 Research Article
Research Article
Directed Collective Breakage of Hydrogen Bond in Ice
Luphi Gao, Victoria Jacob, Chaselynn Jiannotti, Katerina Evangelou and Hai-Feng Ji*
Department of Chemistry, Drexel University, USA
Hai-Feng Ji, Department of Chemistry, Drexel University, Philadelphia, PA 19104, USA.
Received Date: September 17, 2021; Published Date: October 01, 2021
Introduction
We present an observation of directed collective breaking of hydrogen bonds from ice crystals when a force is applied on an ice disk. Most of the ice disks broke into 6 pieces at the point of contact with a 60° angle, indicating the directed collective breaking of the ice crystals. Since the formula of water, H2O, was discovered two centuries ago by Amedeo Avogadro, researchers have remained interested in the properties of water—one of the top questions driving life science in the 20th century. Some fundamental questions are still not fully answered, such as, “How many bonds does each H2O molecule make with its nearest neighbors?”, which was listed as one of the top 100 questions spanning the sciences in 2005.
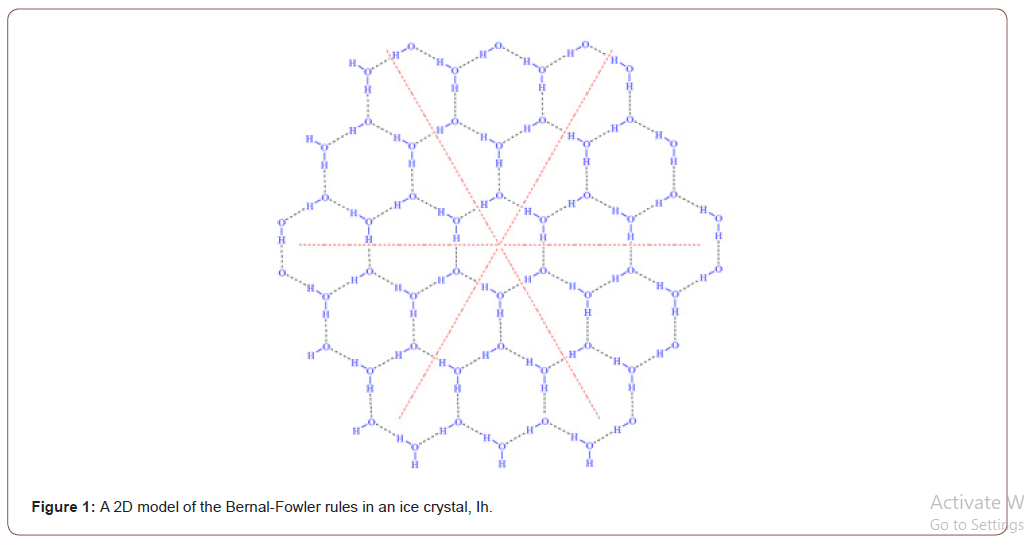
Compared to water, the hydrogen bonding in its ordered solid crystalline phase, ice, is well studied. All ice in the biosphere possesses a hexagonal crystal structure, ice 1h (known as ice one h or ice-phase-one), due to the hydrogen bonding (Figure 1). The structure of ice Ih can be considered as crinkled sheets composed of hexagonal rings with one oxygen atom on each vertex of each ring. Each water molecule makes a maximum of four hydrogen bonds with neighboring molecules in the sheet and between sheets with near tetrahedral bonding angles. The hydrogen bonds are on the edges of the hexagonal rings. The hydrogen-bonded hexagonal rings are the mechanism that causes symmetrical and hexagonal structures in snowflakes (Figure 1).
This crystal structure of crystal ice Ih has been known for almost a century since it was first proposed by Linus Pauling in 1935. One of the remaining fundamental chemistry questions about the crystal structure of ice is whether, under a certain condition, the hydrogen bonds break collectively in a controlled manner, i.e., the breaking of a series of H-bonds along one direction, such as along the dash lines shown in Figure 1. If the hydrogen bonds break collectively along the six directions from the center, it is expected that the ice will break into six pieces with each one having a 60o angle from the center. From a mechanical engineering point of view, the ice should have an anisotropic property starting from any point of the ice. This mechanical property of ice has not been studied. In this short report, we demonstrate that a thin piece of ice does break upon a shock/hit on the ice at the point of contact. The ice breaks into six pieces with the expected angle. This might be the first example of direct observation of the collective breaking of hydrogen bonds along the expected direction in a controlled manner.
In our experiments, 150 mL of water was added to a 251 mL Styrofoam cup. Next, the cup was placed inside the freezer at -6 °C. The ice disk with a diameter of 6.5 cm formed after 2 hours, 3 hours, 4 hours, and 5 hours are roughly 2 mm, 3 mm, 4 mm, and 5 mm, in thickness, respectively. The cup was taken out of the freezer once the desired thickness had formed. The ice disk was then ready for testing. The square ice disk was prepared under a similar condition. The impact force test of ice disks was conducted using a 17 g round steel ball initially released from rest. All experiments were done in pentaplicate. The average impact force at the surface of the ice disk is defined as F = mgh / d, where m is the mass of the falling ball, g is the acceleration of gravity, h is the initial height of the falling ball, and d is the distance traveled after impact.
As shown in Figure 2A, when a round-shape ball hits the ice, the ice cracks into Six pieces when the shock energy at impact is greater than the energy required to break the hydrogen bonds holding the H2O molecules together. The angles were measured to be 60°. When the cracks extended further, the lines were not straight in most samples (Figure 2A and B), which might be due to the slight hydrogen disorder in the ice (Figure 2).
The results are similar when square ice flakes were used. When the ball hit the ice too hard, the ice was smashed into multiple small pieces. When the energy was insufficient, less than six pieces were observed (Figure 3), but the 60° or 2 × 60° angles were still observed.



Conclusion
Anisotropy, in materials science, means the physical properties of a material are directionally dependent. Nearly all single crystals, including ice, are anisotropic regarding mechanical properties. The anisotropic cracking of single-crystal ice is of interest itself in understanding the physical properties of ice. The potential applications of this property of ice is worthy of investigation, specifically in the studies of propagation of seismic waves, the flow behavior of ice, viscosity of the ice, etc.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- D Kennedy, C Norman (2005) “What don’t we know?” Science 309: 78.
- VF Petrenko (1993) Structure of Ordinary Ice Ih. Part 1: Ideal Structure of Ice Paperback. Publisher: PN.
- Pauling (1935) The Structure and Entropy of Ice and of Other Crystals with Some Randomness of Atomic Arrangement. J Amer Chem Soc 57: 2680-2684.
- JD Bernal, RH Fowler (1933) A Theory of Water and Ionic Solution, with Particular Reference to Hydrogen and Hydroxyl Ions. J Chem Phys 1: 515.
- Diez O Eisen (2015) Seismic wave propagation in anisotropic ice – Part 1: Elasticity tensor and derived quantities from ice-core properties.” The Cryosphere 9: 367-384.
- P Pimienta, P Duval (1987) The Physical Basis of Ice Sheet Modelling (Proceedings of the Vancouver Symposium, August). IAHS Publ 170.
-
Luphi Gao, Victoria Jacob, Chaselynn Jiannotti, Katerina Evangelou, Hai-Feng Ji. Directed Collective Breakage of Hydrogen Bond in Ice. Insi in Chem & Biochem. 1(5): 2021. ICBC. MS.ID.000525.
-
Collective Breakage, Hydrogen Bond, Ice, Chemistry, Solid crystalline phase, Water molecule.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






