 Short Communication
Short Communication
Photophysical and Hydrodynamic Properties of Fluorescent Dye Alexa Fluor (AF647) in Reverse Micelle Environment
Daniel Kim1,2, Hannah Spoon1 , and Rajesh K. Nayak1*
1Department of Chemistry, Physics, and Engineering, Cameron University, USA
2Department of Agriculture, Biology, and Health Sciences, Cameron University, USA
Rajesh K. Nayak, Department of Chemistry, Physics, and Engineering, Cameron University, 2800 W Gore Blvd, Lawton, OK, 73505 USA.
Received Date: July 03,2023; Published Date: July 13,2023
Abstract
The photophysical and hydrodynamic properties of the fluorescent probe Alexa Fluor (AF647) were examined by using steady-state UVVis absorption and steady-state fluorescence emission and excitation spectroscopic techniques. The anionic reverse micelles (RMs), sodium di- 2-ethylhexyl sulfosuccinate (AOT) of varying sizes were employed for these investigations and, the results obtained were compared with those in aqueous environment. Our findings suggest that the dye molecules behave very differently in smaller reverse micelles, probably due to their location in interfacial region of RMs. Our findings further suggest that the RMs can be used as a simple model system to probe biomolecule dynamics.
Keywords: Reverse micelles; Confined environment; RM; AOT
Introduction
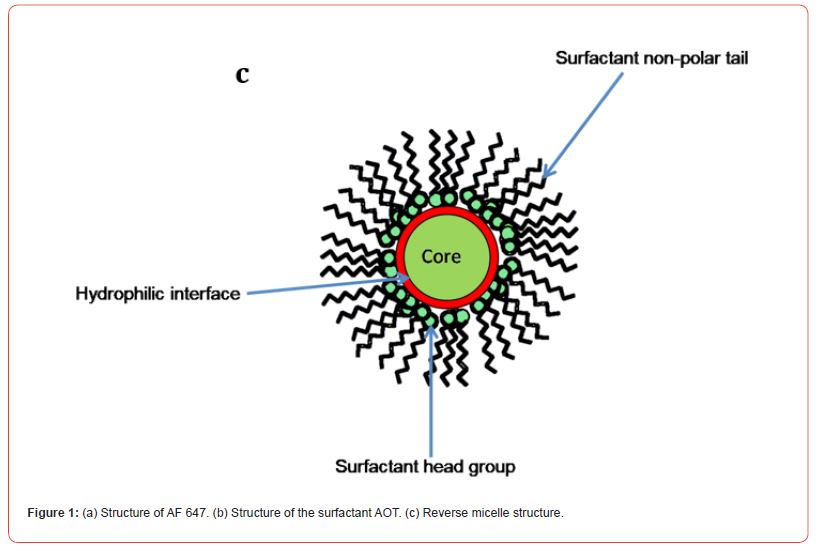
Reverse micelles (RMs) are well known as water- in- oil microemulsions surrounded by surfactant monolayer, described as nanopools of water droplets uniformly dispersed in organic solvents where hydrophilic head groups directed towards polar solvents, often water and hydrophobic tails directed towards nonpolar solvents1,2,3,4 (Figure 1 c). Variety of cationic, anionic and neutral surfactants can form RMs in nonpolar organic solvents but among them Aerosol-OT (AOT) is widely used surfactant5. Aerosol-OT (sodium bis(2-ethylhexyl) sulfosuccinate, AOT) is composed of a negatively charged “head” group and two nonpolar hydrocarbon “tails” (Figure 1 b). The AOT RM is readily producible over wide range of sizes because of its great water solubilisation capacity6,7 and the size of AOT RMs is generally characterized by the parameter w0, which is the molar ratio of water to surfactant.
In last few decades, there have been numerous studies on photophysical properties of various fluorescent dye molecules8-18 and so many questions are answered. But, many questions on photophysics and location of probe molecules are yet to be answered. AF 647 is an excellent fluorescent dye and due to its exceptional photo stability and pH insensitiveness, it has been extensively used in super resolution microscopy and cell bioimaging19. In this work, we present an investigation on photophysical and hydrodynamic properties of fluorescent dye AF 647 in AOT reverse micelles of varying sizes by using steady state absorption and fluorescence spectroscopy. Our findings suggest that AF 647 behave very differently inside confined reverse micelle of smaller sizes as compared to its behaviour in aqueous environment in contrast to the bigger RMs where it exhibits same behaviour as aqueous environment.
Materials and Methods
Isooctane, nanopure water (Invitrogen), AOT (Alfa Aesar), AF647, Fischer Scientific) were purchased and were used without further purification. AOT RMs of varying sizes used for the experiments were prepared by using by the lab designed protocols. UV-Vis absorption spectra were taken by a Shimadzu UV-1800 UV Spectrophotometer with quartz cuvettes of a 1.0cm optical path length. Fluorescence emission and excitation spectra were taken by a Shimadzu RF-6000 spectrofluorometer with a quartz fluorescence cuvette of a 1.0cm path length.
Results and Discussion
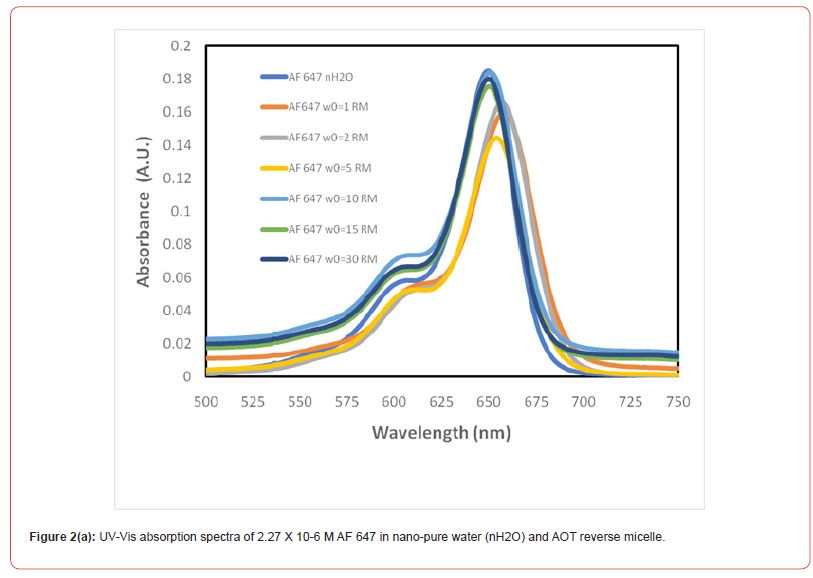
Figure 2(a) shows the steady-state UV-Vis spectra of AF 647 dyes in aqueous solution and in reverse micelles of varying sizes. The result shows that AF647 in aqueous solution exhibits absorption peak, 𝛌max at 650 nm. Once the AF 647 is introduced in reverse micelle of smallest size at w0 =1, the 𝛌max has been significantly red shifted and the dye shows absorption peak at 659 nm. As we go from w0 from 1 to 2 and 5, the red shifting is still significant. For example, at w0 =2, AF 647 shows absorption peak at 657 nm and at w0 =5, the absorption peak is exhibited at 656 nm. But at w0 =10 , 15 and 30, AF 647 absorbs at 650 nm, which means the dye in bigger size of RMs behave very similar to that of aqueous environment. Most importantly, even at moderately bigger size of the RM, i.e. at w0 =10, the dye exhibits similar phtophysical behaviour like the way it behaves in free water.
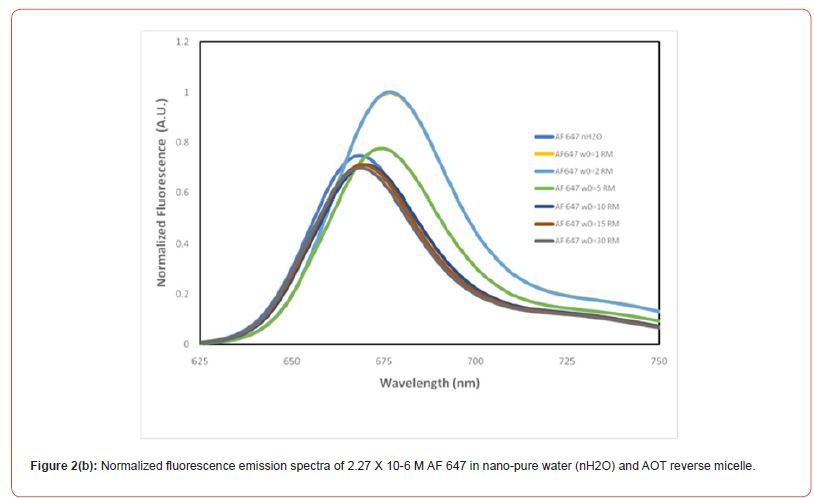
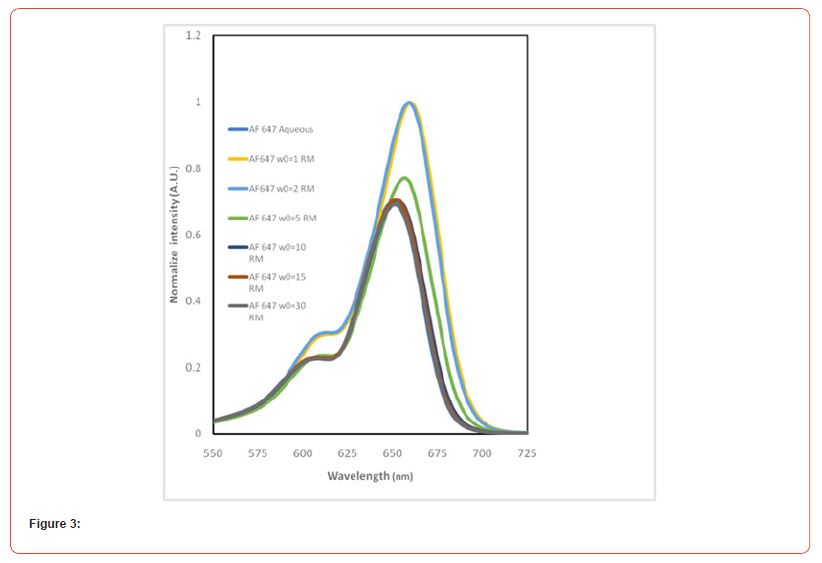
Figure 2(b) and 2(c) show the normalized fluorescence emission and excitation spectra of AF 647 in aqueous environment as well as in RMs of varying sizes. The steady-state emission data shows that the dye exhibits emission maxima at 668 nm but at w0 =1, the emission maxima has been red shifted by 9 nm to 677 nm. The significant red shifting trend continues until it reaches at w0 =10, where the emission maxima are same as water and thereafter at bigger RM size, the emission maxima remain same. Similarly our excitation spectral data supports our findings in UV-Vis spectral data. The photophysical parameters in our findings suggest that AF 647 behaves very differently in smaller and smallest reverse micelles and it behaves very similarly as that in aqueous environment in moderately bigger and the biggest RM sizes. Furthermore, the larger water pool in bigger RMs behaves like bulk water, and therefore the photophysics remains similar to that of the dyes in bulk water. Also, our results further suggest that the confined water dynamics dramatically changes around w0 =106,20. Another interesting fact is that the dye when encapsulated in smaller RMs probably resides in interfacial region in contrast to the location at bigger RMs, where it resides majorly in the core region Figure 3.
Conclusion
In summary, we studied photophysical and hydrodynamic properties of AF647 by using steady-state UV-Vis and steady-state Fluorescence spectroscopic techniques. Our findings suggest that the dye molecules behave very differently in RMs of smaller sizes, probably due to their location in interfacial region of RMs. On the other hand, the dyes in bigger RMs show similar photophysical behaviour to that of dye in aqueous environment. Additionally, our observation gives us a protocol to use reverse micelle as a model system to study complex biomolecule dynamics as it can mimic the cellular environment.
Acknowledgments
The authors would like to thank Cameron University’s Todd and Cindy Sanner endowed lectureship for funding.
Conflict of Interest
No Conflict of interest
References
- M Massimo, S Albel (2015) J Phys Chem Lett 6: 170-174.
- T K De, A Maitra (1995) Adv. Colloid Interface Sci. 59: 95-193.
- S A Tovstun, V F Razumov (2015) Colloid.Polym.Sci. 293: 165-176.
- A Rahdar, M Almasi-Kashi (2017) J. Mol. Struct. 1128: 257-262.
- N E Levinger, L A Swafford (2009) Annu. Rev. Phys. Chem. 60: 385-406.
- Zhu Ruixue, Lu Rong, Yu Anchi (2011) Chem. Phys. 13: 20844-20854.
- M Novaria, M A Biasutti, J J Silber, N M Correa (2007) J. Phys. Chem. 111: 748-749.
- Ferreira B, A Jose (2010) Costa. M. Silvia, Phys. Chem. B. 114: 10417-10426.
- N Nandi, K Bhattacharya, B Bagchi (2000) Chem. Rev. 100: 2013-2045.
- J Faeder, B M Ladanyi (2000) J. Phys. Chem. B. 104: 10603-10613.
- K Bhattacharya (2003) Acc. Chem. Res. 36: 95-101.
- Bozkurt Ebru, Onganer Yavuz (2018) J. Mol. 1173: 490-497.
- A M Dokter, S Woutersen, H J Bakker (2006) Inhomogeneous dynamics in confined water nanodroplets. Proc. Natl. Acad. Sci. U. S. A. 103(42): 15355-15358.
- Bayraktutan Tugba, Meral Kadem, Onganer Yavuz (2014) Photophysical properties of pyronin dyes in reverse micelles of AOT. J Lumin145: 925-929.
- Laia TA Cesar, Costa B A Silvia (1998) Fluorescence quenching of a squaraine dye by water in AOT reversed micelles. J. Com. Soc. , Faraday 94: 2367-2373.
- Bapali Alok, Seth Debabrata (2020) The photophysics of a hydrophilic molecule in the presence of graphene oxide. J Lumin. 217: 116816-116827.
- N E Levinger (2002) Water in confinement. Science. 298(5599): 1722-1723.
- B Bagchi (2005) Chem. Rev. 105: 3197-3219.
- Sanjun Fan, James EA Webb, Ying Yang, Daniel J Nieves, Vinicius R Goncales, et al, (2019) Observing the Reversible Single Molecule Electrochemistry of Alexa Fluor 647 Dyes by Total Internal Reflection Fluorescence Microscopy. Chem.In. 131: 14637-14640.
- A K Satpati, M Kumbhakar, S Nath, H Pal (2009) Chem-PhysChem, 10: 2966-2978.
-
Daniel Kim, Hannah Spoon and Rajesh K. Nayak*. Photophysical and Hydrodynamic Properties of Fluorescent Dye Alexa Fluor (AF647) in Reverse Micelle Environment. Insi in Chem & Biochem. 2(5): 2023. ICBC. MS.ID.000548.
-
Reverse micelles, Confined environment, RM, AOT
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






