 Research Article
Research Article
Cassia Fistula Seeds Nutritional Profile; an Insight into its Therapeutic Potentials
Oyewole TA1*, Oyewole ON2 and Falodun AE3
1Department of Science Technology Biochemistry unit, Federal Polytechnic, Ado-Ekiti, Ekiti State, Nigeria
2Department of Science Technology Chemistry unit, Federal Polytechnic, Ado-Ekiti, Ekiti State, Nigeria
3Central Research Laboratory, Federal Polytechnic, Ado Ekiti, Ekiti State, Nigeria
Oyewole TA, Department of Science Technology Biochemistry unit, Federal Polytechnic, Ado-Ekiti, Ekiti State, Nigeria.
Received Date: June 20, 2022; Published Date: June 29, 2022
Abstract
Cassia fistula has long been thought as ornamental plant due to its attractive leaves and the seeds has been regarded as waste product particularly in Nigeria. However, due to its numerous therapeutic attributes, it has remarkably gained popularity in Asian and African countries. Therefore, this study aimed to assess the important biological metabolites inherent in the Cassia fistula seeds using high throughput analytical instrumentations. The investigated seeds appeared to be good sources of magnesium, potassium, calcium, phosphorus, zinc iron, but the selenium and copper content were deficient when compared with Recommended Dietary Allowance. The essential amino acids contents were found to be relatively high in the seeds with highest values of Arginine of 8.90605%. The essential amino acid profiles of seeds were compared favorably with FAO/WHO (1991) requirement pattern when compared to recommended amino acids scores. Fatty acid profiles revealed that the seed samples is rich in monounsaturated fatty acids with values of 31.8658% and polyunsaturated fatty acids (44.9563%) with low saturated fatty acids. The study provides evidence that Cassia fistula seeds contain essential amino acids, minerals which could be of interest for the development of new drugs and it could be valuable as an additives, supplement for animal feeds, nutraceuticals and therapeutic medicine.
Keywords: Fatty acids; Phytochemicals; Amino acids; Bioactive compounds
Introduction
Cassia fistula is a tree plant commonly found in Africa, Asia and some parts of the world. It is a tracheophytes plants of the family of Fabacea with a scientific name Cassia fistula [1], and is commonly called Golden shower [2]. Despite the traditional claims of the potency of the different parts of Cassia fistula in traditional medicine, more scientific research work could be done on its pharmacological activities in human and poultry animals. The description of the Cassia fistula plant parts are shown in Figure 1.The leaves, barks, pulps etc. of these plants have been used by

It has also been shown that extracts prepared from certain plants could be used as pill, decoction and powder for the management of several diseases [4]. Cassia fistula seeds have been reported to be useful in the management of jaundice [5], skin disorders, gastric distress, swollen throat and many more among some tribes [6]. Evidences have shown that some bioactive compounds in this plant could play key roles in pharmacological activities; increasing lifespan of mice with tumor [7], lowering blood sugar thereby revealing its anti-diabetic property [8], hepatoprotective [9], antioxidant [10] activities among many others. Also, it has been reported to possess anti-fungal activity due to its clearance profile as anti-candidal agents [11].
Researchers have isolated, identified and investigated numerous chemical compounds, and their applications in the prevention and management of diseases have been established [12]. Some primary and secondary metabolites present in different seed plants have been documented to play vital Pharmacological activities on human health and even poultry foods. One of such are the protein building blood called the amino-acids with diverse metabolic roles such as; in the synthesis of protein, they help to stimulate insulin secretion in pancreatic β-cells stimulating glucose clearance from the blood in the management and prevention of Diabetes mellitus. Other aminoacids like arginine, leucine, isoleucine, alanine and phenylalanine have been reported to possess this insulin stimulating effects [13].
Moreover, polyunsaturated fatty acids are the unit of fats essential during pregnancy, early child growth and development, during lactation for the development of the brain at early life. Evidences have linked PUFA to play vital role in the management of coronary heart related diseases when SFA was replaced with PUFA reducing coronary heart diseases in Adult, older men and women [14]. The anti-carcinogenic properties of some conjugated linolenic acid and monounsaturated fatty acids in animal models have also been reported [15].
In addition, some crop plant seeds and their oil have been reported to be rich source of polyunsaturated fatty acids (PUFA) essential in the human diet and in the management of diseases such as cardiovascular related diseases, cancer related diseases, neurological and hormonal disorders [16]. Several minerals from different plant food and parts such as Zn, Cu, Mg, Ca are essential for proper health functioning and since mineral deficiency has been a major health challenge in different part of the world, increasing dietary mineral is a great therapy in combating these challenges in human and even other an [17]. Also, Plants, herbs containing vitamins, minerals, amino-acids used in traditional medicine have been used as a remedy to manage diseases and deficiencies [18]. There is little information on the therapeutic and health related profiles of these C seeds in Nigeria. Therefore Gas chromatography method was used to determine the fatty acid profiles, amino acid profiles of these seeds, which will help to unfold the medicinal and other importance of these seeds in Nigeria and other parts of the World.
Materials and Methods
Plant Material Cassia fistula seed pods were collected from The Federal Polytechnic Ado-Ekiti, Ekiti- State Nigeria. The pods were allowed to dry and the seeds were removed from them and washed with water. The seeds were then air-dried for five days after which it was grinded into powder and stored in an airtight container and all chemicals used were of analytical grade.
Phytochemical analysis
The qualitative and quantitative analysis of Secondary metabolites of aqueous extracts of Cassia fistula seeds was carried out using standard methods as described by Sofowora (1993) and Harborne (1973) [19].
Determination of mineral composition
Analysis of mineral element was determined according to the official method of the Association of Official Analytical Chemists [20]. The sample analysis digestion was carried out using aqua regia (nitric acid and hydrochloric acid mixture in ratio 1:3). 15 ml concentrated acid was added to 1 g of sample in a conical flask of 100 ml, and then make to boil by heating on a fumed cupboard until the solution becomes whitish. The filtered solution was made up to 50 ml with distilled water then transferred into a sample bottles and labeled. The digested sample mineral analysis was determined using atomic absorption spectrophotometer (AAS) (Buck Scientific AAS Model 211 VGP) and Jenway Digital Flame Photometer (PFPT Model).
Fatty acid analysis using Gas chromatography
C seed fatty acid analysis were carried out using GC−flame ionization detection (GC-FID) (HP 6890 Powered with HP ChemStation Rev. A 09.01[1206] Software, with a FID detector equipped with a 30 m × 0.25 mm × 0.25 μm HP INNO Wax column dimension. The temperatures of the injection port and the detector were 250 and 320°C, respectively. Samples were desorbed in the split mode (split ratio 20:1). The oven temperature program was initially held at 60°C and first rampling at 12oC/min for 20 minutes, maintained for 2 minutes and the second rampling at 15oC/minutes for 3 minutes was maintained for 8 minutes. Nitrogen was used as the carrier gas. The peak areas of the target compounds were used to quantify the absolute contents compared to that of calibration samples with known concentrations.
Amino acid analysis
Amino Acid profile Extraction and analysis were carried out following the modified method of AOAC method 982.30, 2006 and Danka et al., (2012) in the sample. 0.5 g of sample was weighed into the 250ml conical flask capacity. Fat content of the sample was defatted by extracting it with 30ml of petroleum spirit three times with thimble equipped soxhlet extractor. For complete hydrolysis to be achieved, the sample was hydrolysed three times for the totality of amino acids recovery. 30ml of the 1M potassium hydroxide was used to soak the pulverized and defatted sample and solution was incubated for 48 hours at 110oC in hermetically closed borosilicate glass container. Hence, the hydolysate was neutralized to get pH in the range of 2.5- 5.0 after the Alkaline hydrolysis. Cation exchange solid-phase extraction was used to purify the solution. The amino acids in purified solution were derivatised with ethylchloroformate before injection to Gas chromatography [20].
Results
Qualitative phytochemical analysis
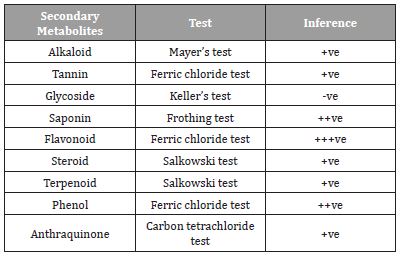
The qualitative analysis of Cassia fistula seed aqueous extracts carried out base on standard protocols, with alkaloid, tannin, saponin, flavonoid, steroid, terpenoid, phenol and anthraquinone are present in the plant while glycoside is absent (Table 1).
Table 1:Phytochemical analysis of C seed. - Absent, +Trace, ++Moderate, +++Abundant.
Quantitative phytochemical analysis
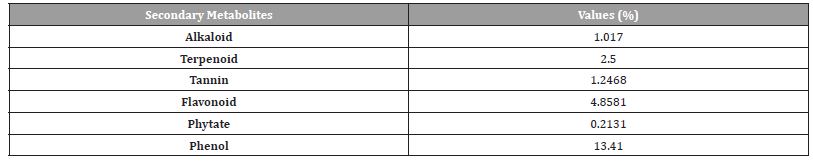
The Cassia fistula seeds aqueous extract shows higher concentration of phenol, followed by flavonoid terpenoids and tannin (Table 2).
Table 2:Phytochemical composition of C seed.
Mineral analysis of Cassia fistula seed
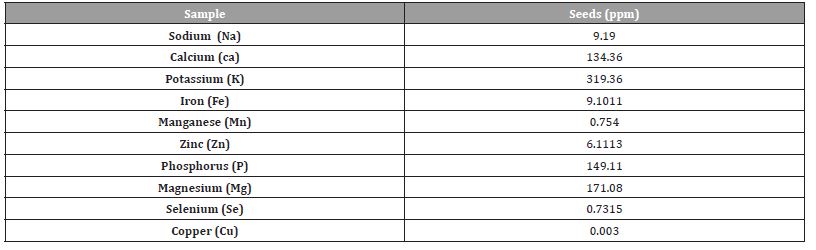
The mineral analysis of the Cassia fistula seeds using the atomic absorption spectrophotometer (AAS). The level of potassium is higher followed by magnesium, phosphorus and calcium as shown in (Table 3).
Table 3:Mineral analysis of digested sample of C seeds.
Fatty acid methyl ester analysis
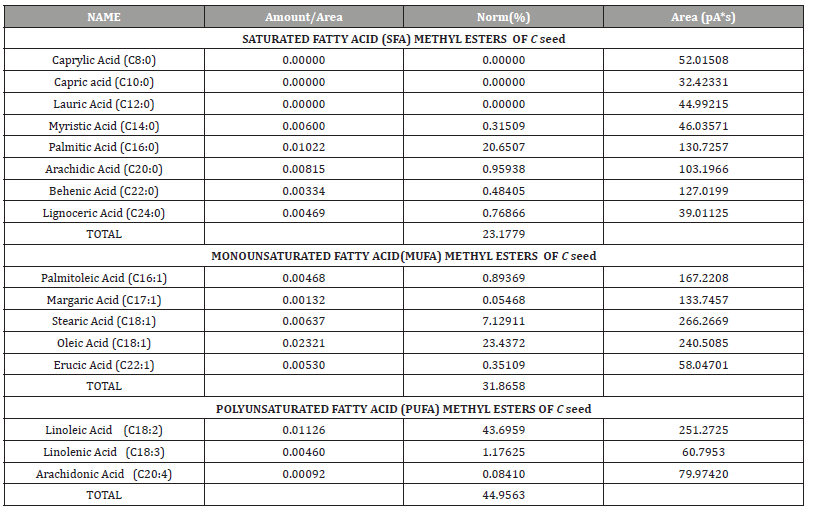
The result and chromatograms of the fatty acid methyl ester analysis of Cassia fistula seed aqueous extracts using GC− flame ionization detection (GC-FID). The level of saturated fatty acid is 23.2%, monounsaturated fatty acid level is 31.9% and polyunsaturated fatty acid level is 45% as shown in (Table 4.0).
Table 4:The quantity of different classes of fatty acid methyl esters profiles in C seed.
Amino acid analysis
The result and chromatograms of the amino-acids analysis of Cassia fistula seed aqueous extracts using Gas chromatography (GC-FID) are shown in (Table 5.0, Table 5.1 and Figure 2).
Table 5:Shows the Essential amino-acids quantities of C seed.
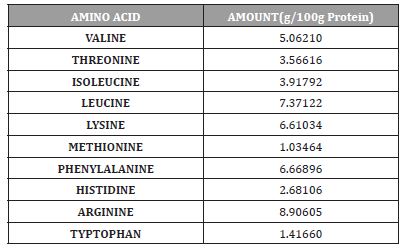
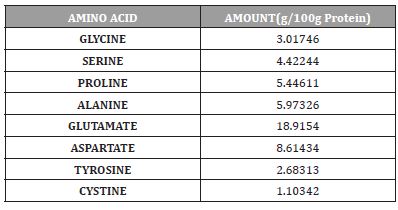
Table 6:Shows the Non-essential amino acids quantities of Cassia fistula seed.
The seed of this plant could be an important tool in discovering therapeutic drugs through isolation of some of these essential metabolites inherent in the seed.
Discussion
The bioactive constituents of Cassia fistula seeds were analyzed and identification of phytochemicals, minerals, saturated, monounsaturated, polyunsaturated fatty acid, and amino acids content were determined using standard procedures (Table 1-5).
The phytochemical screening of the aqueous extracts of Cassia fistula seed revealed the presence of phenol, saponin, tannin (Table 1-2) some of which have been documented to possess antioxidant, anti-inflammatory and antimicrobial activity in some fruits, vegetables and herbs, Flavonoid in some plants, has been recorded to show vasodilating action against cardiovascular diseases. The saponin contents of some plants have been reported to show hypolipidemic activity, anti-microbial activity, antiatherosclerotics, and antimicrobial activity in different studies [21-25].
Calcium, phosphorus, magnesium, potassium etc. are present in larger amounts compare to other mineral constituents present in Cassia fistula seed in (Table 3). Manganese, zinc, copper and selenium are co-enzyme of antioxidant enzymatic systems involve in defense against free radicals. Reduced intakes of certain micronutrients have been reported to increase oxidative stress in the pathogenesis of several diseases [26].
The Gas chromatography analysis of fatty acid methyl ester show high level of PUFA and MUFA in Cassia fistula seed unraveling its therapeutic potentials (Table 4).
Some evidences of PUFA-rich diets have been reported to reduce Total cholesterol and LDL-cholesterol but manage to increase HDLcholesterol among subjects on solid food experiment [27]. And since replacing saturated fat with MUFA in some designed trials, it has proved to improve the CVD markers profiles [28], significantly reduced high blood pressure in overweight model and decrease Triglyceride level among subjects with Diabetes mellitus [29].
The Gas chromatography analysis of Amino acid in Cassia fistula seeds revealed the presence of ten essential amino acids (Table 5.0) and eight non-essential amino acids (Table 5.1).
The use of Amino acids in the management and treatment of diverse diseases have been documented; Histidine has been reported to be used in the management of Arthritis, enhance blood flow and proper functioning of the nervous system [30]. Reports have shown that Arginine provides the substrate for the synthesis of nitric oxide, a mediating factor of vascular homeostasis [31]. Impairment of NO production or bioactivity has been considered in cardiometabolic risk, including that of coronary artery disease, stroke, and diabetes [32]. Omega-3 fatty acids have been documented in the treatment of osteoarthritis and athreosclereosis [32].
Plants due to their phytoconstituents have been a major key factor in the management of human diseases and improving human health over time. Traditional practitioners have always used these medicinal plants in the cure and treatment of diverse infections since old times. Due to these observations of the traditional applications of these plants in health related conditions, carrying out scientific investigations on some of the important bioactive constituents present in Cassia fistula seed was considerable bearing in mind the therapeutic values present in some different seed plants. Several reports have shown the therapeutic values of different parts of Cassia fistula and such therapeutic and pharmacological values includes: anti-inflammatory, anti-microbial, anti-ulcer, anti-tusive, wound healing, antipyretic, antiparasitic, anti-itching activities [33] among many others. Different metabolites inherent in different plant parts have been documented to be a major factor in these diverse activities in some part of the World.
Amino acids, minerals, vitamins, unsaturated fats etc. play vital roles against oxidative stress, supporting the growth and development of poultry animals and reducing their risk of diseases, regulating immune response and maintaining normal metabolic and physiological systems. Since there is a high demands for growth enhancement additives, disease preventing drugs etc. Checking the amounts of these constituents in Cassia fistula seeds could be a step in the right direction [34-39].
Some of these constituents unravel the therapeutic potentials of the seeds as they have been reported to have immense benefits in the management of different diseases. The high level of some aminoacids and minerals have given information on the biological roles the seeds could play in disease treatment and in further isolation and synthesis of drugs. The seeds have shown characteristic possible potential and applications in Nutraceuticals and in drug design.
Conclusion
This plant is commonly use in other part of the World, however the importance of these tree plant have not maximized in Nigeria. The Cassia fistula seed is so rich in essential metabolites such as palmitic acid, linolenic acid, oleic acid, phenols, flavonoids, Arginine, phenylalanine, Valine, lysine, isoleucine and minerals such as K, Ca, Mg, P which have all proved to play different roles in the management of diseases are also present in these cassia fistula seeds. More information of the medicinal and nutritional values of the seed would and the detection of more bioactive compounds and isolating them could be a major step towards understanding the therapeutic actions of this seed in disease management.
Acknowledgement
None.
Conflict of Interest
No Conflict of interest.
References
- Kassahun (2015) “REVIEW OF BAMBOO VALUE CHAIN IN ETHIOPIAwuro zone, P.O. Box 17, Dawuro, Southern Ethiopia,” Int J African Soc Cult Tradit 2(3): 52-67.
- Bekele-tesemma (1985) “Useful trees and shrubs of Ethiopia : Identification , Propagation and Management for 17 Agroclimatic Zones.”
- Y MAAZ Tadesse (2016) BAMBOO SPECIES Biological, Ecological and Management Aspects BAMBOO SPECIES.
- BN Kigomo, JF Kamiri (1985) “Observations on the Growth and Yield of Oxytenanthera Abyssinica (A. Rich) Munro in Plantation,” East African Agric For J 51(1): 22-29
- AK Ray, S K Das, S Mondal, P Ramachandrarao (2004) Microstructural characterization of bamboo. J Mater Sci 39(3): 1055-1060.
- Ensermu Kelbessa, Sebsebe Demissew (2014) Diversity of Vascular Plant Taxa of The Flora of Ethiopia and Eritrea. 13(1): 37-45.
- Wubalem Tadesse, Getachew Desalegn, Abraham (2012) “Forestry and Forest Products: technologies and Issues Ethiopian Institute of Agricultural Research, Addis Ababa, Ethiopia.,” For. For. Prod. Ethiop. Technol. Issues, p. 441, [Online]. Available: http://publication.eiar.gov.et:8080/xmlui/bitstream/handle/123456789/50/forestry
- RL Banik (2015) Bamboo The Plant and its Uses.
- Y Yu, H Wang, F Lu, G Tian, J Lin (2014) “Bamboo fibers for composite applications: A mechanical and morphological investigation.” J Mater Sci 49(6): 2559-2566.
- P Guznay (2004) “Guadua Angustifolia,” Palma Trop. S.A 3(61): 4, [Online]. Available:http://www.4shared.com/office/7cBG17R7/guadua_angustifolia_-_por_guzn.htm?locale=es.
- Walter Liese (1998) The anatomy of bamboo culm, International Network for Bamboo and Rattan.
- ETDELOS Ríos (2014) Polymer Composite Materials.
- E Trujillo, D Perremans, L Osorio, AW Van Vuure, J Ivens, et al. (2014) “Characterization of Unidirectional Discontinuous Bamboo Fibre / Epoxy Composites,”: 22-26.
- Abd Latif Mohmod, Ashaari Hj Amin, Jamaludin Kasim, Mohd Zin Jusuh (2016) “Effects of Anatomical Characteristics on the Physical and Mechanical Properties of Bambusa Blumeana: Forest Research Institute Malaysia Stable URL 6(2): 159-170.
- S Verheyden, I Verpoest, KU Leuven (2012) Microstructural Analysis and Mechanical Behaviour Of Bamboo Fibres: 24-28.
- F Wang, J Shao, LM Keer, L Li, J Zhang (2015) The effect of elementary fibre variability on bamboo fibre strength 75: 136-142.
- Chaowana (2013) “Bamboo : An Alternative Raw Material for Wood and Wood-Based Composites 2(2).
- SEE, SEE (2021) Plant growth and biomass distribution on Guadua angustifolia Kunth in relation to ageing in the Valle del Cauca - Colombia the Journal of the American Bamboo Society.
- D Division (2004) “Developmental Changes in Cell Wall Structure of Phloem Fibres of the Bamboo Dendrocalamus asper: 497-505.
- W Liese, G Weiner (1996) Ageing of bamboo culms. A review 30: 77-89.
- J Lin, X He, Y Hu, T Kuang, R Ceulemans (2002) “Lignification and lignin heterogeneity for various age classes of bamboo (Phyllostachys pubescens) stems Lignification and lignin heterogeneity for various age classes of bamboo (Phyllostachys pubescens) stems,”.
- AN Rao, G Dhanarajan, CB Sastry (1985) Recent Research on Bamboos Proceedings of the International Bamboo Workshop October 6-14.
- SP Pubescens (2007) Chemical Changes with Maturation Of The Bamboo Species Phy-Llostachks Pubescens,” 19(1).
- HN Hisham, S Othman, H Rokiah, MA Latif, S Ani, et al. (2006) Characterization of Bamboo Scortechinii at Different Ages Gigantochloa 18(4): 236-242.
- J Fu, L Fu, Q Zhang, W Gao (2015) Xylanase- and cellulose-aided bioprocessing of bamboo 605-611.
- Depuydt, Delphine EC (2018) Industrial Crops & Products European bamboo fi bres for composites applications, study on the seasonal in fl uence,” 133: 304-316.
- DEC Depuydt, N Sweygers, L Appels, J Ivens, AW Van Vuure (2019) “Bamboo fibres sourced from three global locations: A microstructural, mechanical and chemical composition study,”
- Y Nishiyama, P Langan, H Chanzy (2002) “Crystal Structure and Hydrogen-Bonding System in Cellulose I from Synchrotron X-ray and Neutron Fiber Diffraction,” 9074-9082.
- JI Mora (2008) Extraction of cellulose and preparation of nanocellulose from sisal fibers Extraction of cellulose and preparation of nanocellulose from sisal fibers.
- ACH Barreto, D S Rosa, FJN Maia, AEC Ju (2014) “Thermal and mechanical properties of biocomposites based on a cashew nut shell liquid matrix reinforced with bamboo fibers,”
- A Rachini, M Le Troedec, C Peyratout (2008) Comparison of the Thermal Degradation of Natural , Alkali-Treated and Silane-Treated Hemp Fibers Under Air and an Inert Atmosphere.
- Z Sebestyén, Z May, K Réczey, E Jakab (2013) “The effect of alkaline pretreatment on the thermal decomposition of hemp,” 1-12.
- IOPC Series, M Science (2020) “Changes in the density , specific gravity and dimensional stability of candlenut wood (Aleurites moluccanus (L.) Willd) from several variation temperatures with oil-heat treatment Changes in the density, specific gravity and dimensional stability of candlenut wood (Aleurites moluccanus (L) Willd) from several variation temperatures with oil-heat treatment.
- J Wei, H Liu, Y Chen, H Yu (2016) Functionalized Cellulosic Fibers through Periodate Oxidization of Bamboo Pulp.
- V Placet (2009) A Characterization of the thermo-mechanical behaviour of Hemp fibres intended for the manufacturing of high performance composites. Compos. Part A 40(8): 1111-1118.
-
Oyewole TA, Oyewole ON, Falodun AE. Cassia Fistula Seeds Nutritional Profile; an Insight into its Therapeutic Potentials. Insi in Chem & Biochem. 2(2): 2022. ICBC. MS.ID.000533.
-
Crystallinity, Bambusa, Dendrocalamus, Gigantochloa, Guadua, Phyllostachys, Schizostachyum And Thyrsostachys, Environmental Conditions, Parenchyma Cells, Sclerenchyma, Sclerenchyma Cells.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






