 Research Article
Research Article
The UV/Visible Light Resistance and Photocatalytic Activity of Zinc Oxide Particles Doped with Metal Ions
Yen-Ling Tsai1*, Chih-Ling Shen2 and Chao-chin Su2
1Department of Cosmetic Science, Vanung University, Taoyuan 320313, Taiwan
2Institute of Organic and Polymeric Materials, National Taipei University of Technology, Taipei 106344, Taiwan
Yen-Ling Tsai, Department of Cosmetic Science, Vanung University, Taoyuan 320313, Tiwan.
Received Date:June 01, 2024; Published Date:July 02, 2024
Abstract
Zinc oxide (ZnO) exhibits good sunblock properties, but its effectiveness against UVA and visible light still needs improvement. In this study, a simple chemical precipitation method, combined with introduction of silver ion (Ag+) or ferric ion (Fe3+) was utilized to modify the structure of ZnO and evaluate its effect on the transmittance of ZnO. By adding solutions of silver nitrate/zinc hydroxide and iron sulfate/zinc hydroxide with different molar ratios and subjecting them to annealing at 200°C, then ZnO particles, Ag+-doped ZnO particles, and Fe3+-doped ZnO particles were prepared. X-ray Diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR) measurements showed structural changes in the ZnO crystalline after the addition of Ag+ and Fe3+. UV-Vis spectroscopy testing of the light transmittance of various ZnO particles revealed that the addition of metal ions significantly affected light transmittance. The introduction of Ag+ and Fe3+ indeed effectively reduced the light transmittance of ZnO particles. Both the Ag+-doped and Fe3+-doped ZnO particles exhibiting best protection against UVA, UVB, and visible light. Photocatalytic experiments using methylene blue revealed a decrease in photocatalytic activity of ZnO particles after Ag+ and Fe3+ doping.
Keywords:Zinc oxide; Silver ion; Ferric ion; Sunblock; Photocatalytic activity
Introduction
Inorganic sunscreens are known for their stability and minimal irritation to the skin, making them a popular choice for skin protection. ZnO particles have been approved by the United States Food and Drug Administration (FDA) as qualified ingredients for sunscreen formulations [1]. Ongoing research continues to affirm the safety of these inorganic compounds, both for human use and environmental impact [2]. When the sun’s rays reach the earth, approximately 95-99% of UVA and 1-5% of UVB radiation reach the surface [3]. ZnO absorbs solar radiation at wavelengths of 385nm and below, making it widely used in sunscreen products. Consequently, numerous studies have been conducted to investigate the sunblock properties of ZnO.
The chemical precipitation method is a convenient, cost-effective, reproducible, environmentally friendly, and low-temperature process commonly employed for the preparation of ZnO. By adjusting parameters such as reaction temperature, reaction time, pH value, and precursor concentration in the preparation solution, the size and morphology of ZnO can be controlled [4]. Photocatalysts have been widely applied in various aspects of daily life, such as antibacterial, anti-fouling, water purification, and air purification. The mechanism of photocatalysis is primarily based on the interaction of light with certain substances. When light shines on these substances, the electrons of atoms absorb a certain amount of energy, causing them to transition from the valence band to the conduction band. This process creates positively charged electron holes, forming electron-hole pairs that emit energy [5]. When the photoexcited electrons come into contact with O2 molecules dissolved in a water solution, they can react to generate superoxide radicals (˙O2–) [6]. Simultaneously, the holes can directly oxidize pollutants or H2O molecules to form hydroxyl radicals (˙OH). The ˙O2– and ˙OH produced in these reactions are highly reactive oxidants capable of rapidly decomposing various organic molecules, water molecules, and carbon dioxide molecules [7,8].
Experimental Methods and Details
Preparation of ZnO particles
The appropriate amount of hexahydrate zinc nitrate was added to deionized water and stir for 2 minutes. Then, add an equimolar amount of aqueous ammonia. After 24 hours of reaction, the mixture was centrifuged at a rate of 6000 rpm for 15 minutes. Remove the upper clear liquid after centrifugation and keep the precipitate, which is then placed in a high-temperature furnace at 200°C for 24 hours.
Preparation of ZnO particles doped with metal ions
An appropriate amount of zinc nitrate was added to deionized water to prepare identical solutions. After stirring the mixed solutions at room temperature for 2 minutes, add different weights of zinc nitrate and ferric sulfate separately to the previously mixed solution. Prepare solutions with concentrations of 1x10- 3 and 5x10-3 mole/L for zinc nitrate and ferric sulfate. Stir these solutions until completely dissolved, then add ammonia hydroxide in equimolar ratios to zinc nitrate. After 24 hours of reaction, the mixture is centrifuged at a rate of 6000 rpm for 15 minutes. After centrifugation and removal of the supernatant, treat it at 200°C to obtain ZnO particles.
Sunscreen testing of ZnO particles
Add 0.25g of ZnO particles to 25g of deionized water, stirring at room temperature until the ZnO suspension is uniformly dispersed. Then, add 1.25g of PVA powder into previous suspension and keep stirring to the PVA dissolved, forming a homogeneous suspension. Pour the resulting mixture into a culture dish and dry it in an oven under 60℃. After drying, remove the sample and put it to a cell for UV spectrophotometry testing in the range of 200-800nm.
Results and Discussion
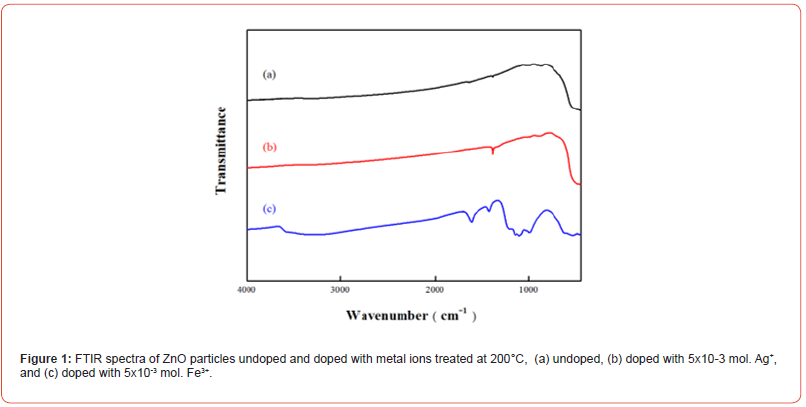
Figure 1 shows the FTIR spectra of ZnO particles undoped and doped with Ag+, Fe3+ respectively. The slight forward shift in absorption observed here is attributed to the doping of Ag+, where some silver partially replace zinc in the crystal lattice of ZnO [16, 17]. It can be observed that the Fe-doped samples exhibit significant absorption peaks at 3500 cm−1 and 1630 cm−1, indicating increased water absorbance of the particles after the addition of ferric ions. This increase in water absorbance is due to the trivalent nature of ferric ions, which results in a higher valency and larger polarity, thus enhancing hydrophilicity. The addition of ferric ions is also reflected in the spectra, with absorption observed in the range of 650 cm-1 to 1500 cm-1, providing evidence that Fe3+ indeed incorporate into the ZnO matrix upon Fe-doping [18, 19] (Figure 1).
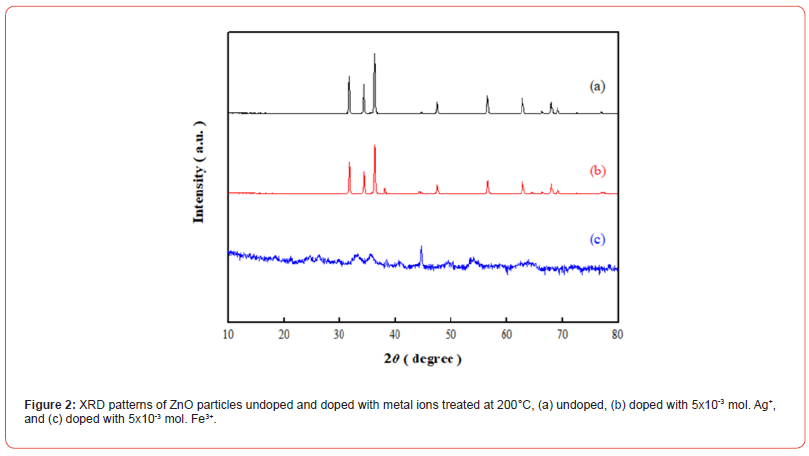
The XRD measurement results of ZnO particles, Ag-doped particles and Fe-doped particles are shown in Figure 2.The crystalline characteristic peaks of ZnO in 2θ are observed at positions 31.64°, 34.45°, 36.23°, 62.97°, 67.85° and 68.97°, corresponding to lattice planes (100), (002), (101), (103), (112), and (201). The crystalline peak positions of the ZnO particles are consistent and match the JCPDS card number (36-1451) [20]. There is a small diffraction peak appears at 2θ = 46.17 (132), matching the characteristic peak of AgO according to JCPDS No. 84-1108, indicating the presence of AgO in the crystalline structure [21]. Additionally, the characteristic peaks of ZnO in this study exhibit a broader tendency, confirming the incorporation of Ag+ into the Zn2+ lattice [22]. The characteristic peaks of ZnO show a shift towards larger 2θ values, attributed to the addition of trivalent Fe3+ ions, which have a smaller ionic radius than Zn2+, causing a reduction in lattice constant [23, 24] (Figure 2).
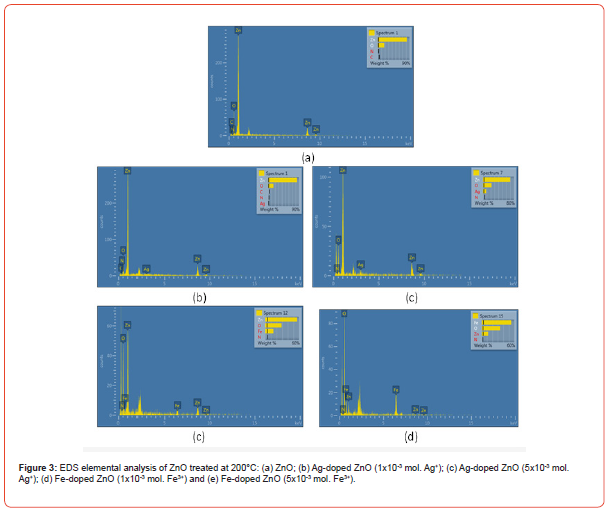
This study also analyzed the composition and content of ZnO particles through energy dispersive spectrometer (EDS) testing. As shown in Figure 3, ZnO particles prepared with different added components and ratios at 200°C exhibit EDS spectra consistent with the expected composition. It is noteworthy that in Fe-doped ZnO particles, the oxygen (O) content significantly increases. Moreover, when a higher concentration of iron is added to form ZnO particles, both the oxygen and iron content increase. Conversely, the zinc (Zn) content decreases. This is attributed to the fact that, after hightemperature 200°C annealing, the iron-containing components on the surface of Fe-doped ZnO particles are oxidized and transformed into Fe2O3 [25] (Figure 3).
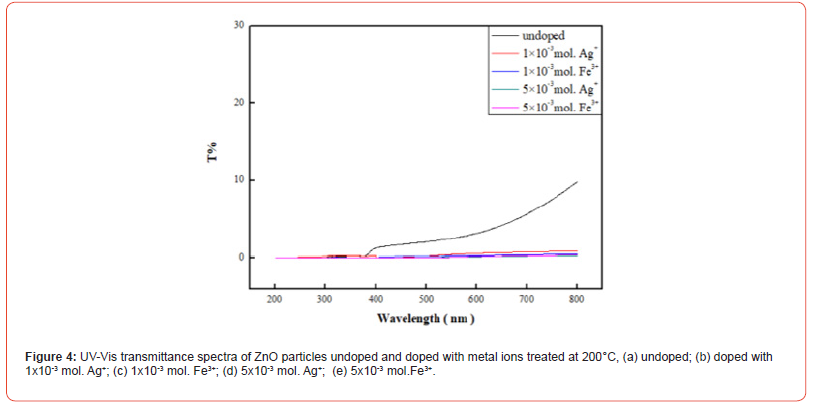
The transmittance of the prepared ZnO, Ag-doped ZnO, and Fe-doped ZnO particles to light ranging from 200 to 800 nm was tested using a UV-Vis spectrophotometer, aiming to evaluate the effects of the concentration of added Ag+ and Fe3+ ions on the particles’ resistance to UVA, UVB, and visible light. Figure 4 illustrates the optical transmittance properties of ZnO particles treated at 200 °C. It can be observed that ZnO particles undoped metal ions exhibit a light transmittance of nearly 10%. ZnO particles doped with Ag+ or Fe3+, regardless of the amount of metal ions added, show a significant reduction in light transmittance. It is speculated that the introduction of Ag+ and Fe3+ alters the crystalline structure of ZnO, thereby affecting its light transmittance (Figure4).
If the skin is exposed to an excess of oxygen and free radicals, it can accelerate skin aging. For mitigating this adverse effect, the various proportions of Ag+ and Fe3+ were incorporated to the preparation solutions to form Ag+ and Fe3+ doped ZnO powders. Ag+ and Fe3+ were used to substitute the Zn2+ in ZnO lattice, aiming to decrease the photocatalytic effect of ZnO powders. In this experiment, methylene blue was utilized for photocatalytic testing [26]. The preparation ratio of methylene blue photocatalytic test solution involved dissolving 0.1g of methylene blue powder in 1000ml of deionized water to form a concentration of 100 ppm methylene blue solution. The prepared particles were uniformly dispersed in this test solution and subjected to continuous stirring and UV light irradiation at a fixed temperature for 1 to 3 hours, followed by detection using a UV-vis spectrophotometer. The absorbance results at the peak wavelength of 660nm for various ZnO particles prepared in this study are listed in Table 1. It can be observed that the photocatalytic activity increases with the duration of UV light exposure when ZnO is not combined with metal ions. However, upon addition of metal ions, the photocatalytic activity of the photocatalyst decreases. This result indicates that the ZnO particles doped with Ag+ or Fe3+ could reduce their photocatalytic activity.
Conclusion
This study employed a simple and easily operable coprecipitation method to synthesize ZnO particles. By introducing Ag+ and Fe3+ metal ions into the ZnO particles, the crystalline structure of ZnO was altered to achieve effective shielding against UVA, UVB, and visible light radiation investigating its impact on the photocatalytic activity of ZnO particles. The results indicate that the incorporation of Ag+ and Fe3+ do indeed alter the crystalline structure of ZnO and enhance its sunblock effectiveness. And their photocatalytic activity was reduced. It’s could ameliorate the adverse effects of ZnO application in sunscreen formulations on the skin.
Acknowledgement
None.
Conflict of Interest
No Conflict of interest.
References
- Osmond MJ, McCall MJ (2010) Zinc oxide nanoparticles in modern sunscreens: an analysis of potential exposure and hazard. Nanotoxicology 4(1): 15‐
- Schneider S, Lim H (2019) A review of inorganic filters zinc oxide and titanium dioxide. Photodermatol Photoimmunol Photomed 35: 442–446.
- Antoniou C, Kosmadaki MG, Stratigos AJ, Katsambas AD (2008) Sunscreens— what’s important to know. J Eur Acad Dermatol Venereol 22(9): 1110–1119.
- Lee T, Ryu H, Lee W (2014) Fast vertical growth of ZnO nanorods using a modified chemical bath deposition. J Alloys Compd 597: 85-90.
- Qiu J, Li M., Xu J, Zhang X-F, Yao J (2020) Bismuth sulfide bridged hierarchical Bi2S3/BiOCl at ZnIn2S4 for efficient photocatalytic Cr (VI) reduction. J Hazard Mater 389: 121858.
- Wang G, Ma X, Wang C, Li S, Qiao J, Zhang H, et al. (2018) Highly efficient visible-light driven photocatalytic hydrogen evolution over Er3+: YAlO3/Ta2O5/rGO/MoSe2 nanocomposite. J Mol Liq 260(15): 375–385.
- Lee M-H, Patil U-M, Kochuveedu S-T, Lee C-S, Kim D-H (2012) The effect of SiO2 shell on the suppression of photocatalytic activity of TiO2 and ZnO nanoparticles. Bull Kor Chem Soc 33: 3767–3771.
- Siddiquey I-A, Furusawa T, Sato M, Suzuki N (2008) Microwave-assisted silica coating and photocatalytic activities of ZnO nanoparticles. Mater Res Bull 43: 3416–3424.
- Xu C, Cao L, Su G, Liu W, Qu X, Yu Y (2010) Preparation, characterization and photocatalytic activity of Co-doped ZnO powders. J Alloys Compd 497: 373–376.
- Ahmad M, Ahmed E, Ahmed W, Elhissi A, Hong Z-L, Khalid N-R (2014) Enhancing visible light responsive photocatalytic activity by decorating Mn-doped ZnO nanoparticles on graphene. Ceram Int 40: 100087–110095.
- Ahmad M, Ahmed E, Zhang Y, Khalid N-R, Xu J, Ullah M, et al. (2013) Preparation of highly efficient Al-doped ZnO photocatalyst by combustion synthesis. Curr Appl Phys 1: 697–704.
- Tsuzuki T, Smith Z, Parker A, He R, Wang X (2009) Photocatalytic activity of manganese doped ZnO nanocrystalline powders. J Aust Ceram Soc 45: 58–62.
- He R, Hocking R-K, Tsuzuki T (2012) Co-doped ZnO nanopowders: location of cobalt and reduction in photocatalytic activity. Mater Chem Phys 132: 1035–1040.
- Tsuzuki T, He R, Wang J, Sun L, Wang X, Hocking R (2012) Reduction of the photocatalytic activity of ZnO nanoparticles for UV protection applications. Int J Nanotechnol Appl 9: 1017–1029.
- Porrawatkul P, Nuengmatcha P, Kuyyogsuy A, Pimsen R, Rattanaburi P (2023) Effect of Na and Al doping on ZnO nanoparticles for potential application in sunscreens. Photochem Photobiol B: Biol 240: 112668.
- Naseer H, Iqbal T (2023) Experimental and theoretical analysis for Ag doped ZnO hotocatalyst for its novel application to improve shelf life of peach. Opt quantum electron 55(5): 471
- Kumaresan S, Vallalperuman K, Sathishkumar S (2017) A Novel one-step synthesis of Ag-doped ZnO nanoparticles for high performance photo-catalytic applications. J Mater Sci Mater Electron 28(8): 5872–5879.
- Ba-Abbad M-M, Kadhum A-A-H, Mohamad A, Takriff M-S, Sopian K (2013) The effect of process parameters on the size of ZnO nanoparticles synthesized via the sol–gel technique. J Alloys Compd 550(15): 63-70.
- Srinivasulu T, Saritha K, Reddy K-R (2017) Synthesis and characterization of Fe-doped ZnO thin flms deposited by chemical spray pyrolysis. Mod Electronic Mater 3: 76–85.
- Zarei S, Hasheminiasari M, Masoudpanah S-M, Javadpour J (2022) Photocatalytic properties of ZnO/SnO2 nanocomposite films: role of morphology. J Mater Res Technol 17: 2305-2312.
- Gauri B, Vidya K, Sharada D, Shobha W (2016) Synthesis and Characterization of Ag/AgO nanoparticles as alcohol sensor. Res J Chem Environ 20(10): 1-5.
- Laksaci H, Belhamdi B, Khelif O, Khelif A, Trari M (2023) Effect of Ag co-doped ZnO on the tartrazine photodegradation under solar irradiation, React Kinet Mech Catal 136: 1689–1704.
- Ba-Abbad M-M, Kadhum A-A-H, Mohamad A-B, Takriff M-S, Sopian K (2013) Visible light photocatalytic activity of Fe3+−doped ZnO nanoparticle prepared via sol–gel technique. Chemosphere 91(11): 1604–1611.
- Elilarassi R, Chandrasekaran G (2017) Optical, electrical and ferromagnetic studies of ZnO:Fe diluted magnetic semiconductor nanoparticles for spintronic applications. Spectrochim. Spectrochim Acta A 186: 120–131.
- Teixeira M-A-C, Piccirillo C, Tobaldi D-M, Pullar R-C, Labrincha J-A, Ferreira M-O, et al. (2017) Effect of preparation and processing conditions on UV absorbing properties of hydroxyapatite-Fe2O3 sunscreen, Mater Sci Eng C 71: 141-149.
- Dao D-V, Bremt M-V-D, Koeller Z, Le T-K (2016) Effect of metal ion doping on the optical properties and the deactivation of photocatalytic activity of ZnO nanopowder for application in sunscreens. Powder Technol 288: 366-370.
-
Yen-Ling Tsai*, Chih-Ling Shen and Chao-chin Su. The UV/Visible Light Resistance and Photocatalytic Activity of Zinc Oxide Particles Doped with Metal Ions. Insi in Chem & Biochem. 3(1): 2024. ICBC. MS.ID.000554.
-
Biochemical, Nutritional Value, Balanites, Aegyptiaca, Laloub, Seed Oil, Biochemistry, protein, Physicochemical, chloroform, benzene, diethyl.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






