 Research Article
Research Article
Phytochemical Investigation and Antimicrobial Activity of Root Bark Extracts of Acacia Oerfota
Mahdi Abdelmageed Mohammed Ali1, RahmaAbubakr Musa2, YagoubAbdella Ali1, Ferial, Mohamed Abu Al bashar Amin1, Osama Osman Adam Yahia1, Tasneem Ezaldeen Taha Hag Eltahir1, Malak Haider Ibrahim Fadell Almoyla1 and Hatim M Y Hamadnalla1*
1Department of Biology and Technology, College of Applied and Industrial Sciences, University of Bahri, Sudan
2Department of Biology, Faculty of Education, University of Zalingei, Sudan
Hatim M Y Hamadnalla, University of Alexandria University, Egypt.
Received Date: January 10, 2022; Published Date: January 21, 2022
Abstract
This study was carried out in Khartoum State-Sudan, in February 2021, Acacia oerfota locally known as (Laot) was chosen for this study to evaluate phytochemical investigation and antimicrobial activity due to it is repeated used in Folk medicine. The plant was collected from Omdurman area washed, dried and extracted successively with (n-hexane, Chloroform, and methanol). Phytochemical screening was carried out and showed that: the root bark extracts contained high amount of alkaloids and saponins in all extracts, moderate amount of flavonoids (in chloroform, methanol extract) and absence of tannins in all extracts. The antimicrobial activity of roots extracts was evaluated against four standard bacteria- Gram positive; (Bacillussubtilis, staphylococcusaureus) and Gram negative; (Escherichia coli, pseudomonas aeruginosa) in addition to one standard fungi (Candida albicans).The results of antimicrobial tests showed that the n-hexane extract had significant inhibition zone against Escherichia coli , pseudomonas aeruginosa, Candida albicans and Bacillus subtilis in concentrations 50% and 25% and no inhibition zone against Staphylococcus aureus and Candida albicans. Methanol extract reported high inhibition zone against Bacillus subtilis in all concentration and no inhibition zone against Staphylococcus aureus. The chloroform extract showed good inhibition zone against Staphylococcus aureus in all concentration and moderate inhibition zone in all concentration against Escherichia coli and Candida albicans and no inhibition zone against Pseudomonas aeruginosa and Bacillus subtilis in all concentrations
Keywords: Acacia oerfota, phytochemical investigation, Antimicrobial activity
Introduction
Medicinal Aromatic Plant (MAPs) plays a valuable and important role in economic, social, cultural and ecological aspects of local communities in the world over. It provides people with medicines to prevent disease, maintain health or cure ailments. Traditional medicinal plants are a therapeutic resource used by the population of the African continent specifically for health care, which may also serve as starting materials for drugs [1,2]. Indigenous people have been using the unique approach of their traditional system of med icine for centuries and among the most renowned are the Chinese, Indian and African systems of medicine [3]. The development of modern scientific medicine is still in use today without any documented evidence of adverse effects this is according to the World Health Organization [4]. The Vachelliao erfota is very important legume tree and browse species for goats and camels in the arid and semiarid zones of the Eastern Sahel and East Africa, where it is commonly found with other acacias such as Acacia mellifera and Acacia laeta [5]. Sudanese medicinal Plants have traditionally occupied an important position in the socio-cultural, spiritual and medicinal area of rural and tribal lives in Sudan. Herbal drugs are of major importance in Sudanese folk medicines previously reported by many researchers in Sudan [6,7,8].
Material and Methods
Collection of sample and Identification
Acacia oerfota roots were collected from south Omdurman area- Khartoum, Sudan- in February 2021. The species was compared with herbarium of Biology and Technology Department, College of Applied Sciences, University of Bahri-Sudan.
Preparation of Plant material
The root bark part was aired and dried in shadow, then powdered by using locally made hammer mill, and were weighed.
Phytochemical screening of the crude extracts: The extracts of root bark, which showed antimicrobial activity was subjected to qualitative chemical screening for the identification of the various classes of phytochemical using methods described [9,1,10,11].
Preparation of crude extracts: An amount of 47g of the dried plant were weighed then extracted with n-hexane by Shaker apparatus for 24hours at room temperature. The n-hexane extract was filtrated by using filter paper then dried. The crude extract was kept at -20 C° in sterile universal bottle. And then extracted with Chloroform by Shaker apparatus for 24 hours at room temperature. The Chloroform extract was filtrated by using filter paper and then dried. The crude extract was kept at -20 C° in sterile bottle. Then extracted with Menthol by Shaker apparatus for 24hours at room temperature. The Menthol extract was filtrated by using filter paper and then dried. The crude extract was kept al -20 C° in sterile bottle.
General phytochemical screening of Acacia oerfota
Preliminary phytochemical screening examination for the active constituent was carried out for crude plantor various extracts with little modification by using the standard method as described by [10,12,1] to tested the following components.
Detection of tannins: An amount of 0.5g of extract was dissolved in10 ml methanol and divided into two test tubesfor caring the following test.
Ferric chloride test: The extracts (4mL) were boiled for 10 mins in 20mL of water in a test tube. A few drops of 5% ferric chloride were added, observed for 10mins for a brownish green or a blue black coloration.
Detection of saponins: The extracts (10 mL) were shaken and heated to boil. Frothing (appearance of creamy mass of small bubbles) shows the presence of saponins.
Detection of alkaloids: About 4 mL of each of the extracts were stirred with 10 mL of 1% aqueous hydrochloric acid on a steam bath for 10mins. 1mL of the extract was treated with a few drops of Mayer’s reagent. Precipitation with these reagents was seen as evidence for the presence of alkaloids.
Detection of flavonoids: The extracts (5mL) were treated with few drops of sodium hydroxide solution. Formation of intense yellow color, which becomes colorless the moment dilute acid is added, indicates the presence of flavonoids.
Detection of carbohydrate: 2ml of extract was treated with a few drops of α-naphthol and Conc. H2SO4 was added carefully in the side of the test tube the formation of purple color or two layers indicates the presence of carbohydrate.
Detection of Amino acids: 50g of different extracts were dissolved in 5ml methanolfiltered. The filtrate was subjected to the following test. 2ml of methanol filtrate was heated with 0.5ml of Ninhydrin solution in boiling water bath for 10 minutes. Appearance of purple color shows the presence of amino acids.
Antimicrobial activity
Culture Media
Preparation of Nutrient Agar
28g of powdered nutrient agar was weighted, dispersed in 1 liter of distilled water and allowed to soak for 10 minutes, swirl to mix then sterilized by autoclaving for 15 minutes at 121c, cooled to 47c, mixed well then poured into petri dishes.
Tested organisms:
Bacterial organisms:
Bacillus subtitles (Gram positive bacteria).
Staphylococcus aureus (Gram positive bacteria).
Escherichia coli (Gram negative bacteria).
Pseudomonas aeruginosa (Gram negative bacteria).
Fungal organisms:
Candida albicans (Fungi).
Preparation of organism’s suspension
One ml aliquots of 24 hours broth culture of the test organisms were aseptically distributed into nutrient agar slopes and incubated at 37C° for 24 hours. The bacteria growth was harvested and washed off with 100ml of Serial normal saline, to produce a suspension containing about 10^10 C.F.U/ml. the suspension was stored in the refrigerator at 4c till used. The number of viable organisms per ml of the stock suspension was determined by means of the surface viable counting technique [13,14].
Sterile dilution of the stock suspension was made in sterile normal saline solution and 0.02ml volume of the appropriated dilution was transferred by micro pipette into the surface of dried nutrient agar plates. The plates were allowed to stand for two hours at room temperature for drops to dry and then incubated at 37c for 24 hours. After incubation, the number of developed colonies per (0.02ml) was multiplied by 50 and by the dilution factor to give the viable count of the stock suspension, expressed as the number of colony forming unit per ml suspension. Each time afresh stock suspension was prepared, all the above experimental condition were maintained constant so that suspension with very close viable count would be obtained.
In vitro testing of extract for antimicrobial activity:
Testing for antibacterial activity:
The cup-plate agar diffusion method [14] was adopted with some minor modifications to assess the antibacterial activity of the prepared extracts. One ml of the standardized bacterial stock suspension 108-109 C.F.U/ml was thoroughly mixed with 100ml of molten sterile nutrient agar which was maintained at 45 C°. 20ml aliquots of the inoculated nutrient agar were distributed into sterile petri dishes. The agar was left to set and in each of these plates 4 cups (10mm in diameter) was cut using a sterile cork borer (No.5) and agar disk were removed.
Alternate cups were filled with 0.1ml sample of the extract dilution in methanol using automatic microliter pipette, and allowed to diffuse at room temperature for two hours. The plates were then incubated in the upright position at 37 C° for 18 hours. Three replicates were carried out for the extract against each of the test organism. After incubation the diameters of the resultant growth inhibition zones were measured, averaged and mean values were tabulated according to [15,16] methods.
Result and Discussion
Phytochemical properties and screening of Acacia oerfota
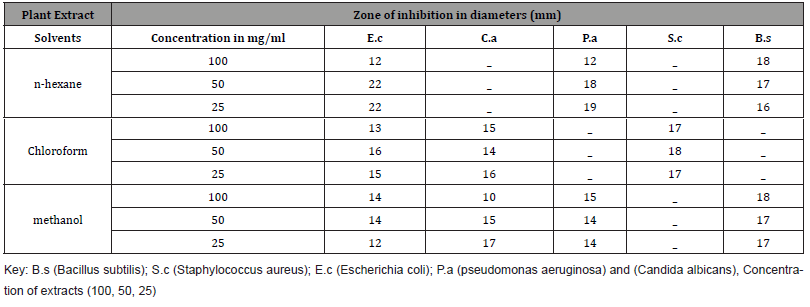
Three solvents were used in successive polarities of Acacia oerfota extract and their properties were showed in the Table (1) the yield values of the species were showed as following, for methanol 3.22% (Brown powder), chloroform 1.24% (Dark brown powder) and n-hexane 0.86% (brown powder). The phytochemical screening result was cited as in Table 2 and the antimicrobial activity was cited as in Table 3.
Table 1:Properties and extractives values of Acacia oerfota root bark extract:
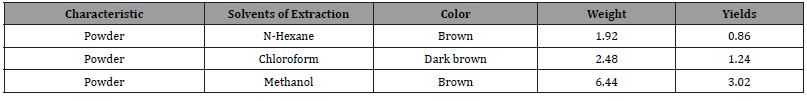
Table 2:Result of phytochemical screening:
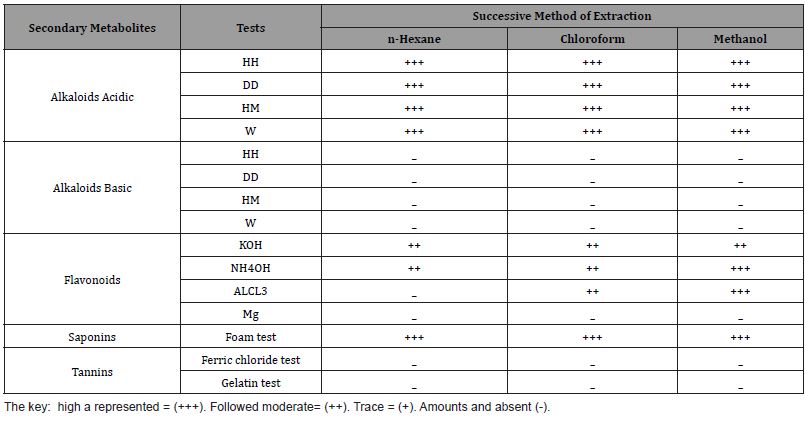
Table 3:Result of Antimicrobial Activities.
Discussion
Extracts properties and yield of root bark of species
Four solvents (n-hexane, chloroform and methanol) were used in to extract secondary metabolites from the plant under investigation. As intable (1), variation was observed in the colors of extracts of different plant species. The colours of variations were reflection of type of solvent, plant species and the part of plant used.
Phytochemical screening
Phytochemical screening of chemical constituents of Acacia oerfota was done and showed contain: high amount of alkaloids in all extract except in general phytochemical, moderate amount of flavonoids (in chloroform, methanol extract), absent of tannins in all extracts, and high amount of saponin. These results disagree with [17]. The result agree with [6,7,5] in test of flavonoid and disagree in other tests. The variation of amount of these secondary metabolites may be due to the nature of the area of collected plant sample.
Antimicrobial activity
The n-hexane extract was more effective and cited a significant inhibition zone against Escherichia coli, pseudomonas aeruginosa Candida and Bacillus subtilis at concentrations 50% and 25% and no inhibition zone against Staphylococcus aureus and Candida albicans. Methanol extract was reported high inhibition zone against Bacillus subtilis at all concentration and no inhibition zone against Staphylococcus aureus. The chloroform extract showed a good inhibition zone against Staphylococcus aureus in all concentration; moderate inhibition zone in all concentration against Escherichia coli and Candida albicans and no inhibition zone against pseudomonas aeruginosa and Bacillus subtilis in all concentration. This result partially agrees with [16] and disagrees with [18].
Conclusion
Acacia oerfota is widely used in Sudanese traditional medicine. The extracts were obtained by extraction of root bark by different solvents and it has shown a good effect on bacteria and fungi. Phytochemical screening was carried out and lead to presence of some secondary metabolites. The root bark result was containing alkaloids, flavonoids, tannins, triterpenes and saponins. The crude extracts were subjected to antibacterial assays using the cup diffusion method and the inhibition zones measured in mm. The root bark extract gave good results ranged between strong to moderate activities against all bacterial and fungal organisms. Overall, Acacia erfota will continues to play important role in herbal medicine, research involving Acacia oerfota root bark and its uses could be expanded greatly in future, leading to new treatment and cure of microbial disease. These results confirm the presence of antimicrobial activity secondary metabolize compounds in Acacia oerfota which gives an opportunity to explore the possible usage of Acacia oerfota parts in the development of antibiotics and insecticides.
Acknowledgement
None.
Conflict of Interest
No Conflict of interest.
References
- Sofowora AE (1993).Medicinal plant and traditional Medicines in Africa...2nd edition. Spectrum Books, Ibadan, Nigeria’s. 289.
- IWU MM, Duncan AR, Okunji CO (1999).New Antimicrobials of plant origin. Injanick, J. (Ed) perspectives in new crops and New Uses. ASHS press Alexandria, V.A. pp 457-462.
- Karunamoorthi K, Ramanujam S, Rathinasamy R. Evaluation of leaf extracts of vitexnegundoL. (Family: Verbenaceae) against Larvae of Culex Tritaeniorhynchus and repellent activity on adult Vector mosquitoes. Parasitol Res. 2012.
- WHO (1977).Assembly, 30 promotion and development of training and research in traditional medicine, World Health Organization. https://apps.who.int/iris/handle/10665/93212
- FAO, 2017.Grassland index. A searchable catalogue of grass and forage legumes. FAO, Rome, ltaly. https://web.archive.org/web/20170120044942
- El Kamali, H.M., Khalid, S.A. the most common herbal remedies in Central Sudan. Fitoterapial 1996.57, 301_306.
- El Ghazali, G.E.B., El Tohami, M.S. and El Egami, A.A.B. (1997). Medicinal plants of Sudan, Part III, Medicinal plants of the eastern Nuba Mountains. Khartoum University Press, Khartoum.
- El – Kamali H H, EI – Khalifa (1999). KK Folk medicinal plants of riverside forests of the Southern Blue Nile district, Sudan. Fitoterapia70: 493-497.
- Martinez A, Valencia G: Marchafitoquimica. (2003). In Manual de prácticas de Pharmacognosy y Fitoquímica: 1999. 1st edition. Medellin: Universidad de Antioquia; Phytochemical screening methods, 2003; 59-65.
- Harborne, J.B., (1984).Phytochemical methods book: A guide to modern technique of plant analysis. Chapman and hall press 2ed End. London, pp: 48-188.
- Khandelwal KR (2008) , practical Pharmacognosy-Techniques and Experiments, 19thed ,NiraliParkashan, pune.
- Farnsworth, N. R. (1983).Natural product and Drug development krogsaard-Larsenandp, Brggerchristensen, S. (ed.), 23.Germplasm Resources Information Network (GRIN). Agricultural Research Service (ARS), United States Department of Agriculture (USDA). Retrieved 19 January 2018.
- Miles, A.A.; Misra, S.S. (1938).The estimation of the bactericidal power of the blood. Journal of Hygiene 38:732.
- Yasmin Hassan Elshiekh* and Mahdi Abdelmageed Mohammed Ali,(2020).PHYTOCHEMICAL AND ANTIBACTERIAL ACTIVITY OF PUNICA GRANATUM (L.)BARK AND PEEL EXTRACTS, International Journal of Modern Pharmaceutical Research, ISSN: 2319-5878.
- Mahdi Abdelmageed Mohammed Ali and Yasmin Hassan Elshiekh,. A study on the chemical constituents, antibacterial and antioxidant activities of Vangueriamadgascariensis (Fruit), The Pharma Innovation Journal 2020; 9(7): 123-126.
- Kavanagh, F. (1972).Analytical microbiology, F. Kavanagh (Ed) vol 11, Academic press, New York & London, pp. 11.
- Cowan MM. (1999) Plant product as antimicrobial agents. S Clinical microbiology review; 12(4):564-582.
- Pineiro S, Chauhan (2013) A, Berhane T-K, Athar R, Zheng G, Wang C et al. Niche partition of bacterio vorax operational taxonomic units along salinity and temporal gradients in the Chesapeake bay reveals distinct estuarine strains. MicrobEcol 65: 652–660.
-
Mahdi Abdelmageed Mohammed Ali, RahmaAbubakr Musa, YagoubAbdella Ali, Ferial, Mohamed Abu Al bashar Amin. Phytochemical Investigation and Antimicrobial Activity of Root Bark Extracts of Acacia Oerfota. Insi in Chem & Biochem. 2(1): 2022. ICBC. MS.ID.000530.
-
Phytochemical, Antimicrobial, Acacia Oerfota, Escherichia coli, pseudomonas aeruginosa, chloride, flavonoids, carbohydrate, Amino acids.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






