 Research Article
Research Article
Conformational Studies on (+)-epi-goniofufrone and Hirsutic Acid Reveals Patterns of Conformer Populations in Triquinane Sesquiterpenes
Sahar Sakhaee1, Mohammad Hossein Sakhaee2, Saghar Sakhaee3, Ahmad Takallou4, Akbar Mobaraki5 and Nader Sakhaee6,7*
1Islamic Azad University, Iran
2Mashhad University of Medical Sciences, Iran
3Medical University of Shahrood, Iran
4Department of Chemistry, Kharazmi University, Iran
5Department of Chemistry, Tarbiat Modarres University, Iran
6Department of Mathematics and Natural Sciences, Harris Stowe State University, USA
7Department of Chemistry, Southern Illinois University Edwardsville, USA
Nader Sakhaee, Department of Mathematics and Natural Sciences, Harris-Stowe State University, St. Louis, MO, 63108, USA & Department of Chemistry, Southern Illinois University Edwardsville, Edwardsville, IL 62025, USA.
Received Date: October 24, 2019; Published Date: October 30, 2019
Abstract
Cyclic compounds are frequently found in natural products, prevailing specially in alkaloids. Whether the choice of nature for these carbonic skeletal frameworks, is conformationally beneficial, has yet to be explored. In this paper we try to show how nature’s choice of cyclic carbonic framework in two triquinane sesquiterpenes is responsible for populating specific conformers to provide bioactivity. Conformational analysis of cisoctahydropentalenes can provide a telltale sign that conformational preference may be a predesigned aspect of both (+)-epi goniofufrone and hirsutic acid. conformational analyses are done, using ωb97xd/6-311+G(d) level of theory. The model was then tested to analyze the stable conformers in, (+)-epi-goniofufrone and hirsutic acid, respectively. The results are in line with our previous findings on chondrosterin J, and further work is underway to find a comprehensive detailed conformational landscape for fused five membered rings.
Keywords: Bioactive conformers; Cis-octahydropentalene; (+)-epi-goniofufrone<; Hirsutic acid; Triquinane sesquiterpenes
Introduction
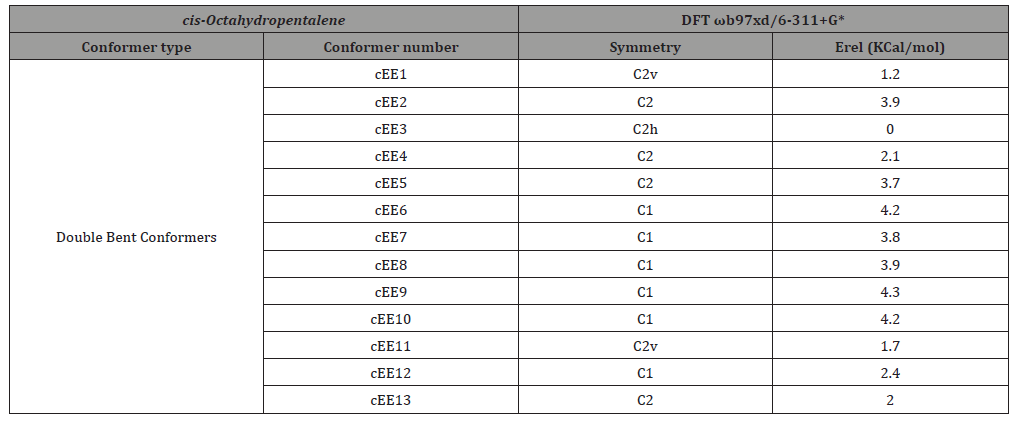
Hoffmann et al. [1] asked the pivotal question of whether natures is deliberately dictating conformational patterns for natural products or not. He suggested that cyclic compounds containing oxygen can attain conformational preferences that are of prime importance to bio activity Still et al. [2]. were among the pioneers to show theses conformational preferences in oxygen containing sixmembered rings can collectively determine the final shape of some natural products. Our new results seems to back up the idea of nature’s deliberation in choosing fused cyclic carbonic skeletons to exploit their conformational space in sculpting bio active molecular shapes Lipnick et al. introduced a wheel model of a of up to 20 conformers [1,3,4] to show cyclopentane conformational mobility. Further attempts [5-12] to classify these conformers mostly proved unsuccessful [13-15]. The concept of a spherical conformational landscape (see SI) decisively classified the conformers into so called bent and twist conformers arranged along two ring coordinates [16]. The model has then developed over the recent years to study the fused cyclic compounds [17]. Figure one shows such a conformational wheel model for cis-octahydropentalene (see SI for conformer legend), recently being studied along with other fused cyclic systems in our studies on conformational analysis Table 1. provides the relative energies of these conformers. Clearly the pseudorotational ring coordinates are not fully accessible in fused cyclic systems [16,17] (Figure 1 & Table 1).
Table 1: Relative energies and symmetry point groups for double envelope conformers in cis-octahydropentalene.
Methods
To study conformational forms in cis-octahydropentalenes as well as hirsutic acid and goniofufrone, density functional methods like xb97xd, in particular yield results similar, if not close, to those obtained via correlational methods like MP2. most local density functional methods, slightly overestimate barriers due to their inability to account for van der Waals attractions in some strained conformers [18,19]. The ωb97xd basis set can reliably result in accurate van der Waals modifications. The geometries computations were done at ωb97xd/6-311+G(d) level [20-22], using Gaussian g09 package [23]. Larger basis sets like 6-311+G(d,p) proved no better accuracy.
Results and Discussion
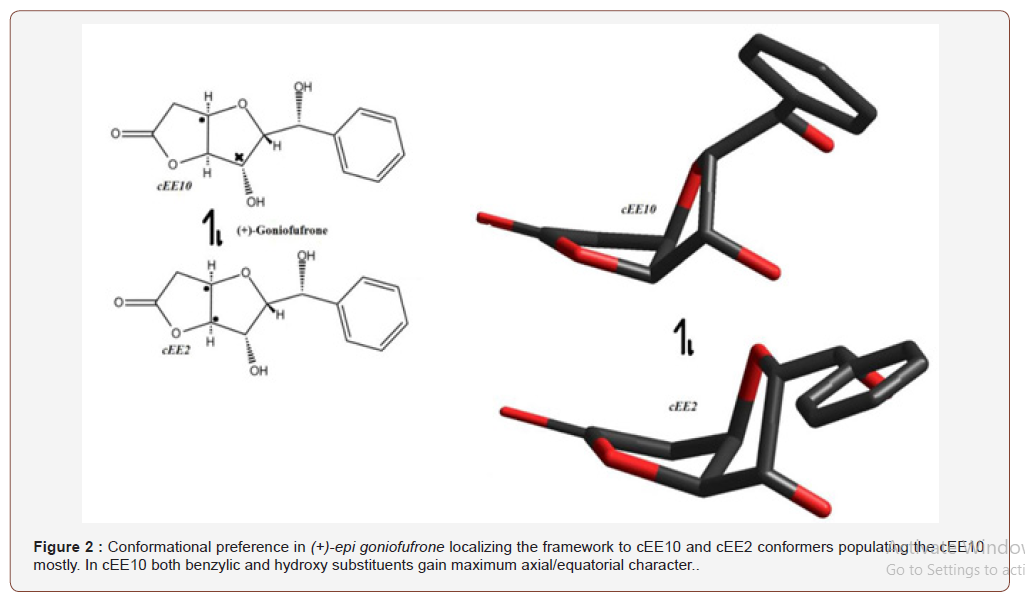
(+)-epi-goniofufrone [24] is one of herbal pharmaceutical polyketide components found in plant family Annonaceae with about 2000 species and have been historically important. This marine natural product is a triquinane sesquiterpene with a main cis-octahydropentalene framework. The molecule switches between conformational forms cEE2 and cEE10, with latter being more fluxional and dynamic in ring coordinate, while cEE2 is rather a trapped conformer with minimal fluxional nature. The two conformers are believed to cooperatively populate the cEE10 in the conformational landscape (Figure 1). It is also noteworthy that more axial/equatorial character is given to hydroxy substituent in cEE10 compared to cEE2, as the substituents are closer the puckered tip of the envelope rather its terminal planar carbons. This more axial/equatorial character may well suggest how nature uses fused cyclic rings to its benefit (Figure 2).
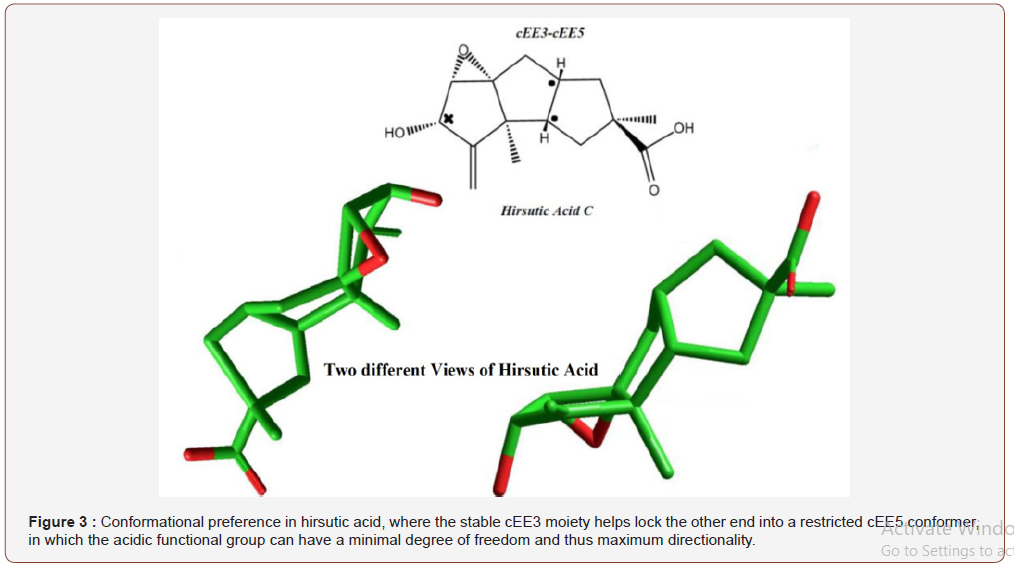
Hirsutic acid is an antibiotic isolated from a fungus, Stereum Hisrsutumm, with the main skeleton of three successive fused cyclopentane rings. Hirsutic acid is also a triquinane sesquiterpene that has three five membered rings fused together in two cis configurations, here a mobile cEE3 part of the molecule seems to balance the other end of the molecule to attain a trapped cEE5 conformation (Figure 3) (Figure S1, S2 & S3).

Conclusion
In Conclusion, two triquinane sesquiterpenes have been shown two adopt pseudorotationally localized conformers, in which their functional substituents can attain maximum axial/equatorial characters. Both compounds possess a cis-octahydropentalene carbonic backbone. Our results show cis-octahydropentalene frameworks can populate desired conformers mainly because of if the intersected ring coordinates in their conformational landscape model. The conformational landscape shown here, successfully explains the bioactive conformers in these two triquinane sesquiterpenes. Further ongoing research is underway to explore a more in depth understanding of such conformational landscapes in cyclic and cyclic fused compounds.
Acknowledgement
None
Conflict of Interest
No conflict of interest.
References
- RW Hoffmann (2000) Conformation design of open‐chain compounds. Angewandte Chemie International Edition 39(12): 2054-2070.
- M Saunders, K Houk, YD Wu, WC Still, M Lipton, et al. (1990) Conformations of cycloheptadecane. A comparison of methods for conformational searching. Journal of the American Chemical Society 112(4): 1419-1427.
- G Ashwell, AG Morell (1974) The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Advances in enzymology and related areas of molecular biology 41: 99-128.
- A Saran, D Perahia, B Pullman (1973) Molecular orbital calculations on the conformation of nucleic acids and their constituents. Theoretica chimica acta 30(1): 31-44.
- JE Kilpatrick, KS Pitzer, R Spitzer (1947) The thermodynamics and molecular structure of cyclopentane1. Journal of the American Chemical Society 69(10): 2483-2488.
- KS Pitzer, WE Donath (1993) Conformations and strain energy of cyclopentane and its derivatives. Molecular Structure and Statistical Thermodynamics: Selected Papers of Kenneth S Pitzer, World Scientific, pp. 98-103.
- D Wertz (1969) Far Infrared Absorption Spectrum and Pseudorotation of the Thiacyclopentane Molecule. The Journal of Chemical Physics 51(5): 2133-2136.
- NL Allinger, JA Hirsch, MA Miller, IJ Tyminski, FA Van Catledge (1968) Conformational analysis. LX. Improved calculations of the structures and energies of hydrocarbons by the Westheimer method. Journal of the American Chemical Society 90(5): 1199-1210.
- L Carreira, R Lord (1969) Ring Puckering Potential Functions of Some Oxygen-Containing Molecules.
- W Green, A Harvey, J Greenhouse (1971) Spectroscopic Determination of the Pseudorotation Barrier in Selenacyclopentane. The Journal of Chemical Physics 54(3): 850-856.
- J Durig, J Willis Jr (1970) Spectra and Structure of Small Ring Compounds. XIX. Vibrational Analysis and the Barrier to Pseudorotation of Germylcyclopentane. The Journal of Chemical Physics 52(12): 6108-6119.
- J Marino, JK Long, New strategies for annulations: a highly convergent and stereoselective synthesis of an octahydronaphthalene synthon for dihydrocompactin. Journal of the American Chemical Society 110(23): 7916-7917.
- J Laane (1969) Far‐Infrared Spectrum and the Barrier to Pseudorotation of Silacyclopentane. The Journal of Chemical Physics 50(5): 1946-1951.
- P Kowalewski, HM Frey, D Infanger, S Leutwyler (2015) Probing the structure, pseudorotation, and radial vibrations of cyclopentane by femtosecond rotational Raman coherence spectroscopy. The Journal of Physical Chemistry A 119(45): 11215-11225.
- G Brügger, HM Frey, P Steinegger, F Balmer, S Leutwyler (2011) Accurate determination of the structure of cyclohexane by femtosecond rotational coherence spectroscopy and ab initio calculations. The Journal of Physical Chemistry A 115(34): 9567-9578.
- N Sakhaee, S Jalili, F Darvish (2016) Spherical conformational landscape shed new lights on fluxional nature of cyclopentane and its derivatives, confirmed by their Raman spectra. Computational and Theoretical Chemistry 1090: 193-202.
- N Sakhaee, H Sakhaee, S Sakhaee, A Takallou (2019) Conformational Analysis of cis-Octahydropentalene, Insights on Bioactive Conformers. Biomedical Journal of Scientific and Technical Research 17(2): 12704-12707.
- TA Halgren (1996) Merck molecular force field. II. MMFF94 van der Waals and electrostatic parameters for intermolecular interactions. Journal of Computational Chemistry 17(5-6): 520-552.
- DJ Giesen, MZ Gu, CJ Cramer, DG Truhlar (1996) A universal organic solvation model. The Journal of organic chemistry 61(25): 8720-8721.
- Y Shao, LF Molnar, Y Jung, J Kussmann, C Ochsenfeld, et al. (2006) Advances in methods and algorithms in a modern quantum chemistry program package. Physical Chemistry Chemical Physics 8: 3172-3191.
- M Frisch, G Trucks, HB Schlegel, GE Scuseria, MA Robb, et al. (2009) Gaussian 09, revision a. 02. Gaussian, Inc, Wallingford, CT 200.
- M Frisch, G Trucks, HB Schlegel, GE Scuseria, MA Robb, et al. (2009) Gaussian 09, revision D. 01. Gaussian, Inc, Wallingford CT.
- HP Hratchian, PV Parandekar, K Raghavachari, MJ Frisch, T Vreven (2008) QM: QM electronic embedding using Mulliken atomic charges: Energies and analytic gradients in an ONIOM framework. The Journal of chemical physics 128(3): 034107.
- L Alves, A Chimiak, M Milewska, T Nomura (2012) Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products. Springer Science & Business Media.
-
Nader Sakhaee, Sahar Sakhaee, Mohammad Hossein Sakhaee, Saghar Sakhaee, Ahmad Takallou, Akbar Mobaraki. Conformational Studies on (+)-epi-goniofufrone and Hirsutic Acid Reveals Patterns of Conformer Populations in Triquinane Sesquiterpenes. Insi in Org & Inorg. 1(1): 2019. IOIC.MS.ID.000501.
-
Bioactive conformers, cis-octahydropentalene, (+)-epi-goniofufrone, Hirsutic acid, Triquinane sesquiterpenes, Chondrosterin J, Pseudorotational, Conformational analysis, Functional methods, Correlational methods, Waals attractions, Annonaceae, Stereum Hisrsutumm.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






