 Research Article
Research Article
A New Reaction/Rearrangement in Quaternary Ammonium Salts
G H Torosyan* and N R Hovhannisyan
Department of General Chemistry& Chemical technology, National Polytechnic University of Armenia, Armenia
Professor Gagik H Torosyan, head of the department General chemistry & Chemical technology of National polytechnic university of Armenia, Armenia.
Received Date: November 23,2022; Published Date: December 12, 2022
Abstract
There are presented newly discovered reactions in the series of quaternary ammonium salts (QUAT) in an alkaline medium or when heated at 140-145°C. It has been shown that QUAT, in which, along with a potential α, β-unsaturated group, there is an allyl type group in an aqueous alkaline medium, undergoes a rearrangement-cleavage reaction. It was established that the alkylation of trimethylphenacylammonium bromide in a two-phase catalytic system in the absence of another QUAT as a Phase transfer catalysis (PTC) catalyst was carried out with the formation of trimethylamine and chalcone. It has been suggested that the ylide is formed from the indicated QUAT, which is alkylated with benzyl chloride followed by elimination. Thermal alkylation of trimethylphenacylammonium salt with dimethylphenylbenzylammonium salt was also carried out. The α-unsaturated ketone (chalcone) and the corresponding amine fractions were isolated as reaction products. It is assumed that αC-alkylation of trimethylphenacylammonium takes place. The resulting QUAT immediately undergoes β-elimination under reaction conditions with the release of chalcone.
Keywords: Quaternary ammonium salt; rearrangement-cleavage reaction; ylid; alkylation-elimination reaction; chalcone; thermal; alkylation
Introduction
There are more than one century that quaternary ammonium salts (QUAT) have been widely studied as attractive organic compounds to study the mechanisms of molecular rearrangements [1,2]. These compounds are used in practice as surfactants, antibacterial drugs to control bacterial growth in clinical or industrial settings and are increasingly being used to treat bacterial infections as prominent class of antibacterial agents [3-5]. Recently, these substances have been offered in the treatment of Covid-19 [6].
In our group, we systematically study chemistry and ways of practical use of QUAT. So recently we have described a few QUAT-s with a wide variety of alkyl and 2-hydroxyethyl groups that have found use [2].
Materials and Methods
The progress of the reaction was followed by thin layer chromatography (TLC), which was carried out on Silufol UV-254, and traces of substances were developed in a desiccator saturated with iodine vapor (chalcone Rf 0.58, eluent benzene:diethyl ether 5:1). TLC for QUAT was carried out under the following eluent conditions: n-butanol: ethanol: acetic acid: water 10:7:3:2. Ultraviolet (UV) spectra were measured on a spectrophotometer SF-46. Infrared (IR) spectra were recorded on a Specord IR-75 spectrophotometer. NMR spectra were recorded on a Varian USA Mercury Plus 1H NMR spectrophotometer (300 MHz).
Alkylation-elimination in an aqueous alkaline medium
To the reaction flask with a system of absorbers with a titrated solution of hydrochloric acid, the equimolar amounts of QUAT and alkyl halide were stirred. Stirring and heating on a boiling water bath for 20 minutes with double molar amount of powder potassium hydroxide in the reaction mixture also. The heating and mixing continued for more than 40 minutes. Then the reaction mixture was cooled, the organic part was extracted by diethyl ether. The ether extract was dried over MgSO4. After distilling of the ether, the reaction products were isolated by distillation. Inverse titration of the contents of the absorbers determined the number of fly amines. In the aqueous layer of the reaction residue, the amount of the formed ionic halide was determined by titration. At the end by neutralization of the aqueous layer, after by evaporation to dryness and extraction with absolute alcohol the non-primary salt was isolated, From 7,74 g (0,03 mol) of trimethylphenacylammonium bromide and 3,8 g (0,03 mol) benzyl chloride and 3,4 g (0,06 mol) of KOH at 90-95oC, it had been obtained 3,2 g chalcone (53,30%), 0,2 g (0,0018 mol) benzyl alcohol, 0,73 g (0,0037 mol) dibenzyl ether and 0,89 g (0,015 mol) trimethylamine. Returned 0,6 g benzyl chloride and 3,6 g initial QUAT. The structure synthesized chalcone was proved by IR, UV, 1H NMR and Mass spectrometry.
Thermal alkylation-elimination reaction
A mixture of equimolar amounts of the initial QUAT in toluene in the presence of catalytic amounts of KOH is heated in an oil bath at 140-145°C, then diluted with water and extracted with ether. The ethereal extract was washed with titrated hydrochloric acid and dried over magnesium sulfate. The ether was distilled. Nonamine reaction products were isolated during the distillation of the rest of the ether extract. The aqueous layer was treated with potash. The resulting volatile trimethylamine during this treatment was absorbed in a system with a titrated solution of hydrochloric acid. By evaporating the aqueous layer to dryness and extracting with absolute alcohol, the unreacted part of the initial QUAT was isolated. A mixture of 10,3 g (0,04 mol) trimethylphenacylammonium bromide-1 (m.p. 191-192°C, Rf 0.52) and 9,9 g (0,04 mol) dimethylphenylbenzyl chloride-2 (m.p. 82-83°C, Rf 0,39) was heated at 140-145°C.
It received 2,5 g (30%) of chalcone m.p. 55-57oC, Rf 0,58, chalcone dimer 1,78 g (21,4%) m.p. 225-227оС, 0,73 g (14,4%) benzyl chloride, 3,2 g (66,6%) dimethylaniline, b.p. 90/91оC / 12 mm, nD20 1,5550. It was received back 6,8 g of a mixture of starting salts with a predominance of QUAT -1 (respectively Rf 0,52 for QUAT -1 and Rf 0,39 for QUAT -2). IR spectrum, ν, cm-1: 730, 770, 1585, 1600, 3700 (phenyl group conjugated with vinyl group), 1665 (C=O). The UV spectrum consists of two major absorption maxima’s, one in the range 340-390 nm, the second relatively minor in the range 200- 270 nm.
-1H NMR spectrum, δ, ppm displays two characteristic signals for αH and βH in the range 6,7-7,4 and 7,3-7,7, respectively as doublets (J =15-17 Hz). 6,38-6,67 & 6,89-6,98 (two phenyl groups).
Results & Discussion
A rearrangement-cleavage reaction of quaternary ammonium salts
It is necessary to present a long-discovered cleavage rearrangement reaction, which had a practical application for the preparation of several carbonyl compounds [7]. Thus, it has been established that in the presence of allyl-type groups in such salts, interaction with aqueous alkalis leads to the formation of carbonyl compounds with the number of carbon atoms in the molecule equal to the sum of carbon atoms in the ordinary and allyl type’s parent salt groups. The formation of a carbon-carbon bond between unsaturated groups apparently proceeds according to a six-membered cyclic mechanism as a result of a nucleophile attack by a hydroxide ion on the α-carbon atom of the α,β-unsaturated group in the enammonium intermediate. It was concluded that a rearrangement-cleavage reaction would take place with all quaternary ammonium salts in which together with the allyl-type group there is also a group which under the action of aqueous alkali becomes α,β-unsaturated. It has been established also that QUAT containing chlorine atom at the β- or γ- position with regards to nitrogen, readily undergo dehydrochlorination reactions.
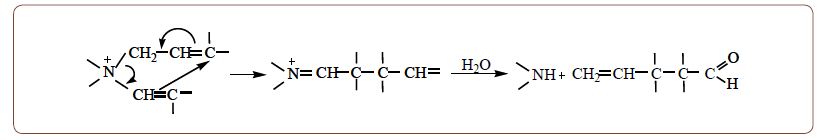
The well-known reactions of the intramolecular rearrangement of Stevens, Sommelett and Wittig in QUAT proceed through the stage of a bipolar ion formation, called by Wittig as ylid [8]. As can be seen from the above scheme of the rearrangement-cleavage reaction also proceed through the stage of a bipolar ion formation, but in this case the anion center is formed not at the α- but at the β-carbon atom, and not at the expense of a proton elimination but due to addition of the nucleophilic agent to the α-carbon atom of the α,β-unsaturated group.
We assumed what will happen to QUAT-s that has the ability to undergo ylid formation, but they do not have the ability for intramolecular rearrangement. For this purpose, we studied QUAT-s with the potential for ylid formation in basic medium / as phase transfer catalysis conditions/, however, in the absence of a path to intramolecular rearrangement, in this case with three methyl groups and in the presence of an alkylating agent in the reaction medium.
Alkylation-elimination reaction in QUAT in an aqueous alkaline medium
It has been studied the possibility of alkylation of QUAT such as trimethylphenacylammonium bromide [8]. We proceeded from the fact that such QUAT cannot undergo Hofmann elimination or rearrangements (Sommel-Hauser, Stevens) even under such milder basic conditions. Alkylation of trimethylphenacylammonium bromide was carried out in a two-phase catalytic system in the absence of another PTC as a PTC catalyst. It has been suggested that the ylide formed from said PBAT will transfer to the organic phase. Subsequently, the ylide will react with benzyl chloride in the organic layer.
It turned out that the studied QUAT is alkylated by benzyl chloride. However, after alkylation, the newly formed salt is immediately eliminated to the chalcone. This process was discovered at the end of the last century and is used in our laboratory to obtain chalcone.
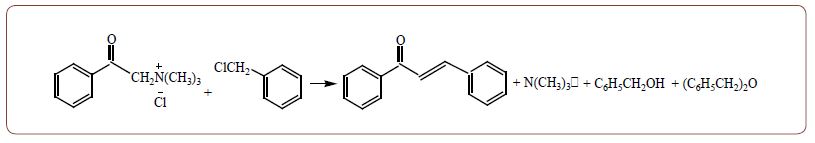
Chalcone is formed with trimethylamine and hydrolysis products of benzyl chloride are also formed in the form of benzyl alcohol and dibenzyl ether. Similar transformations also occurred in the case of a number of other salts with a trimethyl and fourth alkyl group that do not contain active β-hydrogen. Experiments show that elimination occurs quickly, so it is impossible to isolate the starting product of salt alkylation. We have been using the established process in practice for a long time.
We have named this process the alkylation-elimination reaction in QUAT. It should also be noted that our attempt to use trimethylallyl- and trimethylpropargylammonium bromides in this reaction was unsuccessful. These QUAT remain unchanged until the end of the reaction. This also indicates that the hydrogen atoms in these salts are immobile, since this property largely depends on the nature of the group adjacent to the methylene carbon. The impossibility of the formation of ylides excludes the occurrence of this reaction. These details of the mechanism underlie the validity of our assumption about the mechanism of the process found in a two-phase liquid-liquid system.
Alkylation-elimination reaction in QUAT in thermal conditions
Thermal interaction of trimethylphenacylammonium bromide - 1 with dimethylphenylbenzylammonium - 2 (technical name leukotropО) was carried out. QUAT 1 was considered as a C-H acid for possible alkylation. QUAT 2 is an active benzylating agent, in particular at high temperatures. It was assumed that in this case, benzylation of the α-carbon atom of salt 1 is possible, with the formation of a new QUAT with a higher molecular weight. However, as a result of the interaction, chalcone, a dimer of chalcone, are formed with the release of trimethylamine. Probably, α-benzylation of QUAT 1 takes place. The resulting intermediate (expected QUAT) due to the presence of β-hydrogen atoms immediately undergoes Hofmann thermal elimination with the formation of chalcone and trimethylamine. Decomposition products of QUAT 2 are also formed - dimethylaniline and benzyl chloride.
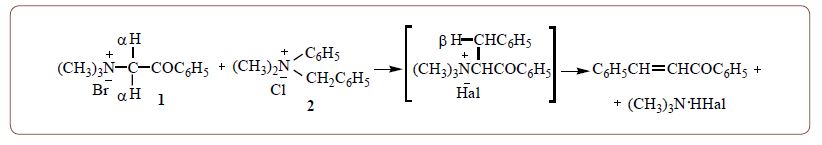
Chalcone dimer was obtained also because of interaction.
Conclusion
It has been shown that quaternary ammonium salts, in which, along with a potential α,β-unsaturated group, there is an allyl type group in an aqueous alkaline medium, undergoes a rearrangement- cleavage reaction. It turned out that QUAT containing with trimethyl and fourth alkyl group with not active β-hydrogen alkylated by benzyl chloride in an aqueous alkaline medium. However, the newly formed intermediate QUAT immediately is eliminated to forms a chalcone & trimethylamine. It has been named this process as alkylation-elimination reaction in QUAT. The possibility of thermal alkylation of a same type quaternary ammonium salt with another ammonium salt with benzyl group also take place with formation chalkone. In our opinion, an alternative method for the synthesis of chalcone was found, in contrast to the previously known ones.
Acknowledgement
None.
Conflict of interest
No conflict of interest.
References
- GH Torosyan, NR Hovhannisyan (2018) The antimicrobial and bactericidal activity of secondary amine with 2-hydroxyethyl group, MOJ Bioorganic & Organic Chemistry 2(3): 142-143.
- GH Torosyan (2018) The Selective N-Alkylation of Monoethanolamine in PTC Condition. MOJ Bioorganic & Organic Chemistry 2(1): 19-21.
- NV Shtyrlin, SV Sapozhnikov, AS Galiullina, and others (2016) Synthesis and Antibacterial Activity of Quaternary Ammonium 4-Deoxypyridoxine Derivatives, BioMed Research International Article ID 3864193.
- KPC Minbiole, MC Jennings, LE. Ator and others (2016) From antimicrobial activity to mechanism of resistance: the multifaceted role of simple quaternary ammonium compounds in bacterial eradication, Tetrahedron 72(25): 3559-3566.
- GH Torosyan (1987) The physiological active compounds 19: 46-48 (AS Ukraine, Kiev).
- M N Nadagouda, Vijayasarathy P, Sin A (2022) Antimicrobial activity of quaternary ammonium salts: structure-activity relationship, Medicinal Chemistry Research 16.
- JV Grigoryan, NR Hovhannisyan, AKh Gulnazaryan (2008) Behavior of ammonium salts containing propargyl-type groups under conditions of a rearrangement-cleavage reaction. J Org Chim Russia 78(2): 344-346.
- GH Torosyan (1987) Reaction of inter- and internal alkylation for compounds with carbonyl and hydroxyl groups, Thesis for chemical doctor degree, Yerevan, Armenia: 358.
-
G H Torosyan* and N R Hovhannisyan. A New Reaction/Rearrangement in Quaternary Ammonium Salts. Insi in Chem & Biochem. 2(3): 2022. ICBC. MS.ID.000537.
-
General Chemistry& Chemical technology, Quaternary ammonium salt; rearrangement-cleavage reaction; ylid; alkylation-elimination reaction; chalcone; thermal; alkylation.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






