 Mini Review
Mini Review
Isosorbide as a Platform for the Generation of New Biobased Organophosphorus Flame Retardants
Bob A Howell* and Yoseph G Daniel
Department of Chemistry and Biochemistry, Central Michigan University, USA
Bob A Howell, Science of Advanced Materials, Center for Applications in Polymer Science, Department of Chemistry and Biochemistry, Central Michigan University, Mt. Pleasant, MI 48859-0001, USA.
Received Date: March 19, 2020; Published Date: April 14, 2020
Abstract
Isosorbide is a bicyclic diether diol readily available from starch derived from a number of cereal crops. Its rigid structure and difunctionality make it an attractive material for conversion to a variety of polymer additives. Principal among these are flame retardants. These may be generated by either direct or indirect means and display good flame retardancy in a variety of polymeric materials.
Introduction
Starch is available, in abundance, annually from a variety of seed grains [1,2]. Starch may be hydrolyzed to provide glucose which, in turn, can be reduced and dehydrated to afford isosorbide, a reactive diol [3,4]. Isosorbide may serve as a base for the preparation of both polymers and polymer additives, particularly plasticizers and flame retardants [5,6]. It may also be used for the generation of therapeutic agents and surfactants. The preparation of biobased phosphorus flame retardants has become increasingly important as threats posed to the environment and human health from the use of traditional organohalogen flame retardants has become widely recognized [7-18].
Results and Discussion
Isosorbide is a diether diol of cupped structure with hydroxyl groups of different reactivity, one endo to the skeletal structure and the other exo. However, both are sufficiently reactive that bis adducts may be formed readily. This has been exploited for the generation of several classes of biobased flame retardants. Isosorbide may be directly converted to phosphorus esters by treatment with an appropriate phosphoryl chloride or with a phosphite in the presence of carbon tetrachloride (Atherton-Todd procedure) [19]. These esters display outstanding flame retarding characteristics when incorporated, at relatively low levels, into DGEBA epoxy resin [20, 21]. Alternatively, isosorbide may be converted to the bis-acrylate. Michael addition of phosphite affords flame-retarding compounds with the phosphorus moiety attached via a P-C bond. These compounds display superior thermal stability and are suitable for use with polymers that process at high temperatures. Oligomeric flame retardants may also be generated from isosorbide [22]. More novel flame retardants may be produced by first treating isosorbide with 10-undecenoic acid, a biomaterial from castor oil, to generate a bis-ester containing terminal unsaturation. This compound smoothly undergoes thiol-ene reaction with 2-hydroxyethanethiol to provide a derivative with terminal hydroxyl groups. Esterification with multiple phosphorus agents generates a series of compounds with good flame-retarding properties [23,24]. These compounds contain sulfur as sulfide. This may readily be oxidized to sulfoxide or sulfone to permit an evaluation of the oxidation level of sulfur on flame retardancy [25].
Reactive vinyl monomers containing phosphorus may also be generated from isosorbide. Treatment of isosorbide with diphenylchlorophosphate may be used to generate a mixture of isomeric diphenylphosphato alcohols. These alcohols may be separated using column chromatography and treated with acryloyl chloride to provide the corresponding diphenylphosphato acrylates [26]. These acrylates behave as typical vinyl monomers and may be incorporated into vinyl polymers to provide flame retardance. Since these materials are covalently bound into the polymer the possibility of loss due to volatilization or leaching is precluded. The level of comonomer required is low enough that the properties of the copolymer are not markedly different from those of the polymer containing no comonomer. Copolymers of styrene containing exo-5-(diphenylphosphato)isosorbide-5-endo-acrylate at a level to provide materials containing 1 and 2% phosphorus have been prepared (Figure 1).
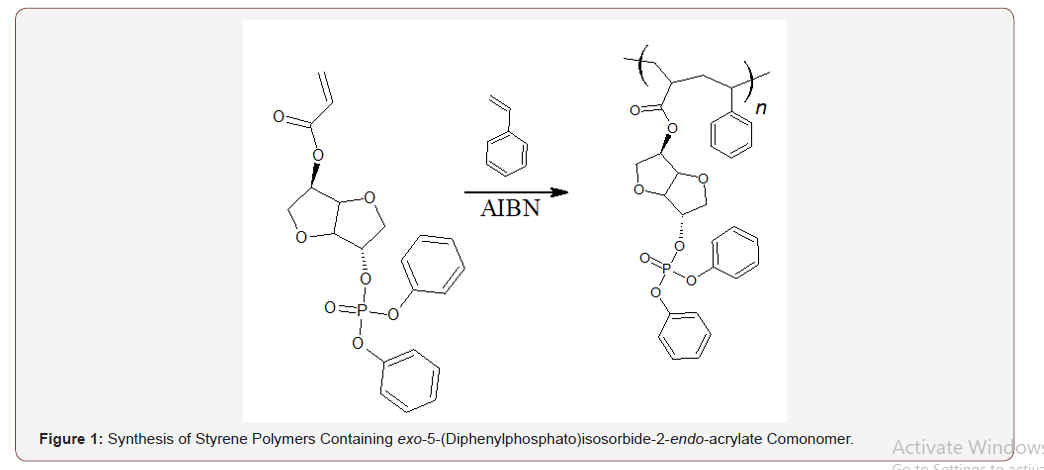
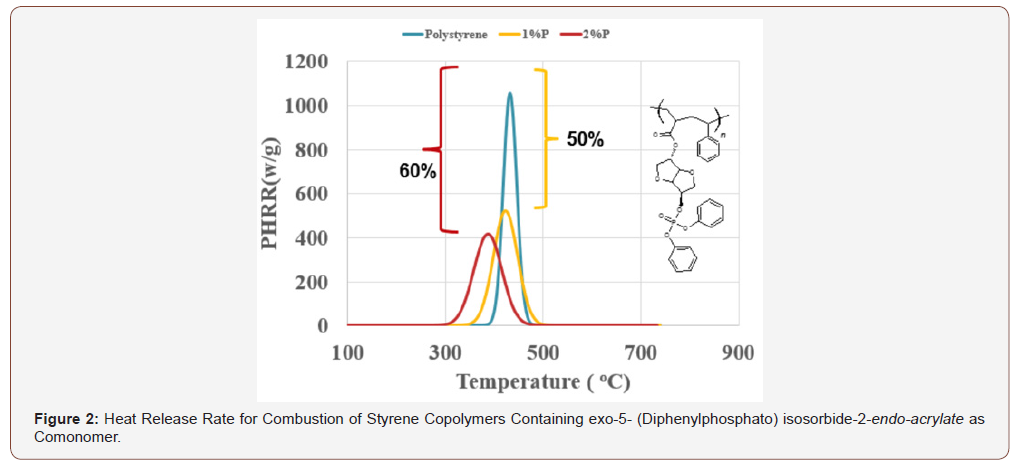
The presence of the comonomer at this level dramatically reduces the peak heat release rate (PHRR) for combustion of the polymer (Figure 2). The presence of 1% phosphorus brings about a 50% reduction in PHRR compared to that for poly(styrene) containing no comonomer.
Conclusion
Isosorbide represents an excellent platform for the generation of biobased phosphorus flame retardants of both the additive and reactive type. Numerous bis-phosphorus derivatives display excellent flame retardant properties as polymer additives. Alternatively, isosorbide may be converted to phosphatoacrylates which function well as reactive flame retardants for incorporation into vinyl polymers.
Acknowledgement
Support for this work from Chemtura Corporation (now Lanxess) is gratefully acknowledged. Diphenylchlorophosphate was provided by ICL-IP America; the diglycidal ether of bisphenol A (DGEBA) by the Dow Chemical Company.
Conflict of Interest
Authors declare that there are no conflicts of interest.
References
- LH Princen (1982) Alternative Industrial Feedstocks from Agriculture. Economic Botany 36: 302-331.
- D Miller (2018) The Bioharvest is Here. The Progressive Farmer: 22-25.
- G Fleche, H Huchette (1986) Isosorbide. Preparation, Properties and Chemistry. Starch Staerke 38: 26-30.
- D Yuan, L Li, F Li, Y Wang, N Zhao, et al. (2019) Solvent-free Production of Isosorbide from Sorbitol Catalyzed by a Polymeric Acid. Chem Sus Chem 12: 4986-4995.
- Arico (2020) Isosorbide as a Biobased Platform Chemical: Recent Advances. Current Opinion in Green and Sustainable Chemistry 21: 82-88.
- Rose, R. Palkovits (2020) Isosorbide as a Renewable Platform Chemical for Versatile Applications –Quo Vadis? Chem Sus Chem 5: 167-176.
- BA Howell, GW Lienhart, VJ Livingstone, D Aulakh (2020) 1-Dopyl-1,2-(4-hydroxyphenyl)ethene: A Flame Retardant Hardner for Epoxy Resin. Polym Degrad Stab 175: 109110.
- R Sonnier, A Taguet, L Ferry, JM Lopez-Cuesta (2018) Towards Biobased Flame Retardant Polymers, Springer International Publishing AG, Cham, Switzerland.
- J Sag, D Goeddere, P Kubla, L Griener, F Schonberger, M Doring (2019) Phosphorus-containing Flame Retardants from Biobased Chemicals and their Application in Polyesters and Epoxy Resins. Molecules 24: 3746.
- L Costas, F Laoutid, S. Brohez, P Dubois (2017) Biobased Flame Retardants: When Nature Meets Fire Protection. Mater Sci Eng R 1-25.
- BA Howell, YG Daniel, EA Ostrander (2018) Flame Retardants from Renewable Sources: Food Waste, Plant Oils and Starch”. In HN Cheng, PB Smith, (Eds.), Green Polymer Chemistry: New Products, Processes and Applications (ACS Symposium Series 1920), American Chemical Society, Washington, DC, USA, 25: pp. 405-421.
- BA Howell, W Sun (2018) Biobased Flame Retardants from Tartaric Acid and Derivatives. Polym Degrad Stab 157: 199-211.
- BA Howell, KL Oberdorfer, EA Ostrander (2018) Phosphorus Flame Retardants for Polymeric Materials from Gallic Acid and Other Naturally Occurring Multihydroxybenzoic Acids. Int J Polym Sci.
- BA Howell, A Alrubayyi (2019) 2-Dopyl-1,4-di(3-dopylpropanoyl)benzene, an Effective Phosphorus Flame Retardant. Polym Degrad Stab 162: 196-200.
- BA Howell, EA Ostrander (2019) Flame-retardant Compounds for Polymeric Materials from an Abundantly Available, Renewable Biosource, Castor Oil. Fire and Materials.
- A Alace, RJ Wenning (2002) The Significance of Brominated Flame Retardants in the Environment: Current Understanding, Issues and Challenges, Chemosphere 5: 579-582.
- A Sjodin, DG Patterson, A Bergman (2003) A Review of Human Exposure to Brominated Flame Retardants – Particularly Polybrominated Diphenyl Ethers. Environ Int 29: 829-839.
- SO Shaw, A Blum, R Weber, K Kannan, D Rich, et al. (2010) Halogenated Flame Retardants: Do the Fire Safety Benefits Justify the Risks. Rev Environ Health 25: 261-305.
- YG Daniel, BA Howell (2017) Synthesis and Characterization of Isosorbide bis-Phosphorus Esters. Heteroat Chem 28: e21369.
- YG Daniel, BA Howell (2017) Flame Retardant Properties of Isosorbide bis-Phosphorus Esters. Polym Degrad Stab 140: 25-31.
- YG Daniel, BA Howell (2018) Phosphorus Flame Retardants from Isosorbide bis-Acrylate. Polym Degrad Stab 156: 14-21.
- C Hu, S Bourbigot, T Delaunay, M Collinet, S Marcill, et al. (2019) Synthesis of Isosorbide Based Flame Retardants: Application for Polybutylene Succinate. Polym Degrad Stab 164: 9-17.
- BA Howell, YG Daniel (2015) Phosphorus Flame Retardants from Esters of Isosorbide and !0-Undecenoic Acid” in H.N. Cheng, R.A. Gross and P.B. Smith, Eds., Green Polymer Chemistry: Biobased Materials and Biocatalysis (ACS Symposium Series 1192), American Chemical Society, Washington, D.C., Ch.21, pp. 339-367.
- BA Howell, YG Daniel (2015) Thermal Degradation of Phosphorus Esters Derived from Isosorbide and 10-Undecenoic Acid,” J. Therm. Anal. Calorim. 121: 411-419.
- BA Howell, YG Daniel (2018) The Impact of Sulfur Oxidation Level on Flame Retardancy. J Fire Sci 36: 518-534.
- BA Howell, YG Daniel (2020) Synthesis and Characterization of Isomeric Diphenylphosphatoisosorbide Acrylates Phosphorus, Sulfur, and Silicon and the Related Elements.
- BA Howell, YG Daniel (2019) Incorporation of Comonomer exo-5-(Diphenylphosphato)isosorbide-2-endo-acrylate to Generate Flame Retardant Poly(styrene). Polymers 11: 2038.
-
Bob A Howell, Yoseph G D. Isosorbide as a Platform for the Generation of New Biobased Organophosphorus Flame Retardants. Insi in Chem & Biochem. 1(1): 2020. ICBC. MS.ID.000509.
-
Isosorbide, Organophosphorus, Flame Retardants, Chemistry and Biochemistry, bis-phosphorus derivatives, isosorbide, Polymer additives.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






