 Review Article
Review Article
Diagnosis of Infectious Diseases: Tuberculosis, Malaria and HIV and Aids – A Review
Nikiwe Mhlanga1,2* and Sinazo Cobongela1,2,3
1DSI/Mintek Nanotechnology Innovation Centre, Randburg, South Africa
2Advanced Materials Division, Mintek, Randburg, South Africa
3School of Chemistry, University of the Witwatersrand, Johannesburg, South Africa
Nikiwe Mhlanga, DSI/Mintek Nanotechnology Innovation Centre, Advanced Materials Division, Mintek, Randburg, South Africa.
Received Date: February 05, 2021; Published Date: February 23, 2021
Abstract
Achieving and sustaining global healthcare is one of the challenges of our time. Healthcare entails sensitive and competent diagnosis of pathogens, treatment and prevention. Infectious diseases have claimed millions of lives and escalated poverty conditions especially in developing regions. Hence, the United Nations Millennium Development Goals (MDGs) between the years 2000 and 2015 included healthcare: the eradication of infectious diseases and rehabilitating societal damages triggered by the diseases. Great efforts have been taken towards this goal; evident by the decline in the statistics of global malaria, human immune deficiency virus (HIV) infections and mortality cases recorded at the end of the MDGs era. However, current infectious diseases statistics from resource-constrained regions are still a sore point. Africa and South East Asia recorded the highest number of both new infections and death cases worldwide. Science and technology face the challenge of producing Point of Care Testing (POCT) devices suitable for such regions to achieve efficient detection of diseases across the global spectrum. This paper reviews traditional and new techniques used for the detection of some infectious diseases: malaria, tuberculosis and HIV. The drawbacks of these techniques are discussed and solutions such as Plasmonic metal-based immuno chemical biosensors are considered. For example, vibrational spectroscopy (Raman), which has a potential for the detection and identification of infectious diseases using traditionally weaker intrinsic Raman signals can be improved by the inclusion of the Plasmonic metals, a phenomenon called Surface Enhanced Raman spectroscopy (SERS). Hence, with innumerable references, this paper also reviews SERS application in biosensing and synergising with new technologies such as microfluidics.
Keywords: POCT; TB; malaria; HIV; SERS; Diagnosis
Introduction
Infectious diseases such as malaria, tuberculosis (TB), human immunodeficiency virus (HIV) and cholera, to name a few, continue to cause indisposition and mortality in the world [1]. Infectious diseases account for roughly 16.2 % of the world’s mortality and children are the most affected group [2]. Globalisation and travelling catalyses the worldwide spread of these infectious diseases [1]. Hence, early detection is vital to make informed treatment and prevention decisions. Over the years, extensive discoveries on diagnostics have been attained, evident by the vast number of assays at our disposal e.g., gold standard methods: microscopy, tissue culture, lateral flow immunoassays, enzyme-linked immunosorbent assays (ELISA) and recently developed polymerase chain reaction (PCR) [1,3]. Although, a decline in mortality cases has been recorded by the World Health Organisation (WHO), developing regions continue to be burdened [1,3]. Disadvantaged regions cannot afford the expensive and infrastructural requirements of the available diagnostic assays which explain their continued struggle [1,3]. The high mortality rates experienced in these regions; Sub-Saharan Africa and India, aggravates the standard of living, i.e., increasing numbers of orphans and poverty. The development of sensitive, robust diagnostics for infectious diseases can greatly reduce mortality, encourage better health, and enhance the productivity of the individuals which encourages poverty alleviation [1]. Hence the urgent demand for affordable, sensitive, robust diagnostic tools as an improvement to the traditional diagnostic techniques encouraging their adoption as point of care testing (POCT) devices administered at the bedside in poverty stricken community [1,3]. An ideal POCT tool should be fast, reliable, inexpensive, multiplexing, and portable [4] and is in accordance with the WHO standard, ASSURED (Affordable, Sensitive, Specific, User-friendly, Robust and rapid, Equipment free and Delivered to the patient) [1,3-5]. This review deliberate on the pros and cons of some of the available diagnostic techniques for malaria, TB and HIV.
Malaria
Malaria is caused by Plasmodium genus protozoan. The Plasmodium genus has five species: vivax, knowlesi, malariae, ovale and falciparum, and the latter is the deadliest of the five [6-8]. Malaria is transmitted by female anopheles mosquitoes mostly found in humid areas. It gets injected into the bloodstream (erythrocytes) in the form of sporozoites which then travels and targets the liver where it multiplies asexually in a period of about 7-10 days [9]. At this stage, the infection remains asymptomatic. In the liver, the protozoan can either be stored in a dormant stage which can relapse after several years or the parasite (merozoite) can disseminate from the liver into the bloodstream where it permeates and multiplies in erythrocytes thus causing rupture and reinfection of more erythrocytes [1,6]. Figure 1 further gives an illustration of the malaria life cycle. Malaria presents with flu-like symptoms such as high fever, shaking chills and sweating 10-15 days following infection which makes it hard to rely on these symptoms for early diagnosis. It is then followed up by more aggressive symptoms, some of which are nausea and/or vomiting, diarrhoea, severe headache, fatigue, jaundice and later seizures, confusion and kidney failure. Clinical manifestations of malaria include severe anaemia, thrombocytopenia, hypoglycaemia, metabolic acidosis, hyperlactatemia and others [10,11].
Malaria likely affects anyone exposed to the parasite. However, children and pregnant women are the most affected due to underdeveloped immune system and lowered immunity, respectively [1]. In such cases, the monocytes produce high levels of cytokines like Tumour Necrosis Factors to regulate the immune response. The immune system produces antibodies against the merozoites or proteins expressed from the infected erythrocyte. The antibodies help in neutralizing the parasite by preventing further infection, limit parasite growth and increase phagocytosis and clearance of the infected cells by macrophages. However, it is a hurdle to develop antimalarial immunity that can completely get rid of the parasite on its own due to the polymorphism of the target malaria antigens [12]. The Plasmodium genome is susceptible to mutations thus diversifies the population on variants [13]. Hence it is important for early diagnosis. Malaria claimed about a million sub-Saharan African and Indian lives in 2010 and continues to claim 548 000 year on year [14]. At the end of the millennium development goals (MDGs) era, 2015, a global decline in malaria cases and mortality across all ages were reported. A 41 % decline was attained between 2000 and 2015 and 21 % from 2010 to 2015. Developing regions showed most malaria prevailing cases at the end of the MDGs; 429 000 global malaria mortality cases surfaced mostly from the WHO African region (92 %), south-east Asia (6 %) and the eastern Mediterranean region (2 %) [15]. The P. falciparum species was responsible for 99 % of the deaths, and 3100 deaths were due to vivax. Commendable strides towards malaria prevention, diagnosis and treatment were taken as confirmed by the global malaria cases and mortality decline. However, the persistent prevalence of cases in developing regions, Africa and South east India is still a major concern. Developing regions suffer from lack of knowledge, poor infrastructure to support available diagnostic devices, and stigmatised views [15]. The current diagnostic tools are not adapted for these regions; hence the urgent need for POCT devices which meet the ASSURED WHO standard [16]. The Malaria global technical strategy (GTS), 2016-2030, focuses on decreasing malaria incidences by 90 % in 2030; eliminating malaria from 35 infected countries; and preventing malaria spread to malaria free regions. The GTS align to the sustainable development goals (SDGs) aiming to eradicate AIDs, TB, malaria and neglected tropical diseases by 2030 [15] (Figure 1).
Malaria diagnosis
Diagnosis and identification of malaria species is paramount to the treatment and attainment of the set envisioned GTS. A gold standard, the “Giemsa-stained blood smear” is used for diagnosis and a series of other techniques ranging from microscopy (fluorescence), spectroscopy (laser desorption mass spectrometry), and molecular (PCR, antigen detecting immunochromatographic strips) [14,18]. Table 1 gives a summary of the various malaria detection assays. Early detection of the causative bacterium can lower mortality, overuse of the malaria drugs and eliminate the issue of drug resistance due to overuse [19]. A number of the economical tools listed in Table 1 show low/poor sensitivity at low parasitemia levels in comparison to their counterparts with enhanced sensitivity, e.g., mass spectroscopy, ATR-IR, and PCR which are not economical. Lateral flow immunochromatographic assays have potential for developing regions although their sensitivity, identification of different strains and co-infections requires improvement [1] (Table 1).
Table 1:Malaria diagnostic assays/tools.
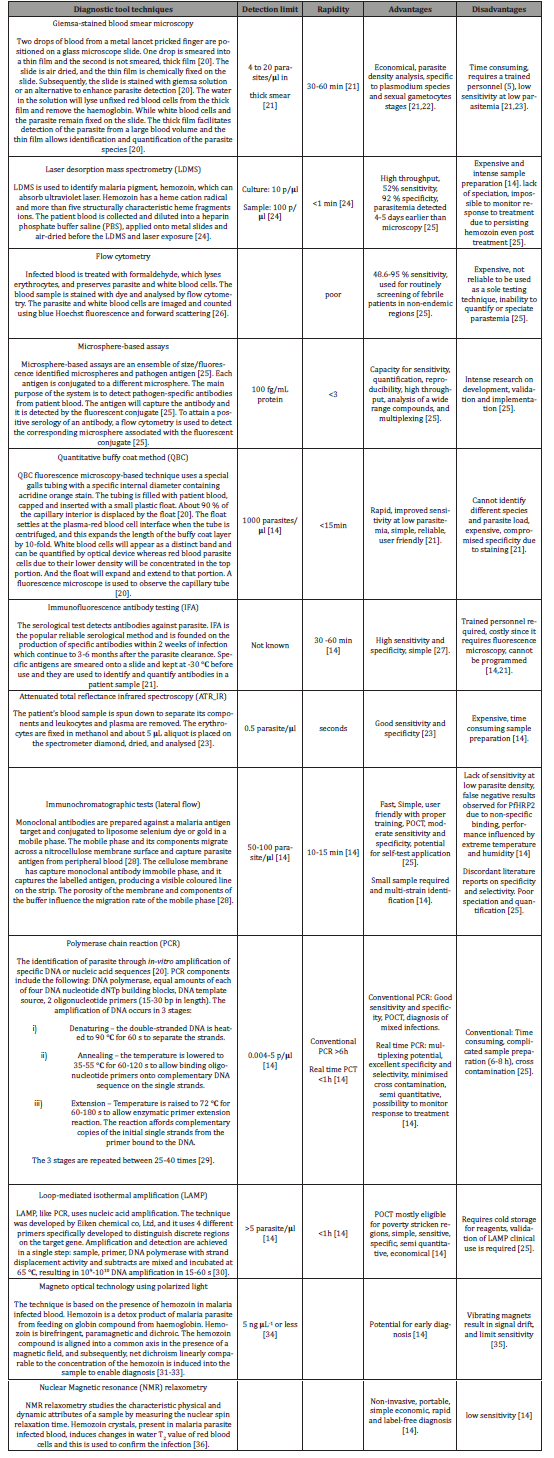
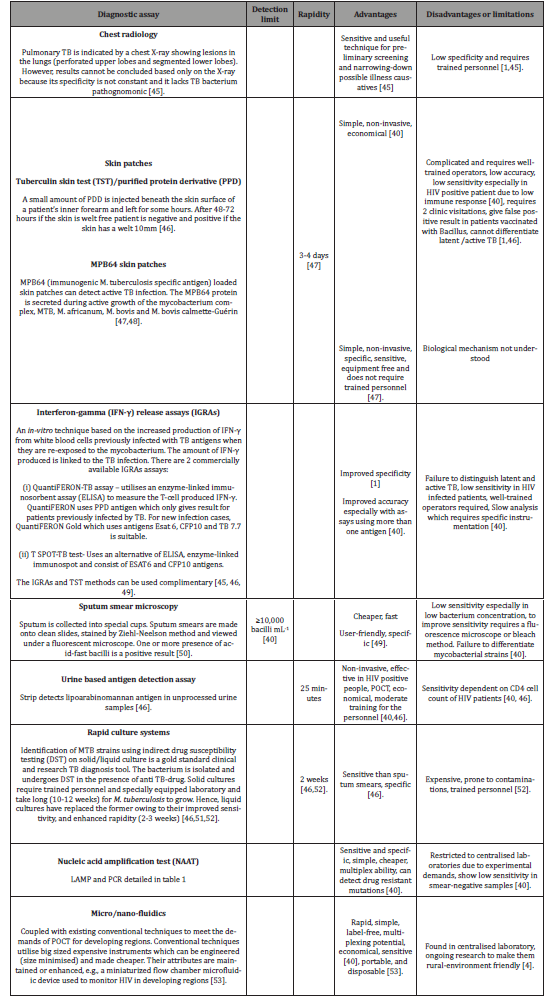
Table 2:TB diagnostic techniques or assays.
Tuberculosis (TB)
TB is a chronic infection caused by a Mycobacterium tuberculosis (MTB) complex [37] and it is one of the leading causes of death globally for the past 25 years [38] evidenced by the subsequent WHO statistics. Primarily TB affects the lungs while secondary extra-pulmonary TB can develop in the central nervous and circulatory systems and other body organs [1,38]. MTB is airborne and thus get released into the air if an infected person coughs or sneezes. Innate immunity is evoked upon inhalation of the MTB tubercle bacilli into the lungs. Ideally, alveolar macrophages should be able to ingest and destroy the bacilli. However, in most cases, the virulence factor of the MTB exceeds the intrinsic microbicidal capacity of the host phagocytes thus will multiply leading to disruption of the macrophages [39]. In about 2-3 weeks post-infection, T-cell immunity produces antigen specific CD4+ and CD8+ T-cells to activate macrophages to kill the intracellular bacilli. The T-cells also helps in increasing the production of cytokines such as primarily gamma interferon [70] which in turn activates macrophages and stimulates natural killer cells and neutrophils [79,214]. In addition, B lymphocytes produce antibodies against lipoarabinomannan which is a glycolipid and a virulence factor of MTB. Figure 2 illustrates the different stages of the growth of the bacterium from the inhalation stage to development and the release to the atmosphere via sneezing of an infected person. Active pulmonary TB is responsible for the transmission of this disease. At times, the tubercle bacilli can be harboured inside the lungs in a dormant state [1,39]. The Latent infection can progress to active infection if triggered by environmental changes which can weaken the immune system such as pregnancy or presence of other pathogens [1,40]. Hence, diagnosis of the TB bacterium is informed by the pathogenicity of the infection, active or dormant.
In 2011 approximately 1.1 million death cases were reported out of 8.8 million reported TB cases; and WHO estimated a 2 million multi-drug resistant TB outbreak between 2011 and 2015 [40]. In 2014, 9.6 million people were estimated to have contracted the TB bacterium worldwide; 5.4 million men, 3.2 million women and 1.0 million children. Of the 9.6 million cases, 12 % were ascribed to HIV-positive patients. However, out of the 9.6 million estimated cases, only 6 million cases of TB were reported in 2014; 37 % of the cases remained unreported and triggers questions on their management [41]. It is such cases that perpetuate the spread of the air-borne bacterium since the patients are not treated. Hence effective diagnostic tools are a necessity to close the existing gap between diagnostics and treatment. The 2014 reported cases had higher mortality cases for men 890 000, 480 000 women and 140 000 children. In 2014, multi drug-resistant (MDR-TB) cases were estimated to be 480 000 but only 123 000 cases were reported. In May 2014, WHO embarked on a global end TB plan; by 2030 countries are expected to lower numbers of TB mortality by 90 % and new incidences by 80 % and lighten financial burden of families affected and infected by the epidemic [41]. The pandemic continued and in 2015, 49 million lives were lost due to TB, 6.1 million new cases and 580 000 eligible for MDR-TB treatment [42]. The recent WHO and United Nations SDGs (2016-2035) continue to strive to end the TB endemic. A 90 % reduction in TB mortality and 80 % reduction in new cases is envisioned by 2030. Technological breakthroughs in diagnosis and treatment of TB, eradication of socio and economic consequences of TB, worldwide TB care and prevention are cogent markers which would aid in the attainment of the aforementioned goals [43] (Figure 2).
TB diagnosis
For an active infection, diagnosis is done by chest radiology or sputum collection and microbiological cell culturing [1,44]. Whereas latent infections diagnosis poses a challenge due to absence of know TB symptoms like coughing, sputum production and fever. To mitigate this challenge tuberlin skin test which measures immune response of a person when injected with more than 200 TB antigens is used for latent infections [1,44]. A positive skin test is shown by the patients’ response to the antigen via the development of inflammation on the elbow where the antigens are usually injected [1]. Table 2 gives a summary of the advantages and disadvantages of some of the TB diagnostic techniques.
Diagnosis of the TB bacterium proffers challenges due to the slow growth of the bacterium which hampers on-site patient diagnosis [40]. Thus, the lack of symptoms and low bacillary burden during the early stages of infection is a concern. The use of sputum compared to urine or blood complicates the process: i.e., sputum collection, transportation and storage is prone to inappropriate handling and storages which results in poor results [40]. Additionally, the lack of reliable and authenticated TB biomarkers hinders diagnosis. Patients at different stages of infection with different immunization records react differently to treatment. Hence, understanding the pathogen and host reaction during infection is a challenge. An ideal POCT diagnostic assay to mitigate all these afor-enumerated challenges is required [40] (Table 2).
Human Immune Deficiency Virus (HIV) and Acquired Immune Deficiency Syndrome (AIDS)
HIV is a retrovirus that infects the immune system cells and renders them dead or compromised [54]. The early stages of infection are without physical symptoms. Increase in HIV replication and a rise in the viral load is subsequently accompanied by fatigue, fever, headaches, night sweats and other symptoms 2 weeks after the infection [55]. The immune system thereby responds by producing anti-HIV antibodies a few weeks after the infection and this helps lower the HIV multiplication rate and progressively lowers the viral load. The progression of HIV infection depends on the viral and host immunity factors. For some individuals, it takes about 34 days to have detectable antibodies in the blood whereas some can take up to 3 months [56]. HIV weakens the immune system and the infected person becomes susceptible to multiple common bacterial and viral opportunistic infections such as pneumonia and meningitis and co-infection such as TB, hepatitis, cancer and others [57]. This, in turn, contributes to a more compromised immune system known as AIDS [54]. The first case of HIV was reported in 1981 and it is one of the formidable challenges of our time [58]. Although impressive strides have been taken in the control, prevention and management of HIV and AIDS using antiretroviral therapy (ART) [59] to a point of undetectable viral load, however, it is still a challenge to completely eradicate HIV. The immune cells, macrophages, dendritic cell, T-helper cells or CD4 are knocked down by this globular ribonucleic acid virus which has a very high mutation rate and this mutation rate creates challenges for the development of treatment and diagnosis assays [1]. Figure 3 summarises the HIV life cycle. The HIV parasite fuses with the CD4+ T-helper cells and integrates with the host DNA, replicates, assembly, buds and matures. The matured HIV strains are then released into the blood to attack more CD4+ T-helper cells and further weaken the defence system [60]. The virus can spread through biological fluids (blood, vaginal fluids, semen breast milk) contacts and to counteract the spread of the virus rapid diagnosis and preventative measures are a requirement [1]. The end of the United Nations (UN) MDGs in 2015 recorded a decline in HIV/ AIDS new cases and mortality [61]. The UNAIDS/WHO estimated a 35 % (2.1 million) reduction of new cases, ascribed to prevention programmes and ARV treatment [61]. The expansion of the ARV resulted in a 45 % (1.1 million) decline in death cases and by mid- 2016 about 18 million people were estimated to be on the ARV treatments [61]. In the MDGs era, ARV extended to middle and low class and their price continued to decrease to make them accessible to everyone and furthermore the SDGs aim to reduce new cases and mortality incidences to less than 500 000 by 2020 [61]. Accurate, sensitive, affordable diagnosis of the virus is one of the key players in achieving the envisioned SDGs.
Standard HIV diagnosis techniques include enzyme immunoassay (EIA) and western blot. EIA is the first generation which detects IgG antibodies and the current third generation which detects IgM antibodies. These techniques are not devoid of challenges as they require trained personnel and twoday visits to the clinic, hence they are not suitable for POCT. Availability of HIV antibodies POCT lateral flows has revolutionized diagnosis with comparable or even better sensitivity (98-100 %) and specificity (86-100 %) to that of the traditional assays [62]. But early detection is still a challenge, as only after 9 days of infection are the HIV antibodies detectable with the POCT lateral flows [62]. Although the 9 days is better compared to 3-6 weeks western blot turnout time [62]. In the United States of America (USA) in July 2012, an OraQuick test (OraSure technologies) self-administered HIV testing kit was approved by Food and Drug Administration (FDA) and it uses saliva from a mouth swab with a turnout time of 20 - 40 min [63]. The OraQuick test was well received in the USA and even developing countries such as Zambia proven by a published study in 2012 on comparison of the OraQuick test with other two blood-based rapid HIV antibody assays [64]. However, there were also concerns with its sensitivity which was lower on individual users than when it is administered by trained personals [65]. This discrepancy can result in false results and it was not the only challenge, early detection of the virus and false negative results during early infection stages were concerns [65].
However, in a case like South Africa, the use of the OraQuick self-HIV testing kit was received with mixed emotions triggering absurd fears of HIV positive people committing suicide because of lack of counselling [63]. South African pharmacies were burnt from supplying the OraQuick test medical devices even though the medicines and related substance control act no. 161 of 1965 had no direct regulatory system for such [63]. Self-administered kits can allow people to test at the comfort of their homes without feeling judged which can eradicate the issue of late diagnosis [63]. In May 2015, after much deliberation the pharmacy council of South Africa lifted the ban, allowing pharmacies to supply the kits under strict minimum standards [66] (Figure 3).

Vibrational Spectroscopy in the Detection Of Infectious Diseases
Optical spectroscopy, i.e., Raman and Fourier transform infrared (FTIR) are fingerprinting tools that can be used in the diagnosis of infectious diseases due to their high sensitivity, specificity in low parasitemia levels, portability, capability and ability to visualise and analyse molecular composition and interactions [68]. The WHO malaria and TB gold diagnosis standards discussed in preceding sections still suffer from low sensitivity and in cases of considerable sensitivity, they are observed to be too complicated and expensive for low-resourced regions. Since the WHO’s ambitious goals include health for all, regardless of geographical socio and economical standards, Raman spectroscopy, although known to be bulky has the potential to be miniaturized into portable sizes and reviewed for diagnosis of infectious diseases. The consideration of the vibrational spectroscopies, Raman and FTIR for detection of infectious diseases such as malaria is not new. Studies have been published confirming their application as detection tools. One of the malaria biomarkers, hemozoin has a distinct fingerprint which can be analysed using the vibrational spectroscopy tools such as Raman and its variants, Surface Enhanced Raman Spectroscopy (SERS), Tip-Enhanced Raman Scattering (TERS) and Resonance Raman spectroscopy (RRS). Figure 4 adapted from wood et al [69] compares the TERS, SERS and RRS on fingerprinting of malaria infected blood cells. The TERS was used to probe the hemozoin crystals from the infected cells vacuole and the AFM images show the hemozoin crystals and characteristic hemozoin Raman peaks were recorded [69]. The imaging of the malaria biomarker, hemozoin is also attained via Raman imaging, a wide field Raman imaging tool using fiber array based spectral translator and tailor made laser illumination system was used to image hemozoin from malaria infected erythrocytes [70]. This approach presented potential for hemozoin analysis in early ring stages of the P. falciparum [70]. Figure 5 details the hemozoin characteristic peaks from the infected cells and images developed using the Raman imaging algorithm. An Attenuated total reflectance-FTIR was coupled with partial least-squares regression models to detect and quantify the malaria parasite at different stages of infection [23] (Figure 4 &5).



The application of the vibrational spectroscopy in the detection of biological fluids is growing evidenced by a growing number of peer reviewed proof of concept publications [71]. However translation of the vibrational spectroscopy concepts into real life applications such as detection of infectious diseases in clinics and POCT is still a bottleneck. The vibrational tools present several challenges from the pre-analysis to the analysis stage. Key areas such as sample volume, dilutions, storage, and processing, interoperator and inter-plate reproducibility need to be standardized to avoid lack of reproducibility. An accurate protocol has to be established via clinical trials [71]. The protocol has to also mitigate the challenges presented by the complexity of the blood i.e., inconsistent data due to variable genetic, ethnicity, age, hormonal differences (gender), and lifestyle (smoking, diet) [71].
Synergising the SERS probes with microfluidics mitigates some of the aforementioned key areas such as sample volume, dilutions, storage, processing, inter-operator and inter-plate reproducibility. Microfluidics detection offers the use of smaller volumes, shorter detection time and this in-turn result in rapidity and reduced cost [72]. The coupling of microfluidics with a powerful fingerprinting tool such as SERS is envisioned to result in fast, highly sensitive and specific diagnostic lab on chip gadgets [72]. Wang et al [73] recently proposed a SERS-based immunoassay with digital microfluidics (DMF-SERS) for rapid, automated and sensitive diagnosis of disease biomarkers. A sandwich approach was used: magnetic beads were conjugated with a capture antibody and used as a solid capture for antigens. A SERS probe was synthesised by labelling detection antibody coated Au core-shell nanostructures with 4-mercaptobenzoic acid. The DMF-SERS immunoassay was tested for quantitative detection of avian influenza virus in buffer and human serum and showed excellent sensitivity and selectivity with less assay time and reduced cost by using low reagent volumes [73]. Figure 6 gives an illustration of the DMF-SERS immunoassay (Figure 6).
Outlook
The world experienced a decline in infectious diseases; especially malaria and HIV and Aids at the end of the United Nations Millennium development goals (MDGs). In the MDGs era, massive strides were taken globally towards better scientific and technological techniques in diagnostics, treatments and prevention of the infectious diseases, i.e., ARV treatments on HIV and Aids patients and malaria and TB medication subsidised by the governments. The MDGs (2000-2015) included compacting diseases, illiteracy, poverty, hunger, environmental deterioration, women abuse or discrimination. It is still a concern that the African region and South East of Asia which are mostly developing regions are still mostly impacted by these infectious diseases, having new and deadly cases recorded at the end of the MDGs era from these regions. Infectious diseases WHO gold standard diagnostic tools discussed in the preceding paragraphs are not resource-constrained region friendly. They are expensive, require trained personnel and at least two visits to the clinic, mainly the sensitive spectroscopy requires a full laboratory with good water supply and electricity. All of these requirements are far-fetched for under-privileged areas which are most impacted by infectious diseases. Hence, the urgent need to produce POCT diagnostic assays which will fit the ASSURED WHO standard. Diagnostic tools should be affordable, sensitive and specific, rapid, require no trained personnel, available to the people and possibly require no use of expensive equipment. Development of these POCT tools is already being explored using nanotechnology, material science and other technologies e.g., development of the self HIV testing kit. The self-testing kit will encourage testing especially in regions where HIV and Aids is still stigmatised. The MDGs achievement is impressing although resource-constrained regions are still burdened by the infectious diseases. Hence the extension of MDGs to 2030’s 17 sustainable development goals persisting on achieving healthy lives across all ages. Vibrational spectroscopy (Raman) coupled with lateral flows or microfluidics can be explored for POCT and with the potential for spectroscopy to be miniaturized this could revolutionize diagnostics. However, we cannot ignore that it will come at a cost and hard work to translate the prototypes to functional reproducible POCT gadgets.
Acknowledgement
The Department of Science and Innovation (DSI), South Africa and Mintek, South Africa.
Conflict of Interest
No conflict of interest.
References
- Hauck TS, S Giri, Y Gao, WC Chan (2010) Nanotechnology diagnostics for infectious diseases prevalent in developing countries. Advanced drug delivery reviews 62(4-5): 438-448.
- Rai M, K Kon (2015) Nanotechnology in diagnosis, treatment and prophylaxis of infectious diseases. Academic Press.
- Markwalter CF, AG Kantor, CP Moore, KA Richardson, DW Wright (2018) Inorganic Complexes and Metal-Based Nanomaterials for Infectious Disease Diagnostics. Chemical reviews.
- Sharma S, J Zapatero-Rodríguez, P Estrela, R O'Kennedy (2015) Point-of-care diagnostics in low resource settings: present status and future role of microfluidics. Biosensors 5(3): 577-601.
- Huppert J, E Hesse, CA Gaydos (2010) What’s the point? How point-of-care STI tests can impact infected patients. Point of care 9(1): 36-46.
- Ragavan K, S Kumar, S Swaraj, S Neethirajan (2018) Advances in biosensors and optical assays for diagnosis and detection of malaria. Biosensors and Bioelectronics 105: 188-210.
- Perez-Guaita D, KM Marzec, A Hudson, C Evans, T Chernenko, et al. (2018) Parasites under the Spotlight: Applications of Vibrational Spectroscopy to Malaria Research. Chemical reviews 118(11): 5330-5358.
- Mathison BA, BS Pritt (2017) Update on malaria diagnostics and test utilization. Journal of clinical microbiology JCM: 02562-16.
- Mawson AR (2013) The pathogenesis of malaria: a new perspective. Pathogens and global health 107(3): 122-129.
- Pérez-Tris J, D Hasselquist, O Hellgren, A Krizanauskiene, J Waldenström, et al. (2005) What are malaria parasites? Trends in parasitology 21(5): 209-211.
- Mockenhaupt FP, S Ehrhardt, J Burkhardt, SY Bosomtwe, S Laryea, et al. (2004) Manifestation and outcome of severe malaria in children in northern Ghana. The American journal of tropical medicine and hygiene 71(2): 167-172.
- Bastos MS, M da Silva-Nunes, RS Malafronte, EHE Hoffmann, G Wunderlich, et al. (2007) Antigenic polymorphism and naturally acquired antibodies to Plasmodium vivax merozoite surface protein 1 in rural Amazonians. Clin. Vaccine Immunol 14(10): 1249-1259.
- Zevering Y, C Khamboonruang, K Rungruengthanakit, L Tungviboonchai, J Ruengpipattanapan, et al. (1994) Life-spans of human T-cell responses to determinants from the circumsporozoite proteins of Plasmodium falciparum and Plasmodium vivax. Proceedings of the National Academy of Sciences 91(13): 6118-6122.
- Chen K, C Yuen, Y Aniweh, P Preiser, Q Liu (2016) Towards ultrasensitive malaria diagnosis using surface enhanced Raman spectroscopy. Scientific reports 6: 20177.
- Organisation WH (2016) World Malaria report 2016.
- Organisation Wh (2015) World Malaria report 2015.
- Klein E (2013) Antimalarial drug resistance: a review of the biology and strategies to delay emergence and spread. International journal of antimicrobial agents 41(4): 311-317.
- Moody A, P Chiodini (2000) Methods for the detection of blood parasites. Clinical & Laboratory Haematology 22(4): 189-201.
- Kong K, C Kendall, N Stone, I Notingher (2015) Raman spectroscopy for medical diagnostics—From in-vitro biofluid assays to in-vivo cancer detection. Advanced drug delivery reviews 89: 121-134.
- Carpenter CC, GW Pearson, VS Mitchell, SC Oaks (1991) Malaria: obstacles and opportunities. National Academies Press.
- Tangpukdee, N, C Duangdee, P Wilairatana, S Krudsood (2009) Malaria diagnosis: a brief review. The Korean journal of parasitology 47(2): 93.
- Maltha J, P Gillet, J Jacobs (2013) Malaria rapid diagnostic tests in endemic settings. Clinical Microbiology and Infection 19(5): 399-407.
- Khoshmanesh A, M Dixon, S Kenny, L Tilley, D McNaughton, et al. (2014) Detection and quantification of early-stage malaria parasites in laboratory infected erythrocytes by attenuated total reflectance infrared spectroscopy and multivariate analysis. Analytical chemistry 86(9): 4379-4386.
- Scholl PF, D Kongkasuriyachai, PA Demirev, AB Feldman, JS Lin, et al. (2004) Rapid detection of malaria infection in vivo by laser desorption mass spectrometry. The American journal of tropical medicine and hygiene 71(5): 546-551.
- Erdman LK, KC Kain (2008) Molecular diagnostic and surveillance tools for global malaria control. Travel medicine and infectious disease 6(1-2): 82-99.
- P H van Vianen, A van Engen, S Thaithong, M van der Keur, H J Tanke, et al. (1993) Flow Cytometric Screening of Blood Samples for Malaria Parasites. Cytometry 14: 276-280
- Chen F, BR Flaherty, CE Cohen, DS Peterson, Y Zhao (2016) Direct detection of malaria infected red blood cells by surface enhanced Raman spectroscopy. Nanomedicine: Nanotechnology, Biology and Medicine 12(6): 1445-1451.
- Moody A (2002) Rapid diagnostic tests for malaria parasites. Clinical microbiology reviews 15(1): 66-78.
- Rapley R (1998) Polymerase chain reaction, in Molecular Biomethods Handbook. Springer: 305-325.
- Site EG (2015) The principle of LAMP method. Eiken Chemical Ltd.
- Orbán Á, Á Butykai, A Molnár, Z Pröhle, G Fülöp, et al. (2014) Evaluation of a novel magneto-optical method for the detection of malaria parasites. PloS one 9(5): e96981.
- Coronado LM, CT Nadovich, C Spadafora (2014) Malarial hemozoin: from target to tool. Biochimica et Biophysica Acta (BBA)-General Subjects 1840(6): 2032-2041.
- Newman DM, J Heptinstall, RJ Matelon, L Savage, ML Wears, et al. (2008) A magneto-optic route toward the in vivo diagnosis of malaria: preliminary results and preclinical trial data. Biophysical journal 95(2): 994-1000.
- Karl S, L Gutiérrez, MJ House, TM Davis, TGS Pierre (2011) Nuclear magnetic resonance: a tool for malaria diagnosis? The American journal of tropical medicine and hygiene 85(5): 815-817.
- G Stephen, RDaRB (2014) Rapid Malaria Detection using the Magneto-Optical Properties of the Malaria Pigment.
- Cistola DP, MD Robinson (2016) Compact NMR relaxometry of human blood and blood components. TrAC Trends in Analytical Chemistry: 53-64.
- Aguilar-Ayala DA, JC Palomino, P Vandamme, A Martin, JA Gonzalez-y-Merchand (2017) Genetic regulation of Mycobacterium tuberculosis in a lipid-rich environment. Infection, Genetics and Evolution 55: 392-402.
- Jennifer furin, HC Madhur Pai, Tuberculosis (2019) The Lancet 393(10181): 1642-1656.
- Van Crevel R, TH. Ottenhoff, JW Van Der Meer (2002) Innate immunity to Mycobacterium tuberculosis. Clinical microbiology reviews 15(2): 294-309.
- Wang S, F Inci, G De Libero, A Singhal, U Demirci (2013) Point-of-care assays for tuberculosis: role of nanotechnology/microfluidics. Biotechnology advances 31(4): 438-449.
- Organization WH (2015) Global TB report.
- Organization WH (2016) Global TB report.
- Organisation WH (2017) Global TB report.
- Won EJ, JH Choi, YN Cho, HM Jin, HJ Kee, et al. (2017) Biomarkers for discrimination between latent tuberculosis infection and active tuberculosis disease. Journal of Infection 74(3): 281-293.
- Luna JC (2016) Update on the diagnosis and treatment of pulmonary tuberculosis. Revista Clínica Española (English Edition) 216(2): 76-84.
- Cheon SA, HH Cho, J Kim, J Lee, HJ Kim, et al. (2016) Recent tuberculosis diagnosis toward the end TB strategy. Journal of microbiological methods 123: 51-61.
- Palomino JC (2005) Nonconventional and new methods in the diagnosis of tuberculosis: feasibility and applicability in the field. European Respiratory Journal 26(2): 339-350.
- Ndubuisi NO, OR Azuonye, NO Victor, OC Robert, O Vivian (2016) Front-loaded sputum microscopy in the diagnosis of pulmonary tuberculosis. International journal of mycobacteriology 5(4): 489-492.
- Bekmurzayeva A, M Sypabekova, D Kanayeva (2013) Tuberculosis diagnosis using immunodominant, secreted antigens of Mycobacterium tuberculosis. Tuberculosis 93(4): 381-388.
- Geisbrecht BV, B Nikonenko, R Samala, R Nakamura, CA Nacy, et al. (2006) Design and optimization of a recombinant system for large-scale production of the MPT64 antigen from Mycobacterium tuberculosis. Protein expression and purification 46(1): 64-72.
- Phunpae P, S Chanwong, C Tayapiwatana, N Apiratmateekul, A Makeudom, et al. (2014) Rapid diagnosis of tuberculosis by identification of Antigen 85 in mycobacterial culture system. Diagnostic microbiology and infectious disease 78(3): 242-248.
- Mani V, S Wang, F Inci, G De Libero, A Singhal, et al. (2014) Emerging technologies for monitoring drug-resistant tuberculosis at the point-of-care. Advanced drug delivery reviews 78: 105-117.
- Lee WG, YG Kim, BG Chung, U Demirci, A Khademhosseini (2010) Nano/Microfluidics for diagnosis of infectious diseases in developing countries. Advanced drug delivery reviews 62(4-5): 449-457.
- Organization WH (2019) WHO in South-East Asia.
- Lindbäck SR, Thorstensson AC, Karlsson M, von Sydow L, Flamholc A. Blaxhult, et al. (2000) Diagnosis of primary HIV-1 infection and duration of follow-up after HIV exposure. Aids 14(15): 2333-2339.
- Zetola NM, CD Pilcher (2007) Diagnosis and management of acute HIV infection. Infectious disease clinics of North America 21(1): 19-48.
- Chang CC, M Crane, J Zhou, M Mina, JJ Post, et al. (2013) HIV and co‐ Immunological reviews 254(1): 114-142.
- Organization WH (2012) Regional Health Sector Strategy on HIV 2011-2015. 2012, World Health Organization.
- Cohen MS, YQ Chen, M McCauley, T Gamble, MC Hosseinipour, et al. (2011) Prevention of HIV-1 infection with early antiretroviral therapy. New England journal of medicine 365(6): 493-505.
- Haseltine WA, F Wong-Staal (1988) The molecular biology of the AIDS virus. Scientific American 259(4): 52-63.
- Organisation WH (2016) Prevent HIV, test and treat all.
- Johnston B, J Conly (2002) Point-of-care testing for HIV: HIV counselling and testing. Canadian Journal of Infectious Diseases and Medical Microbiology 13(2): 85-88.
- Richter M, W Venter, A Gray (2012) Enabling HIV self-testing in South Africa. Southern African Journal of HIV Medicine 13(4): 186-187.
- Zachary D, L Mwenge, M Muyoyeta, K Shanaube, A Schaap, et al. (2012) Field comparison of OraQuick® ADVANCE Rapid HIV-1/2 antibody test and two blood-based rapid HIV antibody tests in Zambia. BMC infectious diseases 12(1): 183.
- Phillips DM, VR Zacharopoulos, X Tan, R Pearce-Pratt (1994) Mechanisms of sexual transmission of HIV: does HIV infect intact epithelia? Trends in microbiology 2(11): 454-458.
- Lopez Gonzalez L, K Polao, V Warby (2018) Home HIV testing gets the green light. Health-e, 2016. 8.
- Commons, C, Attribution 4.0 International (CC BY 4.0). Retrieved from.
- Baker MJ, HJ Byrne, J Chalmers, P Gardner, R Goodacre, et al. (2018) Clinical applications of infrared and Raman spectroscopy: state of play and future challenges. Analyst 143(8): p. 1735-1757.
- Wood BR, E Bailo, MA Khiavi, L Tilley, S Deed, et al. (2011) Tip-enhanced Raman scattering (TERS) from hemozoin crystals within a sectioned erythrocyte. Nano letters 11(5): 1868-1873.
- Brückner M, K Becker, J Popp, T Frosch (2015) Fiber array based hyperspectral Raman imaging for chemical selective analysis of malaria-infected red blood cells. Analytica chimica acta 894: 76-84.
- Leal L, M Nogueira, R Canevari, L Carvalho (2018) Vibration spectroscopy and body biofluids: Literature review for clinical applications. Photodiagnosis and photodynamic therapy.
- Viehrig M, AH Thilsted, M Matteucci, K Wu, D Catak, et al. (2018) Injection-molded microfluidic device for SERS sensing using embedded Au-capped polymer nanocones. ACS applied materials & interfaces 10(43): 37417-37425.
- Wang Y, Q Ruan, ZC Lei, SC Lin, Z Zhu, et al. (2018) Highly sensitive and automated surface enhanced raman scattering-based immunoassay for H5N1 detection with digital microfluidics. Analytical chemistry 90(8): 5224-5231.
-
Nikiwe Mhlanga, Sinazo Cobongela. Diagnosis of Infectious Diseases: Tuberculosis, Malaria and HIV and Aids – A Review. Insi in Chem & Biochem. 1(4): 2021. ICBC. MS.ID.000518.
-
Infectious Diseases, POCT; TB; Malaria; HIV; SERS; Diagnosis, Globalisation, Immunosorbent Assays, Microscopy, Anaemia, Thrombocytopenia, Hypoglycaemia, Metabolic Acidosis, Hyperlactatemia.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






