 Short Communication
Short Communication
Natural Disinfectant Production Stem Kit
Lütfiye Aydın, Kayseri Science Center, Turkey.
Received Date: December 27, 2022; Published Date: January 18, 2023
Abstract
HOCl, which is produced naturally by the defense system, can also be produced artificially and used for cleaning and disinfection purposes. Electrolyzed water (EW), commonly known as hypochlorous acid (HOCl), is a safe and environmentally friendly disinfectant that may abide by food safety requirements. HOCl decomposes in aqueous solution as H+ - OClˉ and denatures proteins. HOCl makes viruses harmless by creating chloramines and nitrogenous radicals by breaking down double-stranded DNA. In the work, A simple electrolysis training kit was created. Hypochlorous acid (HOCl) was electrolyzed in salt- aqueous solution. The device has the capacity to produce concentrations ranging from 5 ppm (1 ppm = 1 mg/L) to 30 ppm. This STEM app using electrolysis technology turns tap water into a safe disinfectant in just four minutes.
Keywords: Hypochlorous acid; Natural Disinfectant; STEM; Science Center
Introduction
Hypochlorous acid (HOCl) is a comprehensive disinfectant that is sustainable and environment-friendly; therefore, it is considered a practical alternative [1]. It is non-toxic, non-irritant and non-corrosive at proper usage concentrations and relatively inexpensive. A potent oxidizing agent is hypochlorous acid (HOCl) [2]. It separates into H+ and OCl1 in aqueous solution, denaturing and aggregating proteins [3]. Viruses can also be eliminated by HOCl by chlorination, which produces chloramines and nitrogen-centered radicals that break both single- and double-stranded DNA, rendering the nucleic acid worthless and the virus harmless. The purpose of this study is to show that it is possible that the hypochlorous acid can be produced with simple substances found at home in everyday life.
Material and Method
How is HOCl produced? On-site production of HOCl is possible via electrolysis, non-iodinated salt, and water. Anode and cathode reactions1,3 are give in scheme 1- 54.
Anode Reactions
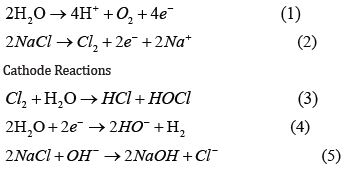
Natural Disinfectant Production Kit is given in figure 1. This Natural Disinfectant Production kit includes two 2B pen tips, a plastic bottle, and a 5V adapter. The system to make HOCl on site is a one-liter container which is filled with water. 2B pen tips were used as electrodes (Figure 1).

A gram of non-iodized salt is added to the water. The system has the ability to make 5 ppm to 30 ppm concentrations depending on which electrolysis time. The electrolyzed solution is completed in 3-5 minutes, when it is ready for use.
Discussion and Conclusion
The amount of existing chlorine obtained from the photometric measurement results with this set is 5-30 ppm. This value indicates that commercially available hypochlorous is suitable for use in hand sanitizer and household disinfectant. It is possible to produce your own disinfectant at home with a simple and inexpensive method.
Acknowledgement
None.
Conflict of Interest
No Conflict of interest.
References
- Yaqub M, Woo C, Lee W (2021) Optimization of hypochlorous acid generation by HCl electrolysis through response surface methodology and artificial neural networks. Journal of Environmental Chemical Engineering 9(5): 105826.
- Pub Chem (t.y.) Hypochlorous acid. Geliş tarihi 24 Aralık 2022, gönderen.
- Block MS, Rowan BG (2020) Hypochlorous Acid: A Review. Journal of Oral and Maxillofacial Surgery 78(9): 1461-1466.
- Huang YR, Hung YC, Hsu SY, Huang YW, Hwang DF (2008) Application of electrolyzed water in the food industry. Food Control 19(4): 329-345.
-
Lütfiye Aydın*. Natural Disinfectant Production Stem Kit. Insi in Chem & Biochem. 2(3): 2023. ICBC. MS.ID.000539.
-
General Chemistry& Chemical technology, Quaternary ammonium salt; rearrangement-cleavage reaction; ylid; alkylation-elimination reaction; chalcone; thermal; alkylation.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






