 Research Article
Research Article
Removal of Micropollutants From Wastewaters by Various Oxidation Processes: A Review
Németh József1, Tatjána Juzsakova1, Le Phuoc Cuong2*, Igor Cretescu3, Sebestyén Viktor1 and Rédey Ákos1
1University of Pannonia, Hungary
2The University of Danang, Vietnam
3Georghe Asachi Technical University, Romania
Le Phuoc Cuong, The University of Danang University of Science and Technology, 54 Nguyen Luong Bang street, Lien Chieu District, Danang 550000, Vietnam.
Received Date: October 23, 2019; Published Date: November 04, 2019
Abstract
In recent years, the micropollutants are increasingly detectable in the aquatic environment and endanger the human health even on shortterm. The impacts of pharmaceuticals and personal hygiene care products and chemicals in the aquatic environment is not clearly understood yet. The drug residues, household chemicals and pesticides are micropolluting components in our waters. The paper deals with different fourth stage wastewater treatment technologies including the advanced oxidation processes for the removal of micropollutants. The micropollutant removal efficiencies are studied and compared in light of the different technological solutions.
Keywords: Removal; Micropollutants; Human health; Oxidation process; Wastewater
Introduction
The task of wastewater treatment is to clean the contaminated waters which are discharged into the environment after treatment. The surface waters must reach a good ecological status according to the Water Framework Directive. It is important not to load the recipients with contminating materials and nutrients which are heavily decomposable. The goal of the sewage treatment is to lower the anthropogenic effects in the hydrological system to the lowest possible level. Thus, the task is typically to reduce the concentration of nutrients, to remove the dissolved oxygen consuming substances and to remove the accumulated organic and inorganic substances from wastewater [1,2].
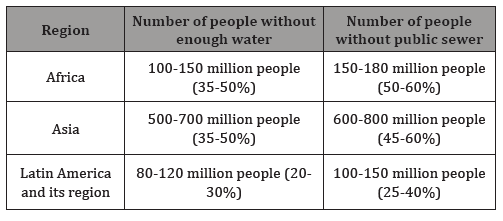
It is clear that in the 21th centur the protection of human health, the implementation of the concept of sustainable development and the protection of the ecosystem continue to play an important role. In many articles [3,4] it is emphasized that urbanization is increasing and the associated wastewater treatment poses a huge burden on the environment [5]. Among the readers of the new scientific results there are only few who have learnt from their own experience the social, economic and environmental problems of water scarcity. However, in many regions Table 1, there are millions of people who lack the water supply or the comfort of the public sewage system. With the introduction of the third stage of the sewage treatment, numerous problems have been resolved. However, there are still many issues that need to be solved. These questions raise the following tasks: the removal of anthropogenic micropollutants,which are often persistent. The WFD guidelines stipulate, the achievement and maintenance of good ecological status, wherever is possible, and the healthy drinking water supply. To solve these problems, many laboratories, research institutions and industrial companies deal with the performance and effectiveness of wastewater treatment technologies (Table 1), [6].
Table 1: Number and proportion of urban dwellers lacking adequate water supply and sewerage (2000) [4].
Micropollutants
Micropollutants are those substances that can be found in waters in magnitude of μg/l and reduce or eliminate the conditions of life processes in natural living waters. In this way the usability of waters as potable waters is drastically reduced or eliminated. Most of these micropollutants entering into living waters have persistent properties. One part of these contaminating materials can be removed in municipal sewage treatment plants, however significant part of these cannot be eliminated in the conventional wastewater facilities [A26]. These micropollutants can be organic micropollutants, inorganic micropollutants, drugs and drug residues, medicines, pesticides, etc. [7]. Therefore a fourth-stage treatment line is necessary to be considered in the wastewater treatment. In recent years, these micropollutants are increasingly detectable in the aquatic environment, as reported in the literature [8]. Christian and Thomas’ work is a good summary of the effects of pharmaceuticals and personal hygiene care products and chemicals in the aquatic environment. Their work suggests that drug residues, household chemicals and pesticides significantly pollute the surface waters. The source of these pollutants can be effluents, untreated waters, drain offs and flood streams [9]. Conventional sewage treatment plants receive a wide spectrum of such pollutants, which cannot be completely removed by conventional treatment procedures, so these materials accumulate in the recipients, as described by Yoon et al. [10].
The concentration of these pollutants is increasing, and today they are increasingly detectable in surface waters. These impurities typically exhibit significant toxicity even at low concentrations. Pollutants with hydrophilic properties also pose a threat to species of aquatic ecosystems [9]. In many cities, it can be observed that the water resources are contaminated in certain extent with effluents, so micropollutants entered into the drinking water bases. Thania and his colleagues dealt with Mexico City’s drinking water base and they identified 17 organic micropollutants whose concentrations were over the limit values, and it was proven that those derived from anthropogenic sources [11]. Yunlong Luo et al. in their 2014 article concluded that the novel tasks of wastewater treatment include maximizing the efficiency of the micropollutant removal during the optimization of sewage treatment plants. Some pollutants cannot be removed at all, others can only be party removed in the traditional wastewater treatment plants. It is mentioned that highly efficient methods, like advanced oxidation processes (AOP), adsorption processes and membrane separation procedures are all suitable for micropollutant removal, but these additional technological steps significantly increase the operating costs of the treatment facilities [7]. According to Maria Gavrilescu et al., these pollutants exhibit a serious challenge for the environment and human health in the future. They also pointed out that the operational treatment technologies cannot handle the new challenges [12].
Pesticides
Pesticides are mixtures of substances to protect plants and thus increase the agricultural productivity. Pesticides are vital elements of the modern agriculture, they play a significant role in highquality production. However, the long-term use of pesticides could exhibit a negative impact on the environment and human health [13]. A significant part of these adverse effects is due to the fact that they are being dispensed in inappropriate doses to agricultural areas or that inappropriate pesticides are used [14]. Mustapha and his associates also revealed a significant fact that in many countries banned pesticides are used [15]. The presence of these components in the environment is a potential source of danger [16].
Pharmaceutical Residues
The presence of pharmaceutical residues and hormones in sewage and surface water has been confirmed in several articles [17]. As the population increases, the use of medicines increases proportionally. In this way the presence of environmentallyunfriendly substances significantly increases in the environment [18]. Consequently, wastewater treatment plants will be heavily burdened with pharmaceutical residues. In the effluent of conventional sewage treatment plants, the concentration of drug substances also increases, which also accumulates in surface waters and accumulates due to their high biological stability [19]. The most frequently detected drug substances in wastewater are the following according to Bush’s work [20]:
Inflammatory and analgesic agents: paracetamol, acetylsalicylic acid, ibuprofen, diclofenac,
Antidepressants: benzodiazepines,
Antiepileptics: carbamazepine,
Lipid-lowering drugs: fibrates
β-blockers: atenolol, propanolol, metoprolol
Antibacterial drugs: antihistamines (ranitidine, famotidine)
Antibiotics: tetracyclines, macrolides, β-lactams, penicillins, quinolones, sulfonamides, fluoroquinolones, chloramphenicol, imidazole derivatives
Other substances: cocaine, barbiturates, methadone, amphetamines, opiates, heroin and other narcotics [20].
These compounds and their metabolites burden the sewage treatment plants. If the sewage treatment plants cannot remove the pollutants, the pollutants enter into the recipients, i.e. into the surface waters. The presence of these substances has a negative impact on the quality of the effluents. Therefore, continuous and targeted monitoring programs can positively contribute to solve this problem, however, additional mitigation measures are necessary.
Processes for the Removal of Micropollutants
Most of the micropollutants are not biologically decomposable or are very difficult to be broken down in the biological wastewater treatment units. For this reason, the micropollutants appear in the recipients unchanged [6]. There are a number of novel technological solutions for the removal of the micropollutants, and a brief summary on these will be given in this paper. Depending on the properties of the micropollutants, the coagulation and flocculation processes can be used to remove some or all of these compounds. From the work of Wray and Andrews, membrane separation processes are not always suitable to withhold the various organic micropollutants with good operational safety. The membrane separation technique can be efficiently combined with coagulation pretreatment [21]. Also due to the chemical and physical properties of the micropollutants those can accumulate in the the wastewater sludge. They are bound to the sludge matrix by chemical or physical adsorption. It is mentioned that during the biological processes the efficiency of the removal is influenced not only by the degradation but also by the sorption processes [22]. The procedures already referred to as fourth stage wastewater treatment are currently installed into the wastewater treatment train, however, these processes have not yet been widely applied in many countries. These proedures are used e.g. in Germany, Switzerland. Stefanos et al., discloses five different oxidation processes and three different post-treatment processes for Swiss treatment plants [23].
Micropollutants in Sewage Treatment Plants
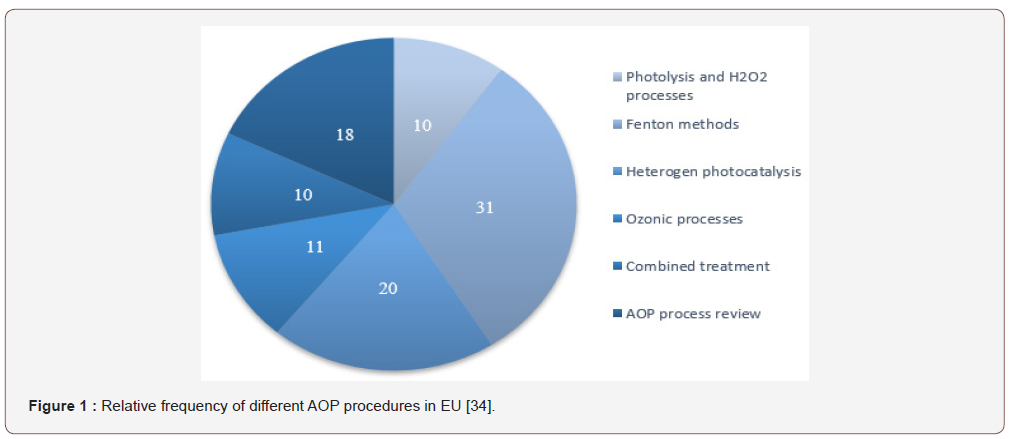
The biological sewage treatments with present technologies are not suitable for removing the micropollutants efficiently. Micropollutants are specific for treatment, and there is no uniform process for their removal due to their different properties as described by Yunlong Luo et al. [7]. A number of articles and reports have been published in this area [24-33], Figure 1. summarizes the relative frequencies of the various AOP techniques used in the European Union between 2004 and 2014. The assessment took into account the groups of substances stipulated in Directive 2013/39 / EU [34], (Figure 1).
Several papers have been published over the last decades on the effectiveness of high efficiency oxidation processes for the removal of micropollutants. Giannakis et al. studied five different oxidation methods for micropollutant removal. The procedures studied were as follows: UV-C, UV-C/H2O2, Solar, Fenton, photo-Fenton procedures. It was found that the UV-C is the most efficient when the pretreated sewage was treated with MBBR (Moving Bed Biofilm Reactor). technology. Even after 10 min treatment the removal efficiency was around 50%. The treatments also exhibited similar results after 10 min of UV treatment, but there were significant differences in the performance after 30 min. After 30 min, the UVC oxidation process showed 70 % removal efficiency after coagulation, and 80% removal efficiency was reached after MBBR purification [23]. The UV/H2O2 process proved to be useful after the coagulation process. The MBBR process was the most effective in micropollutant removal. In case of MBBR after 5 min treatment 85-98 % of the micropollutants were removed, while after 10 min treatment 100% removal efficiency was achieved. It was concluded that the current methods used in wastewater treatment significantly affect the maximum removal efficiency achieved with post-treatment technologies. In the fourth stage of sewage treatment, not only the oxidation processes take place. Adsorption processes are also important to be considered. The oxidation processes combined with adsorption technique have reached outstanding results in the removal of micropollutants. Salvatore et al. studied the removal efficiency of the adsorption combined with high efficiency oxidation process to remove nitrophenol from waste water and a removal efficiency of 70 % was achieved [35].
Oxidation Processes
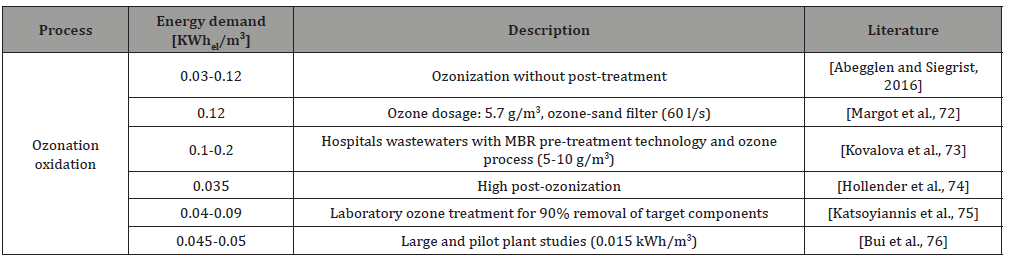
Ozonic oxidation processes are widely used in sewage treatment [36]. Ozone is not stable in an aqueous environment. There are several different solutions to mix the gas-water phases in order that the ozone can oxidize the micropollutants in the water. In the first step free radicals are formed when the ozone is decomposed and reacts with the organic compounds. Oxygen, odor-causing compounds, color-influencing compounds, volatile substances, humic substances, aromatic compounds [37] can easily be oxidized in this way. Advanced oxidation procedures (AOP) have also been extensively studied. According to Riberio et al. the chemical oxidation processes destruct the organic microcontaminants and majority of the complex compounds. During these processes hydroxyl radicals are formed which oxidize the organic pollutants. The products of the oxidation are CO2, H2O and inorganic ions [24]. Ozonization can be used in post-cleaning processes. The reason for this is that the chemical oxidation reduces the concentration of compounds which cannot be removed in the biological units or adsorptive methods. Ozone rapidly reacts with nitrite, so full nitrification takes place which is a prerequisite for the effective ozonation. Additional procedures attached to the conventional sewage treatment technologies to remove the organic micropollutants increase the energy needs. As a result, their environmental and economic impacts are significant. Table 2. summarizes the results of several studies on the energy demands of ozonization. The results of Danièle Mousel et al. show that the ozonic oxidation process used in sewage treatment plants has significantly higher energy demand than that of the adsorption methods [38] in order to remove micropollutants (Table 2).
Table 2: Literature data on the energy demand of ozonization [38].
Hydrogen Peroxide Treatment
In several cases H2O2 can be used to improve the formation of OH radicals [39]. The method is widely spread in the removal of organic pollutants. Rosenfeld et al. also reported that this process is suitable for the removal of pharmaceutical residues [40]. There is a tendency to supplement the hydrogen peroxide processes with UV treatment and other processes. Their effectiveness shows outstandingly good mineralization efficiency, which can be achieved in sevaral cases [41].
UV/H2O2 Combined Processes
Ultraviolet irradiation cleaves the oxygen-oxygen bonds at a suitable wavelength through a photochemical pathway, thus generating OH radicals from H2O2 (Eq. 1) [42]. During the wastewater treatment process, photon and OH radicals oxidize the micro-pollutants [43].
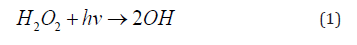
By absorbing a photon (eq.1), the quantum effect of OH inducement is 0.5 at 254 nm wavelength Figure 2, [44,45]. The formation of OH radicals in the UV/H2O2 system is pH and temperature independent [46], however, in practice the efficiency is influenced by the pH of the water since the OH radicals react with the CO3 2- and HCO3 - ions (Eqs. 2 and 3) [33] and other inorganic impurities dissolved in water [47].
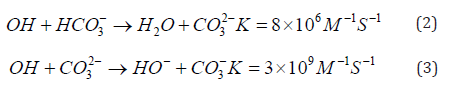
According to Eqs. 2 and 3. the presence of hydrocarbonate and carbonate ions negatively influences the formation of OH radical and the mineralization of the organic materials [48], (Figure 2).
The removal efficiency of 23 different drugs was studied by H2O2/UVC oxidation procedure and the results are summarized in (Table 3).
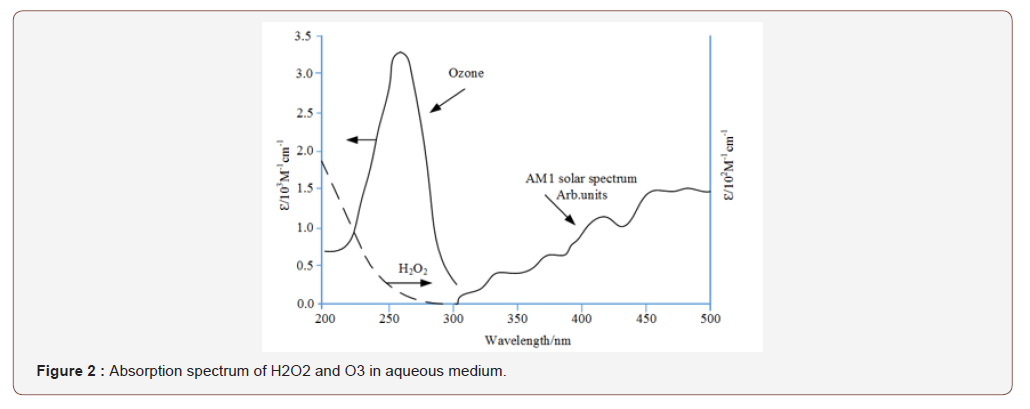
Table 3: The removal efficiency of the UV/ H2O2 oxidation process for 23 micropolluting components at different H2O2 concentrations [Afonso-Olivares et al. 41].
Advanced Ulra-Violet Processes
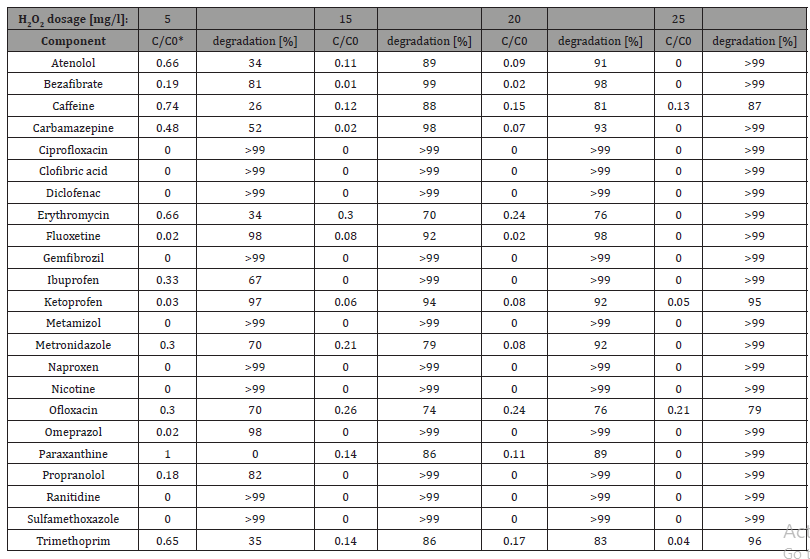
Advanced UV-based oxidation processes have proven to be effective in removing organic micropollutants from waters. In case of heavily contaminated surface waters [47] and wastewaters outstanding results were obtained by this technology [49]. It is apparent from the work of Canonica et al. that the depletion of drug residues at neutral pH was for EE2 compounds was 0.4% for dicl8fenac, 26% for diclofenac and 15% for sulfamethoxazole.The elimination efficiency of 23 pharmaceuaticals are summarized in the following (Table 4), [41]:
*H2O2 dosage / Initial pollutant compound concentration ratio
Table 4: Elimination efficiency for 23 pharmaceuticals by advanced ultra-violet processes [Afonso-Olivares et al. 41].
Fenton Process
High-efficiency oxidation Fenton processes are now widely used in wastewater treatment, organic pollutant removal and other aspects of water protection. These are commonly used in industrial scale to remove non-biodegradable highly-stable materials [50] or for disinfection procedures [51,52]. The Fenton-based procedure is considered to be an efficient and commonly used process [53]. The first Fenton process was elaborated for the oxidation of maleic acid [54,32]. The Fenton process is the most effective at pH=3. The process consits of our steps: oxidation, neutralization, flocculation and sedimentation [55]. Basically, the removal of the organic materials takes place in two steps where the oxidation step and then coagulation occur at first [56]. During the oxidation of organic matter, OH radicals and coagulants are formed. This mechanism can be described according to the following equations (Eq. 4 to Eq 7):
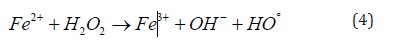
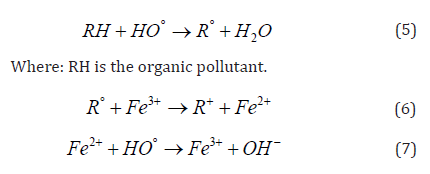
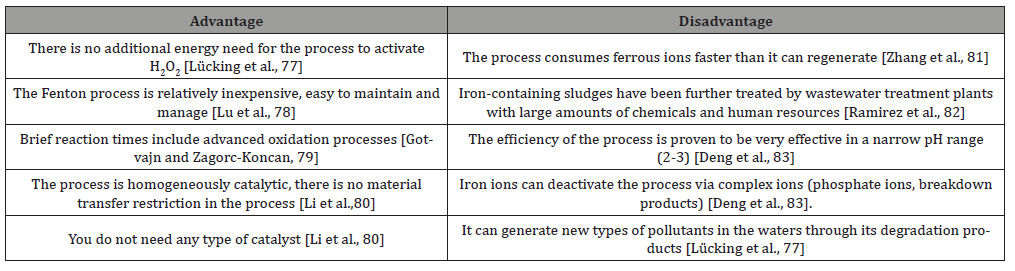
Table 5 summarizes the advantages and disadvantages of the above-mentioned processes according to P.V. Nidheesh and R. Gandhimathi [32], (Table 5).
Table 5: Advantages and Disadvantages of Fenton Processes [Nidheesh and Gandhimathi, 32].
Catalytic Processes
In the course of water purification catalytic processes including heterogeneous, homogeneous catalytic as well as biocatalytic processes are also used. Homogeneous catalysis is an extremely selective process, usually transition metal complex or special organic compound is used with one or more reactants in one phase. Their disadvantage is the operational cost and the separation of the product [57]. The heterogeneous catalyst is immiscible with the reactants, and it forms a separate phase. The catalysts have high specific surface area and the reaction takes place in the pores. The advantage of this catalytic process that it easy to separate the catalysts from the product and catalysts are cheaper than the homogeneous catalysts. Their disadvantage is that in many cases the catalysts are not selective [58]. Biocatalysts are usually protein-type compounds with specific activity and catalyze only the predetermined reaction. Their advantage lies in their high selectivity. Their disadvantage is that they are extremely expensive and sensitive to temperature, pH, solvent, ionic strength, and product concentration [59].
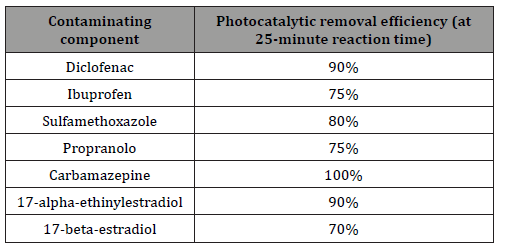
A significant part of the papers dealing with AOP technologies focuses on heterogeneous photocatalysis. It is also apparent from the work of Ana R. Ribeiro et al. that 20% of coloring materials formed during the photocatalytic reaction [34]. Heterogeneous photocatalysis can be considered as a green technology for the removal of organic micropollutants. The most widely used catalyst is doubtless the TiO2. It shows high chemical and photochemical stability. The disadvantage of its use is the broadband energy demand (3.0-3.2 eV), which also covers the UV range in the electromagnetic spectrum [60]. Various combined processes have shown outstanding efficiencies in the removal of various micropollutants. Nuno et al. reported that photocatalytic procedures are the most effective in degradation and mineralization of organic micropollutants [61]. In Table 6, the results of several reaseach groups are summarized on photocatalytic treatments [61], (Table 6).
Table 6: Photocatalytic removal efficiency for drugs [Nuno et al., 61].
Electrochemical Oxidation
The electrochemical oxidation processes have been used in pilot plants in the past and in wastewater treatment projects [62]. In many cases the processes are used to reduce the detrimental discharges into surface water to avoid the ecological damage in the recepients [63]. In the processes hydroxyl radicals are formed directly by electrochemical reactions (anodic oxidation, AO) or with Fenton reagent. In the first case, the OH radicals are generated by the discharge at the anode, while in the latter case the Fenton reaction generates the OH radicals. Based on the experimental results the in situ hydroxyl radicals are the second strongest oxidizing agents according to our present knowledge [64]. Throughout the processes, the OH radicals can be produced in an environmentally friendly way, with a standard reduction potential of E = 2.8 V, which can be used to mineralize the micropollutants [65] during the non-selective oxidation. The processes are widely used to different sewage types. In many cases the processes have resulted in complete mineralization [66]. During the electrochemical oxidation, the OH radicals directly oxidize the pollutants due to the direct electron flow through the anode (Figure 3).
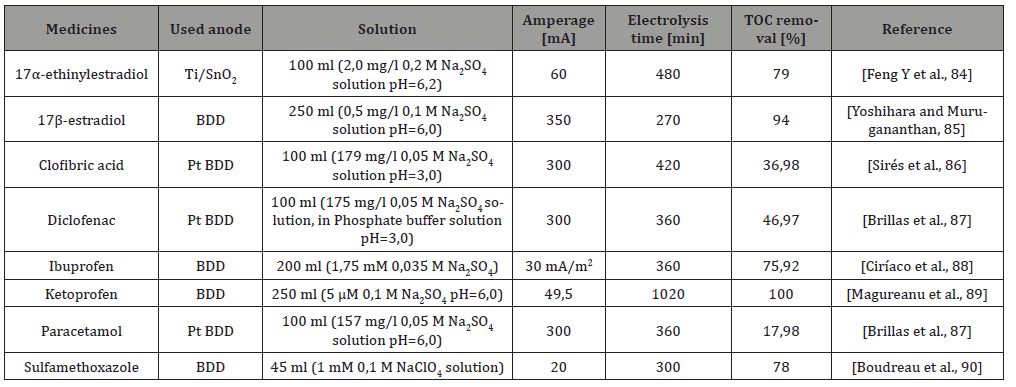
The anodic oxidation reaction is depicted in Figure 3. The reactions (a), (e), (f) show the active anodic reactions (a), (b), (c), (d). The reaction (a) results in the formation of hydroxyl radical M (OH); reaction (b) is the generation of metal oxide (MO), which results in reaction (c), the electrochemical transformation from the organic materials. Step (d) results in the formation of ozone (Michaud et al., 2003), and the formation of hydrogen peroxide can be observed. The electrochemical oxidation processes can also be efficiently used in the field of water purification. It was shown that after 7 hours of treatment, TOC removal of about 99% at 1000 mA current (16.2 mg/l solution) was achieved. It was also found that few intermediate degradation products were generated and effective mineralization was achieved [62]. According to Sirés and Brillas, the anodic electrochemical oxidation methods can be efficiently used for the removal of drug derivatives (Table 7), [67].
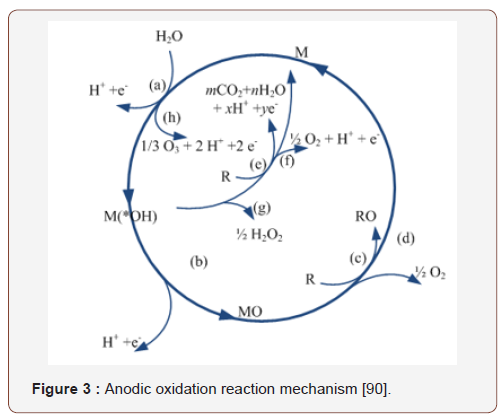
Table 7: Effectiveness of anodic oxidation for medicines Ignasi and Enric [67].
Ultrasonic Irradiation
Ultrasonic irradiation is considered a pollutant-free technology, and its use is widespread. It removes many micropollutants (ibuprofen, ethyl-paraben, paration, methyl-benzotrizole) [68]. The effectiveness of the treatment stems from the following: chemical effects, shock waves and shear stress [68]. Ultrasound spreads in the form of three-dimensional longitudinal waves. Wavefronts pass through the medium and cause pressure increase and decrease. Pressure fluctuation depends on the intensity of the sound waves. The pressure increase may be so high that it interrupts the continuity of the fluid medium by creating microscopic cavities. These rapidly generated cavities pulse through the liquid. As a result, 4000-6000 oC temperature and 300-500 bar pressure may occur locally in the treated wastewater. These extreme values result in the degradation of micropollutants [69].
Microwave Procedures
Microwave technology has gained ground in industrial use in addition to the well known household applications. During oxidation processes the oxidizing agents (potassium-persulfate) used under microwave irradiation will also be able to degrade some of the hardly degradable compounds (e.g.: perfluoro-octanoic acid, pesticides, azo-dyes). Yu-Chi and his colleagues demonstrated the defluorination and degradation efficiencies of compounds by the combination of microwave and oxidation treatment (Table 8) [70]
Table 8: Degradation efficiency of components by microwave treatment.
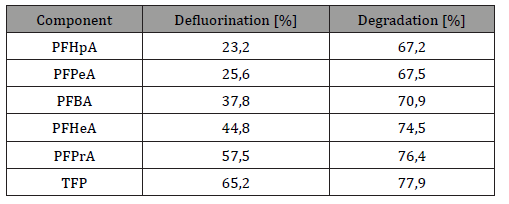
These micropollutants are efficiently broken down during the microwave process. High mineralization rate can be achieved in this way. It has been demonstrated that pH and temperature are important driving elements of this treatment [70]. Yu el al.studied the Fenton reaction combined with microwave treatment of pharmaceuticals of wastewaters. The efficiency varied between 40-60% depending on the Fenton dosage [71-94].
Discussion
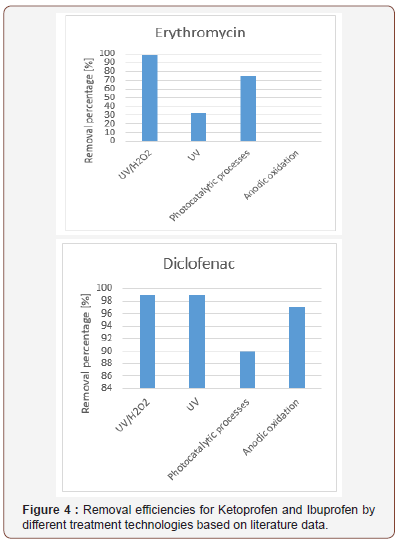
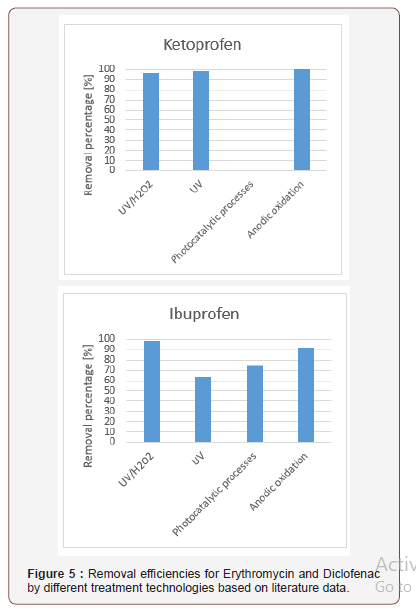
Removal efficiencies of different pharmaceuticals from wastewaters are compared from the point of view of different technological processes used based on literature data. The combined ultraviolet and hydrogen-peroxide process, the ultraviolet process, the photocatalytic process, and the anodic oxidation process are evaluated for micropollutant removal. The removal of drugs (Paracetamol, 17-Beta-Estradiol, 17-Alpha- Ethinyl-estradiol, Trimethoprim, Sulfamethoxazole, Ranitidine, Propanolol, Paraxanthine, Omeprazol, Ofloxacin, Nicotine, Naproxen, Metronidazole, Metamizol, Ketoprofen, Ibuprofen, Gemfibrozil, Fluoxetine, Erythromycin, Diclofenac, Clofibrc acid, Ciprofloxacin, Carbamazepine, Caffeine, Bezafibrate, Atenolol) were studied by the above mentioned micropollutant removal technologies. The removal efficiencies for Ketoprofen, Ibuprofen, Erythromycin and Diclofenac from waters by different treatment technologies are illustrated in Figure 4 and 5. based on literature data since these drugs are widely used in our daily life. It can be concluded that the UV/H2O2 process and the photocatalytic process can be efficiently used for the removal of micropollutants from waters. For the selection of a fourth stage waste water treatment not only the micropollutant removal efficienies, but the financial aspects including the investment and operational costs must be considered as well (Figure 4 and 5).
Conclusion
From the point of view of the sustainable development it is an urgent task to deal with the fourth stage of wastewater treatment since the conventional wastewater treatment facilities cannot cope with the efficient removal of the micropollutants from wastewaters. Therefore it is a challange for the scientists to deal with new solutions to remove drug, pharmaceutical, pesticide residues from waters since the long-term human health impacts of these compounds cannot be clearly and fully understood. However, the preliminary studies on the health implications are alarming therefore it is a must to deal with these issues.
Acknowledgement
None
Conflict of Interest
No conflict of interest.
References
- Kárpáti Árpád (2007) Szennyvíztisztítás alapjai, digitális tanagyag.
- Licskó István (2004) Víz és szennyvíztisztítás, BMEEOVKASG3, segédlet a BME Építőmérnöki Kar hallgatói részé
- Roberto Bagatin, Jiri Jaromír Klemes, Andrea Pietro Reverberi, Donald Huisingh (2004) Conservation and improvements in water resource management: a global challenge. Journal of Cleaner Production 77: 1-9.
- UNICEF (2015) Water and Sanitation, Drinking water.
- Y Sun, Zhuo C, Guangxue W, Qianyuan Wu, F Zhang, et al. (2016) Characteristics of water quality of municipal wastewater treatment plants in China: implications for resources utilization and management. Journal of Cleaner Production 131: 1-9.
- Barjenbruch M, Firk W, Peter Fröhlich A (2014) Antropogén nyomanyagok eltávolításának lehetőségei a kommunális szennyvíztisztító Hírcsatorna 11-12.
- Yunlong Luo, Wenshan Guo, Huu Hao Ngo, Long Duc Nghiem, Faisal Ibney Hai, et al. (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Science of the Total Environment 473-474: 619-641.
- Gregor Knopp, Carsten Prasse, Thomas A, Ternes, Peter Cornel (2010) Elimination of micropollutants and transformation products from a wastewater treatment plant effluent through pilot scale ozonation followed by various activated carbon and biological filters. Water Research 100: 580-592.
- Christian G, Daughton, Thomas A, Ternes (1999) Pharmaceuticals and Personal Care Products in the Environment: Agents of Subtle Change? Environ Health Perspect 107(Suppl 6): 907-938.
- Yeomin Yoon, Jaena Ryu, Jeill Oh, Byeong Gyu Choi, SA Snyder (2010) Occurrence of endocrine disrupting compounds, pharmaceuticals, and personal care products in the Han River (Seoul, South Korea). Science of the Total Environment 408(3): 636-643.
- Thania E Félix Cañedo, Juan C Durán Álvarez, Blanca Jiménez Cisneros (2013) The occurrence and distribution of a group of organic micropollutants in Mexico City’s water sources. Science of the Total Environment 454-455: 109-118.
- Maria Gavrilescu, Kateřina Demnerová, Jens Aamand, Spiros Agathos, Fabio Fava (2015) Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnology 32(1): 147-156.
- Pimentel D (2005) Environmental and economic cost of the application of pesticides primarily in the United States Environ Dev Sustain 7(2): 229-252.
- Dasgupta S, Meisne C, Huq M (2007) A pinch or a pint? Evidence of pesticide overuse in Bangladesh. J Agric Econ 58(1): 91-114.
- Mustapha FA Jallow, Dawood G Awadh, Mohammed S Albaho, Vimala Y Devi, Binson M Thomas (2017) Pesticide risk behaviors and factors influencing pesticide use among farmers in Kuwait. Science of the Total Environment 574: 490-498.
- Boix C, Ibanez M, Sancho JV, Rambla J, Aranda JL, et al. (2015) Fast determination of 40 drugs in water using large volume direct injection liquid chromatography-tandem mass spectrometry. Talanta 131: 719-727.
- Thomas A Ternes (1998) Occurrence of Drugs in German Sewage Treatment Plants And Rivers. Wat Res 32(11): 3245-3260.
- Wooten J M (2012) Pharmacotherapy considerations in elderly adults. South Med J 105(8): 437-445.
- Brillas E, Garcia Segura S, Skoumal M, Arias C (2010) Electrochemical incineration of diclofenac in neutral aqueous medium by anodic oxidation using Pt and boron-doped diamond anodes. Chemosphere 79(6): 605-612.
- Bush K (1997) Antimicrobial agents. Curr Opin Chem Biol 1 :169-175.
- Heather E Wray, Robert C Andrews (2014) Optimization of coagulant dose for biopolymer removal: Impact on ultrafiltration fouling and retention of organic micropollutants. Journal of Water Process Engineering 1: 74-83.
- Falas P, Longree P, La Cour Jansen J, Siegrist H, Hollender J, Joss A (2013) Micropollutant removal by attached and suspended growth in a hybrid biofilm-activated sludge process. Water Research 47(13): 4498-4506.
- Stefanos Giannakis, Margaux Voumard, Dominique Grandjean, Anoys Magnet, Luiz Felippe De Alencastro, et al. (2016) Micropollutant degradation, bacterial inactivation and regrowth risk in wastewater effluents: Influence of the secondary pre-treatment on the efficiency of Advanced Oxidation Processes. Water Research 102: 505-515.
- Marc Pera Titus, Verónica Garcia Molina, Miguel A Baños, Jaime Giménez, et al. (2004) Degradation of chlorophenols by means of advanced oxidation processes: a general review. Applied Catalysis B Environmental 47(4): 219-256.
- Feng L, Van Hullebusch ED, Rodrigo MA, Esposito G, Oturan MA (2013) Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review Chem Eng J 228: 944-964.
- Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions. Adv Environ Res 8: 501-551.
- Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95: 69-96.
- Ikehata K, El Din MG (2005) Aqueous Pesticide degradation by ozonation and ozone-based advanced oxidation processes: a review (part I). Ozone Sci Eng 27: 83-114.
- Joseph CG, Li Puma G, Bono A, Krishnaiah D (2009) Sonophotocatalysis in advanced oxidation process: a short review. Ultrason Sonochem 16(5): 583-589.
- Levec J, Pintar A (2007) Catalytic wet-air oxidation processes: a review. Catal Today 124: 172-184.
- Mishra S, Mahajani VV, Joshi JB (1995) Wet air oxidation. Ind Eng Chem Res 34(1): 2-48.
- Nidheesh PV, Gandhimathi R (2012) Trends in electro Fenton process for water and wastewater treatment: an overview. Desalination 299: 1-15.
- Nick Serpone, Satoshi Horikoshi, Alexei V Emeline (2010) Microwaves in advanced oxidation processes for environmental applications. A brief review. Journal of Photochemistry and Photobiology C Photochemistry Reviews 11(2): 114-131.
- Ana R Ribeiro, Olga C Nunes, Manuel FR Pereira, Adrián MT Silva (2015) An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environment International 75: 33-51.
- Salvatore Cataldo, Adriano Iannì, Vittorio Loddo, Eliana Mirenda, Leonardo Palmisano, et al. (2016) Combination of advanced oxidation processes and active carbons adsorption for the treatment of simulated saline wastewater. Separation and Purification Technology 171: 101-111.
- Andreozzi, Caprio V, Insola A, Marotta R (1999) Advanced oxidation processes (AOP) for water purification and recovery. Catal Today 53(1): 51-59.
- Kiss Zsolt László (2015) Előkezelések szűrési paraméterekre gyakorolt hatásának vizsgálata olajtartalmú szennyvizek illetve termálvizek membránszűrése során (doctoral dissertation), Szegedi Tudományegyetem, Szeged.
- Daniele Mousel, Laurence Palmowski, Johannes Pinnekamp (2017) Energy demand for elimination of organic micropollutants in municipal wastewater treatment plants. Science of The Total Environment 575: 1139-1149.
- Yaal Lester, Imma Ferrer, E Michael Thurman, Karl G Linden (2014) Demonstrating sucralose as a monitor of full-scale UV/AOP treatment of trace organic compounds. Journal of Hazardous Materials 280: 104-110.
- Pereira VJ, Linden KG, Weinberg HS (2007) Evaluation of UV irradiation for photolytic and oxidative degradation of pharmaceutical compounds in water. Water Research 41 (19): 4413-4423.
- Afonso Olivares C, Fernández Rodríguez C, Ojeda González RJ, Sosa Ferrera Z, Santana Rodríguez JJ, et al. (2016) Estimation of kinetic parameters and UV doses necessary to remove twenty-three pharmaceuticals from pre-treated urban wastewater by UV/H2O2. Journal of Photochemistry and Photobiology A: Chemistry 329: 130-138.
- Aleix Benito, Aida Penadés, Josep Lluis Lliberia, Rafael Gonzalez Olmos (2017) Degradation pathways of aniline in aqueous solutions during electro-oxidation with BDD electrodes and UV/H2O2 treatment. Chemosphere 166: 230-237.
- Cristina Postigoa, Susan D Richardson (2014) Transformation of pharmaceuticals during oxidation/disinfection processes in drinking water treatment. Journal of Hazardous Materials 279: 461-475.
- Hunt JP, Taube H (1952) J Am Chem Soc 74: 5999-6004.
- Weeks J L, Matheson M S (1956) J Am Chem Soc 78: 1273-1275.
- Dore M (1989) Oxidant chemistry and water treatment. Lavoisier, Paris.
- Wols BA, Hofman Caris CHM, Harmsen DJH, Beerendonk EF (2013) Degradation of 40 selected pharmaceuticals by UV/H2O2. Water Res 47(15): 5876-5888.
- Serpone N, Horikoshi S, Emeline AV (2010) Microwaves in advanced oxidation processes for environmental applications. A brief review. J Photochem Photobiol C Photochem Rev 11: 114-131.
- Ilho Kim, Naoyuki Yamashita, Hiroaki Tanaka (2009) Photodegradation of pharmaceuticals and personal care products during UV and UV/H2O2 treatments. Chemosphere 77: 518-525.
- Choi H, Al Abed SR, Dionysiou DD, Stathatos E, Lianos P (2010) TiO2 based advanced oxidation nanotechnologies for water purification and reuse. Sustainability Sci Eng 2: 229-254.
- Stefanos Giannakis, María Inmaculada Polo López, Dorothee Spuhler, Jose Antonio Sánchez Pérezc, Pilar Fernández Ibáñez, et al. (2016) Solar disinfection is an augmentable, in situ-generated Photo-Fenton reaction—Part 1: A review of the mechanisms and the fundamental aspects of the process. Applied Catalysis B Environmental 199: 199-223.
- Stefanos Giannakis, María Inmaculada Polo López, Dorothee Spuhler, Jose Antonio Sánchez Pérezc, Pilar Fernández Ibáñez, et al. (2016) Solar disinfection is an augmentable, in situ-generated photo-Fenton reaction—Part 2: A review of the applications for drinking water and wastewater disinfection. Applied Catalysis B: Environmental 198: 431-446.
- Shaobin Wang (2008) A Comparative study of Fenton and Fenton-like reaction kinetics in decolourisation of wastewater. Dyes and Pigments 76: 714-720.
- Fenton H J (1884) Oxidative properties of the H2O2/Fe2+ system and its application. J Chem Soc 65: 889-899.
- Kochany J, Lipczynska Kochany E (2009) Utilization of landfill leachate parameters for pretreatment by Fenton reaction and struvite precipitation-a comparative study. J Hazard Mater 166: 248-254.
- Kang YW, Hwang KY (2000) Effects of reaction conditions on the oxidation efficiency in the Fenton process. Water Res. 34(10): 2786-2790.
- Mortreux A, Petit F (1988) Industrial Applications of Homogeneous Catalysis. Kluwer Academic Publishers Group, Holland.
- Evnin AB, Rabo JA, Kasai PH (1973) Heterogeneously catalyzed vapor-phase oxidation of ethylene to acetaldehyde. Journal of Catalysis 30(1): 109-117.
- Andreas S Bommarius, Bettina R Riebel (2004) Biocatalysis, Fundamental and Applications. Wiley VCH Verlag GmbH & Co. KGaA Weinheim.
- Huang GF, Ma ZL, Huang WQ, Tian Y, Jiao C, et al. (2013) Article ID 371356. J Nanomater 1-8.
- Nuno FF Moreira, José M Sousa, Gonçalo Macedo, Ana R Ribeiro, Luisa Barreiros, et al. (2016) Photocatalytic ozonation of urban wastewater and surface water using immobilized TiO2 with LEDs: Micropollutants, antibiotic resistance genes and estrogenic activity. Water Research 94: 10-22.
- Clement Trellu, Yoan Pechaud, Nihal Oturan, Emmanuel Mousset, David Huguenot, et al. (2016) Comparative study on the removal of humic acids from drinking water by anodic oxidation and electro-Fenton processes: Mineralization efficiency and modelling. Applied Catalysis B: Environmental 194: 32-41.
- Enric Brillas, Carlos A, Martinez Huitle (2015) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Applied Catalysis B: Environmental 166-167: 603-643.
- Latimer WM (2006) Oxidation Potentials, Prentice Hall.
- Enric Brillas, Miguel Angel Banos, Marcel Skoumal, Pere Luıs Cabot, Jose Antonio Garrido, et al. (2007) Degradation of the herbicide 2,4-DP by anodic oxidation, electro-Fenton and photoelectro-Fenton using platinum and boron-doped diamond anodes. Chemosphere 68(2): 199-209.
- Beytul Balci, Nihal Oturan, Richard Cherrier, Mehmet A Oturan (2009) Degradation of atrazine in aqueous medium by electrocatalytically generated hydroxyl radicals. A kinetic and mechanistic study. Water Research 43(7): 1924-1934.
- Ignasi Sirés, Enric Brillas (2012) Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environment International 40: 212-229.
- Cheng Liu, Zhen Cao, Jie Wang, Zhehao Sun, Siyuan He, et al. (2017) Performance and mechanism of phycocyanin removal from water by low-frequency ultrasound treatment. Ultrasonics Sonochemistry 34: 214-221.
- Németh Zsolt, Kárpáti Árpád (2005) Ultrahanggal végzett iszapkezelés és hatásai a szennyvíztisztításban, Iszapmaradék csökkentése, rothasztókapacitás növelése és iszapstabilizáció. Környezetvédelem, vízgazdálkodás és szennyvizek, 08 (in Hungarian).
- Yu Chi Lee, Shang Lien Lo, Pei Te Chiueh, Der Guang Chang (2009) Efficient decomposition of perfluorocarboxylic acids in aqueous solution using microwave-induced persulfate. Water Research 43(11): 2811-2816.
- Yu Yang, Peng Wang, Shujie Shi, Yuan Liu (2009) Microwave enhanced Fenton-like process for the treatment of high concentration pharmaceutical wastewater. Journal of Hazardous Materials 168(1): 238-245.
- Margot C, Kienle A, Magnet M, Weil L, Rossi ALF, et al. (2013) Treatment of Micropollutants in Municipal Wastewater: Ozone or Powdered Activated Carbon? Science of the Total Environment 461-462: 480-498.
- Kovalova L, Siegrist H, Von GU, Eugster J, Hagenbuch M, et al. (2013) Elimination of micropollutants during post treatment of hospital wastewater with powdered activated carbon, ozone and UV. Environ Sci Technol 47(14): 7899-7908.
- Hollender J, Zimmermann SG, Koepke S, Krauss M, Mc Ardell CS, et al. (2009) Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ Sci Technol 43(20): 7862-7869.
- Katsoyiannis IA, Canonica S, Von GU (2011) Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res 45 (13): 3811-3822.
- Bui XT, Vo TPT, Ngo HH, Guo WS, Nguyen TT (2016) Multicriteria assessment of advanced treatment technologies for micropollutants removal at large-scale applications. Science of the Total Environment 563-564: 1050-1067.
- Lücking F, Köser H, Jank M, Ritter A (1998) Iron powder, graphite and activated carbon as catalysts for the oxidation of 4-chlorophenol with hydrogen peroxide in aqueous solution. Water Res 32(9): 2607-2614.
- Lu MC, Zhang H, Huang YY, Wang SY (2005) Influence of inorganic ions on the mineralization of 2, 4-dinitrophenol by the Fenton reaction. Fresenius Environ Bull 14(2): 101-104.
- Gotvajn AZ, Zagorc Koncan J (2005) Combination of Fenton and biological oxidation for treatment of heavily polluted fermentation waste broth. Acta Chim Slov 52: 131-137.
- Li W, Zhou Q, Hua T (2010) Removal of organic matter from landfill leachate by advanced oxidation processes: a review. Int J Chem Eng 1-10.
- Zhang H, Zhang D, Zhou J (2006) Removal of COD from landfill leachate by electro-Fenton method. J. Hazard. Mater. 135(1-3): 106-111.
- Ramirez JH, Costa CA, Madeira LM, Mata G, Vicente MA, et al. (2007) Fenton like oxidation of Orange II solutions using heterogeneous catalysts based on saponite clay. Appl Catal B 71: 44-56.
- Deng J, Jiang J, Zhang Y, Lin X, Du C, et al. (2008) FeVO4 as a highly active heterogeneous Fenton-like catalyst towards the degradation of Orange II. Appl Catal B 84(3): 468-473.
- Feng Y, Wang C, Liu J, Zhang Z (2010) Electrochemical degradation of 17-alpha-ethinylestradiol (EE2) and estrogenic activity changes. J Environ Monit 12: 404-408.
- Yoshihara S, Murugananthan M (2009) Decomposition of various endocrine-disrupting chemicals at boron-doped diamond electrode. Electrochim Acta 54(7): 2031-2038.
- Sirés I, Cabot PL, Centellas F, Garrido JA, Rodríguez RM, et al. (2006) Electrochemical degradation of clofibric acid in water by anodic oxidation: Comparative study with platinum and boron-doped diamond electrodes. Electrochim Acta 52(1): 75-85.
- Brillas E, Sires I, Arias C, Cabot PL, Centellas F, et al. (2005) Mineralization of paracetamol in aqueous medium by anodic oxidation with a boron-doped diamond electrode. Chemosphere 58(4): 399-406.
- Ciriaco L, Anjo C, Correia J, Pacheco M, Lopes A (2009) Electrochemical degradation of ibuprofen on Ti/Pt/PbO2 and Si/BDD electrodes. Electrochim Acta 54: 1464-1472.
- Magureanu M, Piroi D, Mandache NB, David V, Medvedovici A, et al. (2010) Degradation of pharmaceutical compound pentoxifylline in water by non-thermal plasma treatment. Water Res. 44(11): 3445-3453.
- Boudreau J, Bejan D, Li S, Bunce NJ (2010) Competition between electrochemical advanced oxidation and electrochemical hypochlorination of sulfamethoxazole at a boron-doped diamond anode. Ind Eng Chem Res 49(6): 2537-2542.
- Abegglen C, Siegrist H (2016) Micropollutants in municipal wastewater. Processes for further elimination on wastewater treatment.
- Emma Gracia Lor, Juan V Sancho, Felix Hernandez (2010) Simultaneous determination of acidic, neutral and basic pharmaceuticals in urban wastewater by ultra-high-pressure liquid chromatography-tandem mass spectrometry. Journal of Chromatography A 1217(5): 622-632.
- Gregor Knopp, Carsten Prasse, Thomas A. Ternes, Peter Cornel (2016) Elimination of micropollutants and transformation products from a wastewater treatment plant effluent through pilot scale ozonation followed by various activated carbon and biological filters. Water Research 100: 580-592.
- Michaud PA, Panizza M, Ouattara L, Diaco T, Foti G, et al. (2003) Electrochemical oxidation of water on synthetic boron-doped diamond thin film anodes. Journal of Applied Electrochemistry 33(2): 151-154.
-
Németh József, Tatjána Juzsakova, Le Phuoc Cuong, Igor Cretescu, Sebestyén Viktor, Rédey Ákos. Removal of Micropollutants From Wastewaters by Various Oxidation Processes: A Review. Insi in Org & Inorg. 1(1): 2019. IOIC.MS.ID.000502.
-
Removal, Micropollutants, Human health, Oxidation process, Wastewater, Ecosystem, Urbanization, Anthropogenic micropollutants, Laboratories, Micropollutants, Pharmaceuticals.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
- Abstract
- Introduction
- Micropollutants
- Pesticides
- Pharmaceutical Residues
- Processes for the Removal of Micropollutants
- Micropollutants in Sewage Treatment Plants
- Oxidation Processes
- Hydrogen Peroxide Treatment
- UV/H2O2 Combined Processes
- Advanced Ulra-Violet Processes
- Fenton Process
- Catalytic Processes
- Electrochemical Oxidation
- Ultrasonic Irradiation
- Microwave Procedures
- Discussion
- Conclusion
- Acknowledgement
- Conflict of Interest
- References






