 Research Article
Research Article
Chemical properties and antibacterial effect of the oil from seeds of Citrullus colocynthis
Ali Abd Ellahi Elltayeib*, Bothina Khalil Mohamed, Rayan Mohamed Elshami and Safa Abdullah Adam
Department of Chemistry, University of Kordofan, Sudan
Ali Abd Ellahi Elltayeib, Department of Chemistry, Faculty of Science, University of Kordofan, El-Obeid, Sudan.
Received Date: June 22,2020; Published Date: July 06, 2020
Abstract
The study aimed to determine chemical properties, chemical composition and antibacterial activity of the oil from seeds of Citrullus colocynthis. Samples were collected from Alsont in North kordofan state. Chemical properties tested were fatty acid value (2.26mg), saponification value (280.5mg), ester value (278.24mg) and peroxide value (0.21mg). The oil yield was 15.7%. The analysis by GC/MS showed ten compounds in the extracted oil. The compound 9,12-octadecadienoic acid (z,z) methyl ester showed high area percentage (51.34) compared to other compounds detected. The extracted oil tested against two types of bacteria (Streptococcus pyogenes and Pseudomonas aeruginosa) with different concentrations (40, 60, 80 and 100mg/ml) and the inhibition zone were (1.5 mm, 2.5mm, 4.5mm and 8mm) and ( 4.5mm, 8mm, 6.5mm and 10mm) respectively.
Keywords: Chemical properties; Antibacterial effect; Oil extract; Citrullus colocynthis
Introduction
Plants have potent biochemical and have components of phytomedicine. Since time immemorial man is able to obtain from them a marvelous assortment of industrial chemicals. Plant based natural constituents can be derived from any part of the plant like bark, leaves, flowers, roots, fruits, seeds, etc. Any part of the plant may contain active components. The beneficial medicinal effects of plant materials typically result from the combinations of secondary products present in the plant. The medicinal actions of plant are unique to particular plant species or groups and are consistent with this concept as the combination of secondary products in a particular plant is taxonomically distinct. Arid and semi-arid plants are good sources for the production of various types of secondary metabolites which make them resistant to various environment stress e.g. scarcity of water, salinity, pathogens etc. They are also important for the primary metabolism of plants. These compounds include alkaloids, flavonoids, steroids, phenolices, terpenes, volatile oils etc. Man has been exploiting these natural plant products for use in medicines, cosmetics, dyes, flavors and food [1]. Citrullus colocynths common Arabic name: handhal, English: bitter-apple, bitter-cucumber, colocynth, vine-of-sodom, wild gourd. It was native to dry areas of North Africa and it has been known in the Mediterranean region since Biblical time. The traditional medicine of this plant has been used to treat constipation, edema, bacterial infections, fever, cancer, diabetes and an abortifacient. The root was used in inflammation of the breasts, joints pain; externally it was used in ophthalmic and in uterine pains. A paste of the root is applied to the enlarged abdomen of children. The fruit and root were rubbed with water and applied to boils and pimples. The fruit was also used in ascites, biliousness, jaundice, cerebral congestion, colic, constipation dropsy, fever, worms and sciatica, cough, asthma, oedema, bacterial infections, cancer and diabetes. Oil from seeds is used for poisonous bites, bowel complaints, epilepsy and also for blackening the hair [2].
Citrullus colocynth was analyzed for its chemical composition including bioactive secondary metabolites, dietary vitamins and transition elements. The results confirm the presence of these bioactive chemical constituents comprising flavonoids (1.39mg/100g), saponins (0.52mg/100g), alkaloids (1.64mg/100g), phenolic contents (1.22mg/100g) [3]. The seeds of C.colocynths contain edible oil, 56% of which Contained Linoleic acid and 25% of which contained oleic acids 4 [4].
Material and Methods
Plant seeds
Citrullus colocynths seeds were collected in March 2018 from Alsond-North Kordofan State- Sudan. The plant was authenticated by a plant taxonomist in Kordofan University to be Citrullus colocynths. The seeds were shade-dried, cleaned and grinded by a mechanical grinder.
Oil extraction
50.0g of the powdered seeds were extracted by hexane using a soxhlet apparatus for 7 hours. Hexane was evaporated on a hot water bath.
Fatty acid content
3ml of the oil was added to 50ml of ethanol in a 250ml conical flask. The flask was swirled, then a few drops of phenolphthalein were added and the contents were titrated against sodium hydroxide till permanent light pink color appeared.
The Blank sample
50ml of ethanol in 250ml conical flask was swirled, then a few drops of phenolphthalein were added and the contents were titrated against sodium hydroxide till permanent light pink color appeared.
Weight of fatty acid= Volume of NaOH*Molarity of NaOH*Molecular weight of NaOH/Volume of the oil
Peroxide value
3.0 ml of the oil was dissolved in a solvent mixture (50ml) of acetic acid and chloroform (3:2), Warmed with 1ml of potassium iodide and then titrated with sodium thiosulphate using 1ml starch indicator.
The Blank sample
50ml of solvent mixture of acetic acid and chloroform (3:2), Warmed with 1ml of potassium iodide and then titrated with sodium thiosulphate using 1ml starch indicator.
Peroxide value= Volume of Sodium thiosulphate*Molarity of sodium thiosulphate/ Volume of the oil
Saponification value
5ml of the oil was added to 50ml of potassium hydroxide (0.5M) in a conical flask. The flask was warmed in a water bath for 2 hours and left until cooled to room temperature and then titrated with hydrochloride acid (0.5M) using a few drops of phenolphthalein indicator.
The Blank sample
50ml of potassium hydroxide (0.5M) in a conical flask was warmed in the water bath for 2 hours and left until cooled to room temperature and then titrated with hydrochloride acid (0.5M) using a few drops of phenolphthalein indicator.
Saponification value= Volume of KOH*Molarity of KOH*Molecular Wight of KOH/Volume of Oil
Esters value
Esters value=Saponification value Fatty acid value.
GC/MS analysis conditions
The qualitative and quantitative analysis of the samples was carried out by using GC/MS technique model (GC/MS-QP2010- Ultra) from Japans ’Simadzu Company, with serial number 020525101565SA and capillary column (Rtx-5ms-30 m × 0.25 mm × 0.25 μm). The sample was injected by using split mode, helium as the carrier gas passed with flow rate 1.61 ml/min, the temperature program was started from 60°C with rate 10°C /min to 300°C as final temperature degree with 3 minutes hold time, the injection port temperature was 300°C, the ion source temperature was 200°C and the interface temperature was 250°C.The sample was analyzed by using scan mode in the range of m/z 40-500 mass to charge ratio and the total run time was 27 minutes. Identification of components for the sample was achieved by comparing their retention index and mass fragmentation patents with those available in the library, the National Institute of Standards and Technology (NIST).
Sample preparation
2ml of the sample was mixed thoroughly with 7ml of alcoholic sodium hydroxide that was prepared by dissolving 2g in 100ml methanol. 7ml from alcoholic sulfuric acid (1ml H₂SO₄ to 100ml methanol) was then added. The mixture was shaken for 5 minutes. The content of the test tube was left to stand overnight. 1ml of super saturated sodium chloride was added and the contents were shaken. 2ml of normal hexane was added and the contents were shaken thoroughly for three minutes. The n-hexane layer (the upper layer of the test tube) was taken using disposable syringe. 5μl from the n-hexane extract was diluted with 5ml of diethyl ether. The mixture was filtered through syringe filter (0.45) μm and dried with 1g of anhydrous sodium sulphate. 1ml of the diluted sample was injected in the GC-MS instrument.
Preparation of media
Blood agar base was prepared by dissolving 40g of media in 1 liter distilled water, heated in a water bath to boiling (for mixing the solution), after dissolved completely then sterilized by autoclaving at 121°C for 15 minutes, cooled to 47°C and ascetically added 10% detibrinated blood mixed with gentle rotation and poured in a clean and sterilized Petri dishes. A sample of two kinds of bacteria isolated is brought as negative gram stain (Streptococcus pyogenes and Pseudomonas aeruginosa). The bacteria is cultured by using loop and burner by making lines at the surface of the Petri dishes, and then incubated at 37°C for 24 hours. Little holes of known diameters (8mm) are made, and then filled with different concentrations of the solution (100%, 80%, 60%, and 40%). The Petri dishes were incubated at 37°C for 18 hours. The results were registered by measuring the spread of the solution minus the diameter of the hole (8mm) 4 [5].
Bacterial microorganisms
Streptococcus pyogenes
ATCC 27853 (Gram -ve bacteria) Pseudomonas aeruginosa
American Type Culture Collection (ATCC) Rockville, Maryland, USA.
Results
(Tables 1-3)
Table 1:Chemical properties of the extracted oil.
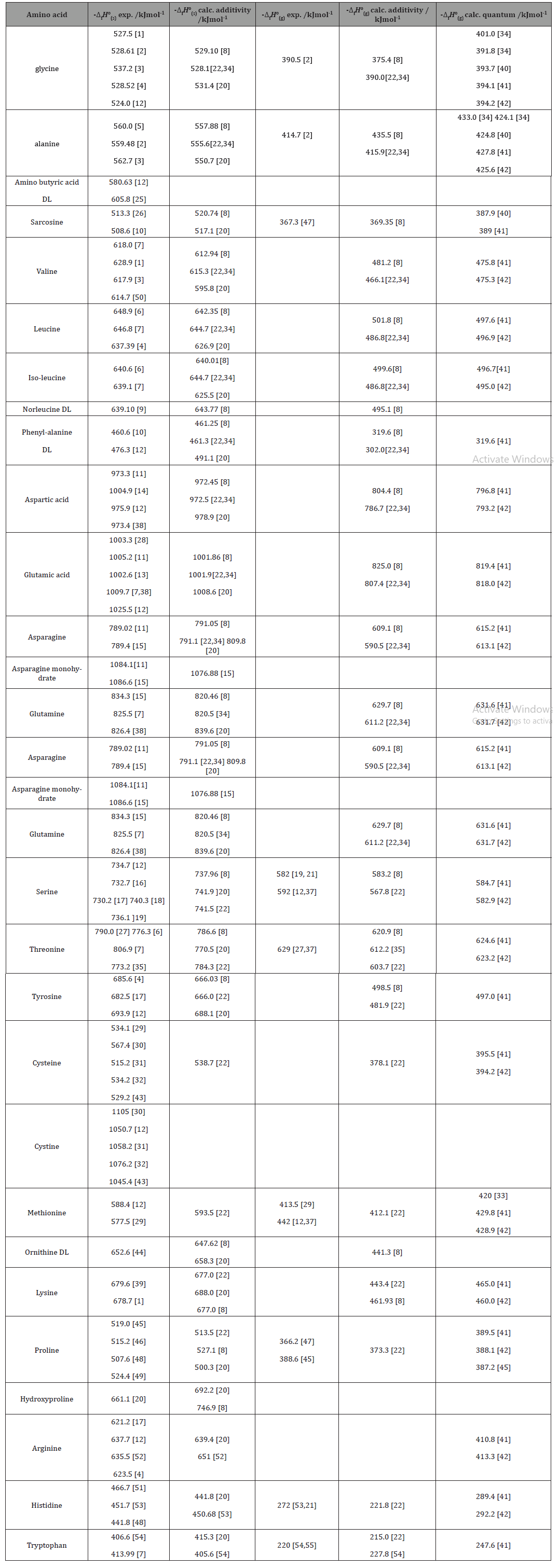
Table 2:The chemical composition of the extracted oil by GC-MS.
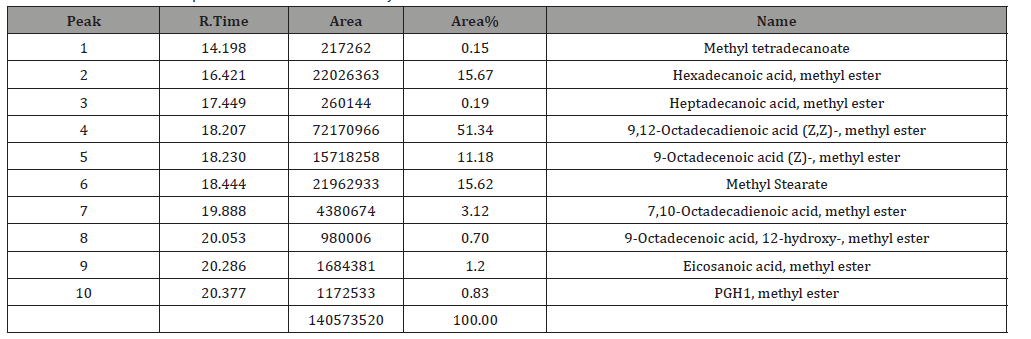
Table 3:Antibacterial effect of the extracted oil against Streptococcus pyogenes and Pseudomonas aeruginosa (inhibition zone in mm).
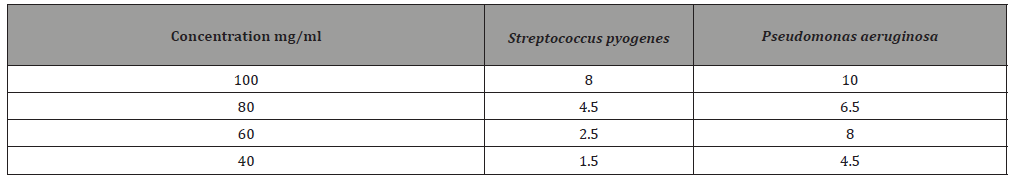
Discussion and Conclusion
The percentage of the oil 15.7%, lower compared with oil percentage produced by Kamalakar et al. [6] research 19%. Fatty acid value 2.26 nearly similar to that obtained by Kamalakar et al. [6] 2.2. Peroxide value 0.21 higher compared with the result obtained by Kamalakar, et al. [6] 0.1. Saponification value 205.887 this value higher compared with the result obtained by Kamalakar et al. [6] 189.7. Ester value 203.627 higher to that obtained by Kamalakar et al. [6] 187.5. Analysis of the oil by GC-MS revealed ten compounds and mainly composed of 9,12-Octadecadienoic acid (Z,Z)methyl ester 51.34% of lower percentage compared with the same compound in the oil extracted by Alsnafi (2016) 58.81%, Hexadecanoic acid methyl ester 15.67%, higher compared with Alsnafi [7] research 10.40%, Methyl stearate15.62% higher compared with Alsnafi [6] research 6.25%, and 9-Octadecenoic acid (Z) methyl ester11.18% higher compared with Duhan et al. [8] 1.70%. The difference between the values obtained in this research and other research values and the compounds detected may be due to differences in weather, climate and the kind of the soil…etc. 100% concentration of the oil is more effective towards pasedomonas (10mm) but toward sterbetococcai has moderate effect (8mm). Other concentrations have lower or no effect toward both types of bacteria.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Meena MC, V Patni (2008) Isolation and Identification of Flavonoid “Quercetin” from Citrullus colocynthis (Linn) Schrad, University of Rajasthan, Jaipur, India.
- Delazar A, Naseri M, Nahar L, Moghadam S, Esnaashari S, et al. (2007) GC-MS analysis and antioxidant activities of essential oils of two cultivated Artemisia species. Chem Nat Compds 43: 112-114.
- Asyaz S, Khan FU, Hussain I, Khan MA, Khan IU (2010) Evaluation of chemical analysis profile of Citrullus colocynthis growing in Southern area of Khyber Pukhtunkhwa, Pakistan. World applied Sciences Journal 10(4): 402-405.
- Jaime A, Silva T, Hussain A (2017) Citrullus colocynthis (L) Schrad. (Colocynth): Biotechnological perspectives. Emirates Journal of Food and Agriculture 29(2): 83-90.
- Forbes S, Thornton S, Glassey B, Forbes M, Buckley NJ (1997) Why parent birds play favourites. Nature 390: 351-352.
- Kamalakar K, Manoj GNVTS, Prasad RBN, Karuna MSL (2015) Thumba (citrulluscolocynthis L) seed oil: a potential bio lubricant base stock. Grasas Y Aceites 66(1): oo17-3495.
- Alsanfi AE (2016) Chemical constituents and pharmacological effects of Citrullus colocynthis. A review. IOSR Journal of pharmacy (e) 6(3): 57-67.
- Duhan A, Duhan S, B Kumari (2012) Effect of chemical refining on Citrullus colocynths and Pongamia pinnate seed oil. African Journal of Food Agriculture 12(3).
-
Ali Abd Ellahi Elltayeib, Bothina Khalil Mohamed, Rayan Mohamed Elshami. Chemical properties and antibacterial effect of the oil from seeds of Citrullus colocynthis. Insi in Chem & Biochem. 1(3): 2020. ICBC. MS.ID.000512.
-
Antitumor Activity, Novel Azole compound, Carcinoma, Swiss Albino Mice.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






