniline and the nitro group of nitrobenzene and the H-peak of zeolite. The sorption of aniline and nitrobenzene from organic solutions / terrachloromethane or hexane/was studied. The results are shown in Tables 3 & 4.
Conclusion
It was revealed that zeolites are good materials for the adsorption of phenol, aniline, nitrobenzene from organic and aqueous solutions. These sorbents can withstand many regeneration cycles, after working out they do not contain harmful components and can be used as building material. The studied sorbents are designed for dynamic sorption in flow filters
Acknowledgement
This study is supported by the Special State Scientific Program of National polytechnic university of Armenia.
Conflict of Interest
No conflict of interest.
References
- GH Torosyan, AA Isakov, AR Aleksanyan, DH Hovhannisyan (2006) Phenol removal from solutions by alumosilikates & further conversion. Chemical Journal of Armenia 59(2): 53-59.
- S N Sargsyan, A Sh Grigoryan, SA Harutjunyan, GH Torosyan (2000) Phenol sorption from wastewater. The Bulletin of Armenian Constructors 2(18): 30-34.
-
Gagik Torosyan, Dezi Hovhannisyan. Sorbents for Fine Treatment of Wastewater from Organic Pollutants. Insi in Org & Inorg. 1(1): 2020. IOIC.MS.ID.000503.
-
Aluminosilicates, Sorption, Chemical affinity, Sorbents, Organic compounds, Natural and synthetic zeolites, Wastewater Treatment, Organic solutions, Organic pollutants, Phenol, Aniline, Nitrobenzene.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






