 Mini Review Article
Mini Review Article
Advances in the Multicomponent Synthesis and Biological Relevance of Cyclic Cyanoguanidines
Hulme Ríos-Guerra* and Jorge Medina-Chargoy
Department of Chemical Sciences, Faculty of Higher Studies Cuautitlán-UNAM, México
Hulme Ríos-Guerra, Department of Chemical Sciences, Section of Organic Chemistry, Faculty of Higher Studies Cuautitlán-UNAM, Cuautitlán Izcalli, EdoMex, CP 54740, Mexico.
Received Date: November 12, 2022; Published Date: November 29, 2022
Abstract
This review addresses recent multicomponent synthesis to assemble biologically active cyclic cyanoguanidine compounds featuring cyanoiminopirimidine core. Accordingly, the most relevant one-pot chemical methods and important aspects of their bioactivity are highlighted here.
Keywords: Multicomponent chemistry; Bioactive 2-cyaniminopyrimidines; A2BAR antagonist; Biological targets
Introduction
The cyanoimine motif (=NCN) has recently gained ground as a relevant precursor in the field of organic synthesis and pharmaceutical development due to its unique bioactivity and chemistry properties. Its ability to function as a bioisosteric replacement for the carbonyl group has led to the development of potent therapeutic acyclic drugs capable of modulating the histamine H2 receptor (cimetidine) or potassium channel (pinacidil), just to name a few. Furthermore, due to its peculiar chemical reactivity, it can lead to the generating molecular diversity (appendage diversity or scaffold diversity) to give rise to libraries of structurally diverse and functionally important azaheterocyclic compounds [1]. However, despite this growing interest in this class of drug development, efficient general multicomponent methods for the synthesis of diversely decorated 2-cyanopminopyrimidines as well as their O-bridged derivatives are still lacking, and only a limited number of successful synthetic routes has been reported. Consequently, efficient methods for the synthesis of different chemotypes of cyanoiminopyrimidines from readily available building blocks are in high demand but remain a challenging synthetic task. For this reason, this document reviews the most relevant works published in the field of multicomponent chemistry field for the assembly of said compounds (Figure 1).
Discussion
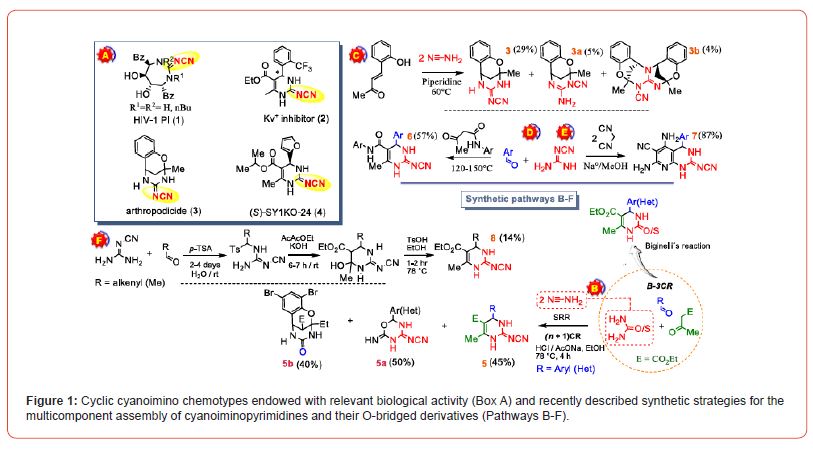
It should be noted that several chemotypes of cyclic cyanoguanidines (Figure 1, Box A) have been recognized as key ligands in diverse physiological processes, standings out as human immunodeficiency virus type 1 protease inhibitors (HIV-1 PI) and promising drug candidate 1 [2], Kv+1 channel modulator 2, arthropodicide 3 [3] and antagonists of human A2Bsub> adenosine receptor (A2BAR) 4 intended to development of novel drugs or drugs candidates to treatment of various pathologies, including prostate cancer and diabetes [4]. The most promising A2BAR antagonist based on 2-cyanoimino-3,4-dihidro-1H-pyrimidine scaffold 5 assembled by a novel multicomponent reaction design is SY1KO-24, which was initially identified as highly selective and potent nonxhantine A2BAR antagonist but is now screening as anticancer agent able to arrest key functions during tumor proliferation, metastasis, angiogenesis, immune suppression and chemoresistance [5]. Access to this novel privileged bioactive scaffold was successful achieved from the cyanamide-based Biginelli reaction by increasing the (n+1) CR chemical dimensionality, consisting of the catalyzed condensation of an (Het)aromatic aldehyde, a β-ketoester and a bifunctional d2a1 component as a surrogate of the 1,3-dinucleophile (Figure 1, pathway B) [6]. Notably, the single reactant replacement design shows the complex nature of the transformation, which depends on multiple factors. As the amount of cyanamide introduced into the reaction increases, the 4-cyanoimino-2-imino-3H-5,6-dihydro- 1,3,5-oxadiazine 5a is obtain as the only product. Conducting the reaction with salicylaldehyde leads to the formation of the hydrated product O-bridged structure, 5,6-dihydro-2H-2,6-methano- 1,3,5-benzoxadiazocin-4(3H)-one 5b. In this regard, when an functionalized Michael acceptor as (E)-4-(2-hydroxyphenyl)but- 3-en-2-one react with a excess of cyanamide under solventless condition in presence of piperidine (Figure 1, pathway C), the insecticide 4-cyanoimino-2,3,5,6-tetrahydro-2,6-methano-1,3,5- benzoxadiazocine 3 is synthesized, along with two additional O-bridged cyanocompounds 3a-b as major side products [2]. Meanwhile, 4-alkenyl derivatives of 2-cyanoiminopyrimindines 8 have been synthesized in a 3-steps sequence by reacting -tosylsubstituted N-cyanoguanidines with a β-ketoester according to the approach disclosed by Shutalev et. al. (Figure 1, Pathway F) [7]. In addition, the Shutalev group has taking advantage of this approach to synthesize 4-amino-2-cyanimino-1,5-dihydro-2H-imidazoles by the orthodox route. In this sense, 2-cyanoguanidine has also been incorporated as a key building block in a three-component reaction to assemble the framework of the azaheterocycle 6 under solventless condition at 150°C, as well as the 2-cyanoimino- 3,4-dihydro-1H-pyrido[2,3-d] pyrimidines scaffolds (Figure 1, Pathways D-E) that featuring the core structure of promising drugs for the treatment of life-threatening diseases [8,9].
Conclusion
The cyclic cyanoimine motif is considered an important precursor in organic and medicinal chemistry due to its unique characteristic of reactivity and bioactivity. Thus, the cyanoiminopyrimidine core, which exhibits subtle structural and stereoelectronic diferences from the privileged dihydropyridine(thione) scaffold, could play an essential role for the pharmacological development of new bioactive agents capable of addressing the “classical” biological target or even those “undruggable”. Notwithstanding the foregoing, the limitation of the multicomponent methods known to date to generate large libraries of azaheterociclic compounds or molecular diversity remains the main problem to be overcome in the quest for new compounds of medicinal importance that are highly enriched in nitrogen atoms.
Acknowledgements
The author thanks the research program FES-UNAM Heterocyclic Chemistry CI2221.
Conflict of Interest
The author declares no conflict of interest.
References
- Soliman AM, Mohamed SK, El-Remaily MAA, Abdel-Ghany H (2014) Synthesis of Novel Modified Guanidines: Reaction of Dicyandiamide with Amino Acids, Amides, and Amines in Aqueous Medium. J Heterocyclic Chem 51: 1322-1326.
- Jadhav PK, Woerner FJ, Lam PYS, Hodge CN, Eyermann CJ, et. al. (1998) Nonpeptide Cyclic Cyanoguanidines as HIV-1 Protease Inhibitors: Synthesis, Structure-Activity Relationships, and X-ray Crystal Structure Studies. J Med Chem 41(9): 1446-1455.
- Shkurko OP (2022) Bridged 1,3(1,5)-benzoxazocines and 1,3,5-benzoxadiazocines as products of the Hantzsch and Biginelli reactions. Chem Heterocycl Compd 58(6/7): 279-300.
- Carbajales C, Azuaje J, Oliveira A, Loza MI, Brea J, et. al. (2017) Enantiospecific Recognition at the A2B Adenosine Receptor by Alkyl 2 Cyanoimino-4-substituted-6-methyl-1,2,3,4-tetrahydropyrimidine-5-carboxylates. J Med Chem 60: 3372-3382.
- Tay AHM, Prieto-Díaz R, Neo S, Tong L, Chen X, et al. (2022) A2B adenosine receptor antagonists rescue lymphocyte activity in adenosine-producing patient-derived cancer models. J Immunother Canc. 10(5): e004592.
- Hulme R, Zamora ODP, Mota EJ, Pastén MA, Delgado F, et al. (2008) Cyanamide: a convenient building block to synthesize 4-aryl-2-cyanoimino-3,4-dihydro-1H-pyrimidine systems via a multicomponent reaction. Tetrahedron 64: 3372-3380.
- Shutalev A D (2000) Fourth International Electronic Conference Synthetic Organic Chemistry ECSO-4 2000: 1-30.
- Hussein BRM, Moustafa AH (2019) A regioselective and convenient one-pot multicomponent synthesis of polyfunctionalized 4-aryl-2-cyanoimino-3,4-dihydro-1H-pyrido[2,3-d]pyrimidines. Synth Commun 49 (18): 2401-2410.
- Gein VL, Zamaraeva TM, Dmitriev MV (2017) Synthesis and Structure of N,6-Diaryl-4-methyl-2-cyanoimino-1,2,3,6-tetrahydropyrimidine-5-carboxamides 87(2): 350-352.
-
Hulme Ríos-Guerra* and Jorge Medina-Chargoy. Advances in the Multicomponent Synthesis and Biological Relevance of Cyclic Cyanoguanidines. Insi in Chem & Biochem. 2(3): 2022. ICBC. MS.ID.000536.
-
Betulonic Acid, Sars-CoV-2 Spike, Protein-Mediated, Cell Entry, Betulinic acid, SARS-CoV-2, Pseudotype, Virus entry, Antivirals.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






