 Review Article
Review Article
Recent Advancements in Photocatalysis of Zirconates: an Approach to Minimize Environmental Toxicity
Maryam Haider1, Ali Raza Kashif2*, Ali Naqi1, Muhammad Usama Younas2, Adil Usman2, Farasat Haider3 and Talal Akhtar4
1Department of Chemistry, Government College University Faisalabad, Faisalabad, Pakistan
2Department of Chemistry, Division of Science and Technology, University of Education, Lahore, Pakistan
3Graduate School of Nanoscience and Technology, Chulalongkorn University, Bangkok, Thailand
4Chemical Engineering Harbin Institute of Technology Shenzhen, China
Ali Raza Kashif, Department of Chemistry, Division of Science and Technology, University of Education, Lahore, Pakistan.
Received Date:April 06,2025; Published Date:May 26, 2025
Abstract
Photocatalysts play a crucial role in eliminating hazardous pigments and harmful environmental pollutants. Rare earth zirconates nanostructures, particularly light rare earth zirconates Ln2Zr2O7 (Ln = Eu, Nd, La, and Gd), are highly effective and promising heterogeneous photocatalysts due to their exceptional photo-reactivity. This analysis focuses on synthesizing and applying these materials for sensor purposes using liquid-based methods, followed by three consecutive operations for dehydration. Cobalt oxide is used as a p-type semiconductor, enhancing the photocatalytic activity of other semiconductors across the entire visible light spectrum (400 nm < λ < 850 nm). The determination of the optical band gap of a material depends on various parameters, including the lattice parameter, average particle size distribution, degree of crystallinity, concentration of substitution, and the type of carriers. Further investigation is necessary to advance the development of catalysts capable of effectively utilizing visible light to catalyze methane’s oxidative coupling with Ln2Zr2O7 through photocatalysis.
Keywords: Photocatalyst; Rare-Earth Oxides (REOs); Lead-free zirconate titanate (PZT); Nanocrystals; Strontium zirconium oxide
Introduction
Principle of Photocatalysis
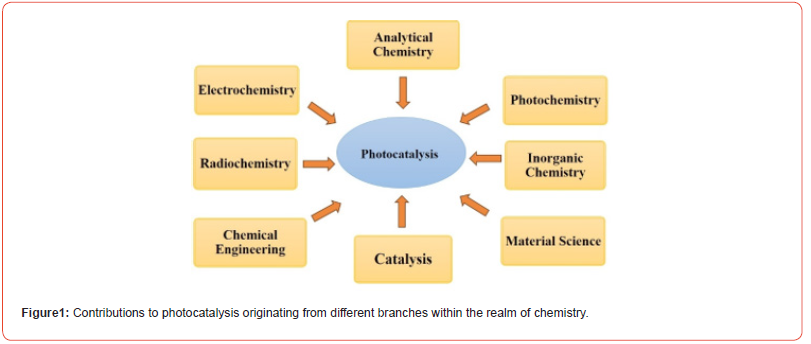
Photocatalysts are crucial for removing hazardous substances from the environment [1]. Photocatalysis and photo electrocatalysis (PEC) are gaining traction as sustainable, cost-effective methods to convert solar radiation into chemical energy. Heterogeneous photocatalysis, an innovative “Advanced Oxidation Process” (AOP) from the late 20th century, has proven highly effective in fine chemicals, green chemistry, and other emerging Advanced Oxidation Processes. Notably, it allows for specific and mild oxidation in organic fine chemistry [2]. The significance of photocatalysis as a significant research topic has grown through collaborative knowledge exchange among scientists from various disciplines (surface science, electrochemistry, radiochemistry, analytical chemistry, electronics, catalysis, material chemistry, and photochemistry), as shown in Figure 1.
Semiconductors in Photocatalysis
Thin films are typically described as materials with a two-dimensional structure and a thickness within the nanometer to micrometer range. Two-dimensional (2D) materials are characterized by the confinement of electrons in one direction and unrestricted electron mobility in the other two directions within the material [3]. Based on semiconductors, photocatalytic and photo-electrocatalytic (PEC) systems have been created to facilitate solar-powered reactions for various purposes. These include generating H2 through water splitting, converting CO2 into low-carbon fuels, fixing N2 into NH3, and decomposing organic pollutants in wastewater [4]. Photocatalysts play an essential role in separating contaminants using solar energy, making photocatalysis a reasonable option [5].
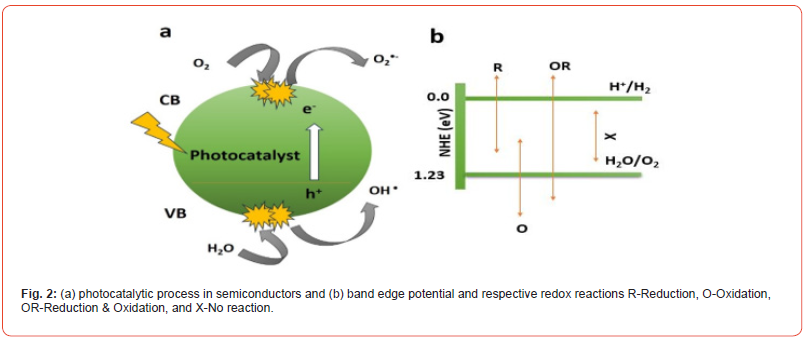
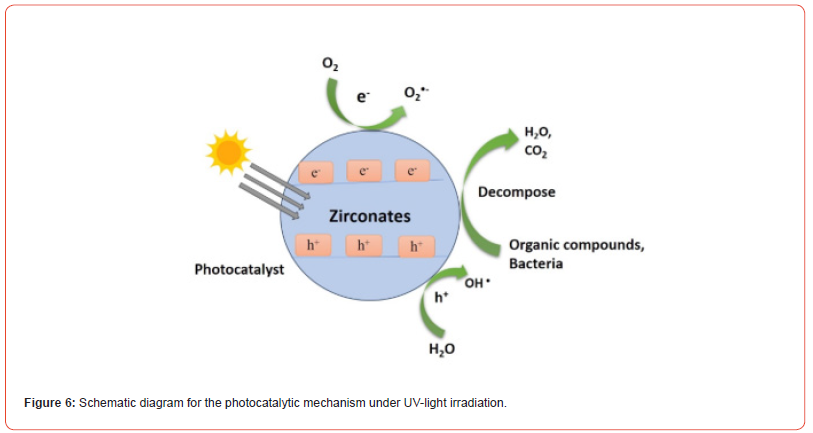
To address environmental issues, nanomaterials have become a key player, mainly by using conductor-photocatalysis. This technique effectively addresses ecological pollution, presenting a greener and more justifiable tactic. It quickly oxidizes organic compounds such as dyes by binding solar energy. The excitation of electrons occurs from the valence band to the conduction band when exposed to semiconductor photocatalysis during the photocatalytic process, leaving holes behind in the valence band. The electron and hole engaged in oxidation and reduction reactions with water molecules after excitations, corresponding to the creation of superoxide, hydroxyl radicals, and anions [6]. The resulting radicals contribute to the degradation and related processes collectively called the photocatalytic process, as shown in Figure 2 (a, b).
The photocatalytic process for pollutant degradation, water splitting, and CO2 conversion into CH4 example is given below [3].

Biomass as Photocatalysts Support
Biomass is usually chosen as a catalyst sustenance because of the abundance of carbon and silica in its waste components. These molecules can be extracted using physical, chemical, or a combination of both processes, including carbonization, pyrolysis, gasification, and hydrolysis procedures. Using biomass as a supporting material is advantageous compared to metallic materials because of its exceptional chemical stability, cost-effectiveness, and environmentally benign nature. Preparing a biomass-supported photocatalyst is crucial for achieving a superior performance and quality catalyst. The commonly employed preparation methods for incorporating the catalyst into the supports include a sol-gel method, ultrasound sonication, thermal polycondensation, solvothermal method, and hydrolysis [7].
Zirconates as Photocatalysts
Rare earth zirconates nanostructures, recognized for their remarkable photo-reactivity, serve as effective heterogeneous photocatalysts. The electrical conductivity of Ln2Zr2O7 nanostructures emphasizes the form and impact of size on rare-earth-based compound features and applications [8]. Various zirconate compounds, such as Al2ZrO5, Ln2Zr2O7, and CoZrO3, act as catalysts and photocatalysts [9]. Zirconium oxides containing rare earth pyrochlore (Ln2Zr2O7, rare earth) exhibit isomorphism, while perovskite-type substances display a luminescence band attributed to crystal structure flaws or faults creating intermediate states within the band gap [10]. Complex oxides with the composition A2Zr2O7, where A represents trivalent rare earth elements, are of great interest due to exceptional thermal stability, radiation stability, high melting point, ionic solid conductivity, notable thermal expansion coefficient, low thermal conductivity, chemical resistance, and favorable sintering rate. The research indicates nearly identical thermal conductivities among all rare-earth zirconates. Doping or co-doping with oxides decreases heat conductivity by creating defect clusters [11]. Perovskites such as calcium zirconate (CaZrO3), strontium zirconium oxide (SrZrO3), and barium zirconate (BaZrO3) display a range of features, such as ferroelectricity, piezoelectricity, and superconductivity. Calcium zirconate (CaZrO3 or CZO) demonstrates notable features such as photoluminescence, catalysis, and dielectric properties in hydrogen sensors, luminescence hosts, capacitors, and catalysts [12]. The substance SrZrO3, with ferroelectric properties and a wide band gap, is used in photocatalysis [13]. Three transition phases exhibit SrZrO3:

Asymmetrical cubic perovskite structure (belonging to the Pm3m space group) characterized by a substance barium zirconate (BZO), under the effect of exposure to Ultraviolet light (UV), the splitting of water molecules takes place by effective photocatalytic capabilities 1s5.
A comprehensive study focused on the photocatalytic ability of BaZrO3 in CO2 reduction under Ultraviolet light (UV), noticing the shortage of previous research in this area. Several analytical methods, such as X-ray diffraction, Transmission Electron Microscopy, Brunauer-Emmett-Teller, surface area measurement, and Ultraviolet light visible diffuse reflectance spectra, were used to study the BaZrO3 sample prepared through the Pechini method [7]. The investigation conducted a detailed assessment of BaZrO3 photocat alytic effectiveness in reducing CO2 under Ultraviolet light employing various cocatalysts [16]. To improve the effectiveness of magnesium zirconate (MgZrO3) in the process of photocatalysis under solar light and to promote the separation of charge carriers, a small quantity of cerium (Ce) can be placed into its structure (Mg1−xCex- ZryO3). The investigation is being obtained on metal/sulfide nanomaterials for their ability to effectively degrade the organic dye through the photocatalysis technique to degrade the organic dye through the photocatalysis technique. These signs of their excellent quality include large surface area, harmlessness, quantum confinement effect, and chemical resilience. Perovskites AZrO3 (A = Ca, Sr, and Ba) display ferroelectric, piezoelectric, and superconducting characteristics [17]. Lanthanum zirconate (Ln2Zr2O7) falls under the classification of pyrochlore oxides A2B2O7, where A and B denote rare-earth elements and tetravalent cations, respectively [18].
Properties of Zirconates
Zirconia acts as a photocatalyst and is classified as an n-type semiconductor because of its negative conduction band potential (-1.0 V), stability, and moderate band energy (5 eV). Furthermore, by causing surface changes and band structure flexibility, doping zirconia with transition metal ions improves its photocatalytic ability. However, stability, low catalytic effectiveness, and inefficient use of visible light have limited the application of zirconium and zirconia-based catalysts [6]. Due to its innocuous chemical nature, increased oxidative potential, high corrosion resistance, and reduced manufacturing, zirconium oxide (ZrO3) has attracted interest. Improved electrical, thermomechanical, catalytic, and optical qualities are only a few of the exceptional attributes that high-entropy ceramics have demonstrated [19]. Because of its simplicity and efficiency, combining bacterial suppression with photocatalytic oxidative destruction of organic pollutants has proven to be a popular strategy. The structural flexibility and simplicity of zirconium- based oxides make them a prominent class of dielectric materials [20]. Incorporating an efficient electron acceptor function like an electron sink and limiting the recombination effect is another method for creating an efficient photocatalyst. The localized half-filled f orbitals of a few rare earth elements such as La, Ce, Gd, and Sm serve as electron trap centers and lower the rate at which charge carriers recombine. This is linked to higher photocatalytic activity caused by the dopant ions occupancy of the metal oxide lattice, resulting in various structural defects [21]. Barium zirconate (BaZrO3 or BZO) is an electrolyte in hydrogen pumps, solid oxide fuel cell devices, and sensors. They are doped by trivalent cations, for example, In3+ ,Y3+ ,Gd3+ , Yb3+ , etc. The most commonly employed approach to increase the proton conductivity of the BZO material. Yttrium-doped BZO exhibits improved mechanical stability and chemical and superior proton conductivity [6]. As a result, studies are conducted using unlike doping concentrations and manufacturing processes to create better performance doped BZO material. Moreover, achieving sinter ability in doped BZO remains a significant challenge for large-scale production despite its potential as an oxide material. The doped BZO was mixed with several sintering aids like copper oxide or cupric oxide (CuO), Nickel oxide (NiO), and Zinc Oxide (ZnO) to reduce the necessary sintering temperature. Among the intriguing proton-conducting oxides, BZO is a desirable material for electrolytes. Techniques, including using dopants, sintering aids, and synthesis procedures, are essential to improving the material production process of BZO and proton transport characteristics. BaZrO3 doped with Y (BaZr1-xYxO3-) has good stability and high proton conductivity at high temperatures. The experimental photoluminescence emission is schematically labeled using the band diagram (Figure 3a) and energy level diagram (Figure 3 b) as guides. Figure 3 a. Displays the photoluminescence emission spectra between 400 and 500 nm (violet-blue region) when excited at 350 nm. In the violet region, an intense broad band is detected at around 420 nm - 422 nm (2.95 eV) for all the samples with the peak centered. This emission occurs when the carrier transitions from the conduction band edge to the valence band in BaZrO3 due to the electronic band structure, which includes Ba(5d), Zr(4d), and O(2p) states. Therefore, a hybridization effect or modest covalent bonding contribution is anticipated in the BZO crystal, as shown in other A2+B4+O32– perovskites. It calculates the density of states near the bottom of the conduction band and the top of the valence band. The wide band in the violet-blue area is caused by shallow flaws in the band gap, leading to the formation of electron traps. The localized levels are dispersed, allowing different energies to activate the trapped electrons. Blue light with a wavelength of around 480 nm is produced by recombining electrons and holes. This occurs when an excited electron in the conduction band loses energy and moves to energy levels in the valence band inhabited by localized defect levels.
According to reports, silver zirconate (Ag2ZrO3) is a potential photocatalyst because of its non-toxicity, thermal stability, and capacity to separate photoexcitation. However, Ag2ZrO3 aggregation may reduce its exposure to visible light, leading to low photocatalytic effectiveness. Thus, creating a hybrid heterojunction based on Ag2ZrO3 on a 2D-supported sheet may be a more effective way to raise Ag2ZrO3 photocatalytic effectiveness [22]. Silver-based photocatalysts possess potent antibacterial properties, exhibit robust photocatalytic efficiency, and demonstrate significant sensitivity to light [21]. In this study, we introduce an eco-friendly and straightforward Sono-Chemical method to synthesize Nd2Zr2O7 nanostructures and nanocomposites utilizing a unique capping agent derived from Morus nigra extract. Nd2Zr2O7-based ceramic nanostructure substance efficiency has remained associated for the first time with NOx reduction. Different methods were employed to measure the purity and to study the properties of Nd2Zr2O7-based nanostructures prepared using Morus nigra extract. Results showed that nanostructures of Nd2Zr2O7 and nanocomposites were efficiently synthesized by using Morus Nigra extract and the Sono-Chemical technique. We can prepare every nanostructured sample using a 60 W ultrasonic probe (18 KHz). The created Nd2Zr2O7-based ceramic nanostructure materials Nd2Zr2O7 - ZrO2 had a 70% conversion of NOx to N2 and can also be used like prospective nano catalysts with adequate performance for propane Selective catalytic reduction (SCR-NOx) [23]. Hagiwara studied the connection between oxide ion conductivity and crystal structures in Nd2Zr2O7 [8].
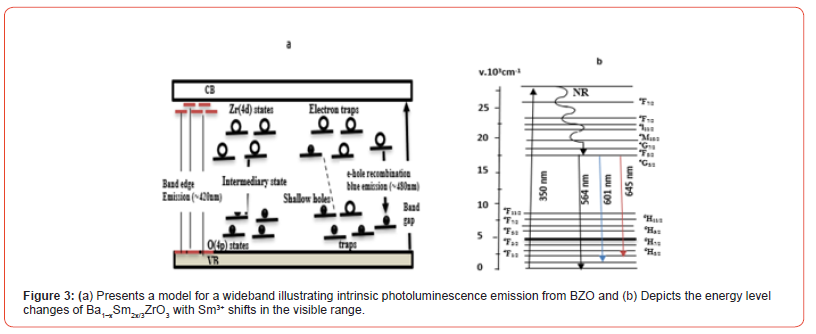
Due to its high curie points and exceptional thermal stability, Ln2Zr2O7 possesses high-temperature piezoelectric characteristics. Adding nickel ions to pure Ln2Zr2O7 oxide increases optical characteristics and photoluminescence, which can be used as photoactive materials to remove dangerous chemicals from environments [18]. Among the intrinsic difficulties of eight rare earth oxides (R2O3, R = Er, Yb, Eu, Sm, Gd, Y, Ce, and Dy) are high energy consumption and phase transformation in the multi-step hydrophobic coating fabrication, thermal coating, development of aromatic contaminants in the magnetic entropy, catalysis, shift in refrigeration and photo cor rosion [24]. The current effort involves the design and synthesis of high-entropy rare-earth zirconates (Yb0.2Nd0.2Sm0.2Eu0.2Gd0.2)2 Zr2O7 and (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7. At 1600ºC, both high-entropy ceramics have excellent phase stability and a single pyrochlore structure. Furthermore, the system includes a high coefficient of thermal expansion of 10.52 × 10− 6 K-1, RT~1500ºC, and Yb has a low thermal conductivity of 1.003 W⋅m-1 K-1, 1500ºC. It also exhibits superior sintering resistance [25]. Up to 1400ºC, the single orthorhombic phase of CaZrO3 fibers remained unchanged. CaZrO3 or CZO fibrous membrane also shows outstanding alkaline resistance. Rendering it a desirable option for high-temperature applications, the CaZrO3 fibrous membrane has exceptional heat stability and flexibility. CaZrO3 or CZO fiber membranes are viable for elevated temperature uses in gas filtration, catalyst support, and thermal insulation due to their exceptional flexibility, alkaline resistance, and thermal stability [17].
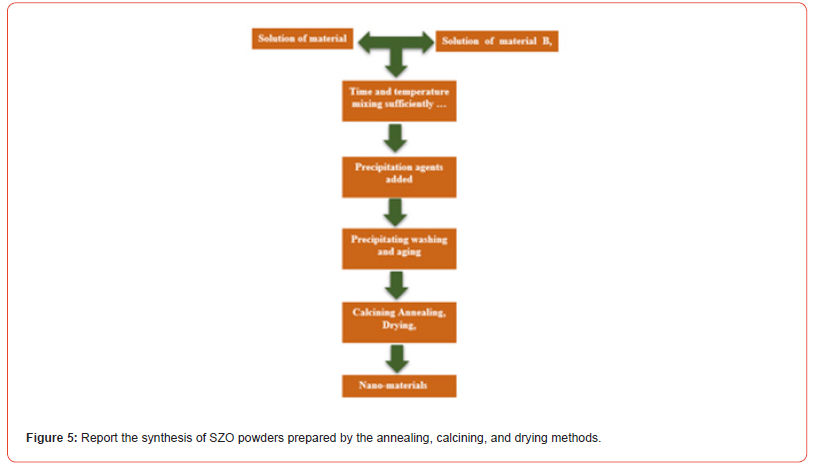
Synthesis of Zirconates Photocatalysis
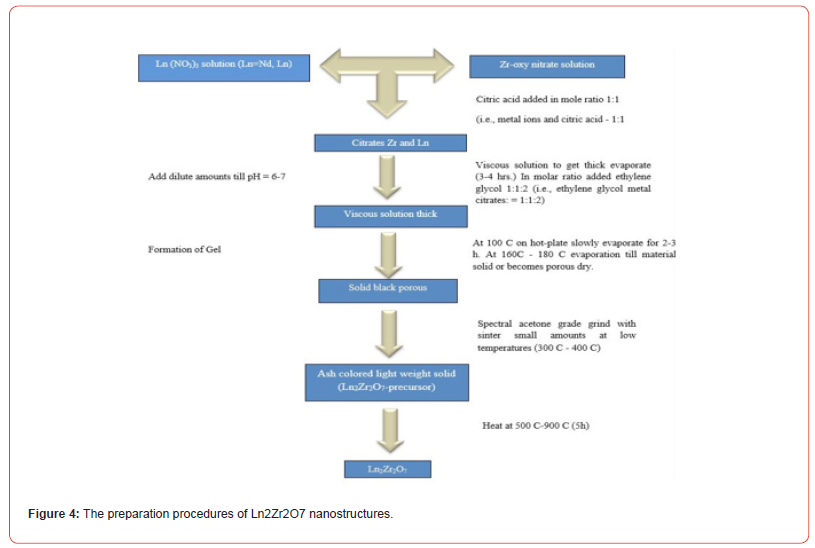
Ln2Zr2O7 zirconates light rare earth synthesis and sensor application of earth (where Gd, Eu, Sm, Nd, La = Ln) are analyzed. After being manufactured via a liquid approach, the materials underwent three calcination operations to dry. The composition and microstructure were verified using XRD and SEM studies. The electrical study was conducted at varying frequencies under various humidity conditions to identify rare earth zirconate compositions that would be good candidates for sensor applications. When light rare earth zirconates were used as active components in humidity sensors, high sensitivities, quick reaction times, and recovery times were observed, particularly for zirconate materials containing elements Ln, based on the right side of light rare earth [26]. Recent research has examined the rare earth zirconates of type Ln2Zr2O7, such as Gd2Zr2O7, Dy2Zr2O7, Nd2Zr2O7, and La2Zr2O7. The findings demonstrate the importance of ceramic materials in coatings of thermal barriers. The conductivity of electrical electrolytes of the oxide influenced various parameters, including the concentration of the oxygen vacancies, structure like a crystal, and radius ionic of doped elements. The electrical conductivity increases in the fluorite phase range as the ionic radius ratio increases. Due to their high curie points and exceptional thermal stability, Ln2Zr2O7 possesses high-temperature piezoelectric characteristics. Adding nickel ions to pure Ln2Zr2O7 oxide increases optical characteristics and photoluminescence, which can be used as photoactive materials to remove dangerous chemicals from environments [18]. A notable benefit of the hydrothermal approach that can produce powders with tiny particle sizes without requiring the calcination stage is homogeneous nucleation processes. However, because of the sluggish reaction rate, it isn’t easy to prepare Ln2Zr2O7 solely using hydrothermal. To speed up a solid reaction and the creation of nanostructures, zirconate rare earth, a mild stage of heating at low temperature is required following the hydrothermal stage [8]. Re2Zr2O7, where Re is a rare earth, is a pyrochlore type of rare earth zirconium oxide that can be made via co-precipitation, ceramics, inorganic sol-gel, hydrothermal, and hydrazine processes. Re2Zr2O7 was prepared utilizing the solid-state approach, which involved synthesizing La2Zr2O7 and Yb2Zr2O7 powders using high-purity raw ZrO2 and Re2O3 powders [27]. This study reports on manufacturing bulk and nano-sized pyrochlore-type rare earth oxides (La2Zr2O7 and Nd2Zr2O7) by sol-gel technique employing an organic gelatin reagent at significantly lower temperatures [10]. It is possible to make mixed metal oxides of lanthanides (ln) and tin (Sn) [28]. The Eu2Zr2O7 and La2Zr2O7 were found to have the highest and lowest conductivities, respectively. The rare-earth-doped ZrO2 system Eu- 2Zr2O7 has the lowest activation energy and the highest electrical conductivity.
Wang et al. (2022) used a hydrothermally assisted technique that involves two stages: hydrothermal and calcination, to synthesize La2Zr2O7 nanoparticles with pyrochlore structure. They used C19H42BrN (CTAB), zirconium nitrate, lanthanum (III) nitrate, and NaOH solution as beginning ingredients. The steps involved in creating La2Zr2O7 nanoparticles are replicated. The form of the synthesized La2Zr2O7 nanoparticles was cubic. Initially, SrZrO3 (SZO) powder was made by a traditional reaction in solid-state between ZrO2 and SrCO3 for 48 hours and at 1473 K while intermittently grinding [32]. Numerous issues, including coarser powder impurity contamination and inhomogeneity distribution with the nonuniform size, arise from the SZO powders generated using this approach. Wet chemical techniques like co-precipitation [16], hydrothermal processes [21], and sol-gel [8] are used to create powders SZO with appropriate chemical, stoichiometry, and physical properties to reduce these kinds of issues. The following steps in pyrolysis of the zirconyl oxalate strontium precursor can be used to produce SZO fine powders:
(a) oxalate, (b) dehydration breakdown, (c) carbonate decomposition into zirconate strontium. Hendekhale & Mohammad-Khah (2020) discovered that during oxalate heat treatment at low temperatures in the air, 1173 K trapped carbon dioxide in a solid. Oxalate strontium zirconyl precursor production is somewhat complex due to heterogeneous equilibria [27]. Several techniques have also been used to maximize the oxalate precursor pathway using additional external solvents [14]. Reverse co-precipitation was used in the current study to create rare-earth high-entropy zirconates (Yb0.2Sm0.2Eu0.2Nd0.2)2 Co2ZrO5 (YbHZ) or (La0.2Sm0.2Gd0.2Nd0.2Eu0.2)2 Co2ZrO5 (LaHZ) [25].
Characterizations and Synthesis of BaZr1-xCexO3-x/2 Nano- Ceramics
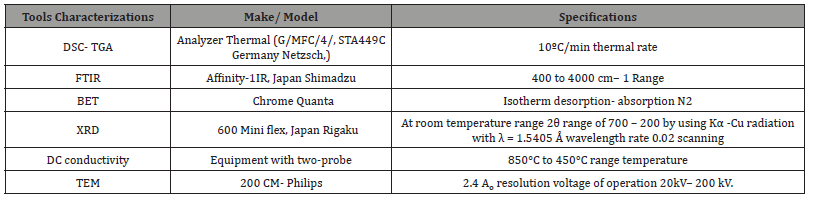
Nano-ceramics BaZr1-xCexO3-x/2 were made utilizing sol-gel, the green approach auto ignition technique [33]. This method has benefits like a synthesis of green, i.e., processing low temperatures, no toxic gases, distribution of homogenous reactants, and ultrafine particle construction. The present work characterization of the employed technique is summarized in Table 2 [33]. The current work used electrospinning and sol-gel techniques to form a CaZrO 3 fibrous membrane with good flexibility at 1100°C and excellent stability at 1200°C. Electrospinning is also used to successfully create fibrous membranes made of calcium zirconate (CZO). Li et al. (2016) alkaline earth cerates and zirconates such as strontium zirconium oxide (SrZrO3), strontium cerium oxide (SrCeO3), and barium cerium oxide (BaCeO3) have high protonic conductivity in a hydrogen gas atmosphere at elevated temperatures. Barium zirconates (BaZrO3 or BZO) and barium-cerates (BaCeO3) with trivalent cation substitutions are being researched as promising proton conductors. Trivalent dopant cations like ytterbium ion (Yb3+) and yttrium ion (Y3+) are crucial in creating oxide ion vacancies that produce protons in a hydrogen gas setting. Four primary categories of perovskite structured oxides, including zirconate-based proton conductors, single-doped zirconate, and hybrid-doped zirconate– cerate-based proton conductors, have exceptional high proton conductivity.
Table 1:Describes the photocatalytic performances of the La2Zr2O7 materials.
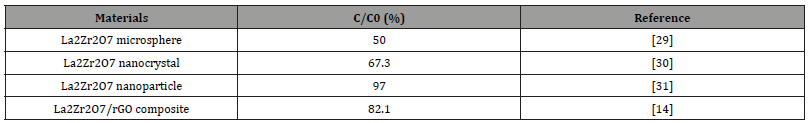
Table 2:Characterization of Employed Techniques.
Problems for SOFCs are fascinating for proton-conducting oxide
fuel cells (H+-SOFCs), which function at the low/intermediate
range of the material’s temperatures. The primary requirement is
the electrolyte materials’ capacity to conduct the ions lacking electron
conduction—solid electrolytes of the SOFCs, in general, need
to meet the specifications listed below [35].
1. Elevated ionic conductivity and density.
2. Behavior of low electrical conductivity.
3. Stable chemically in environments with reducing and oxidizing
agents.
4. Minimal interference with electrode materials and appropriate
mechanical characteristics in harsh operating environments.
5. Exceptional heat resistance and a minimal difference in thermal
expansion compared to other cell components.
6. Sturdy, affordable, and readily accessible.
Advantages and Disadvantages of H+ - SOFC
Perovskite-structured oxides exhibit excellent and high proton conductivity. They can be categorized into four main groups: single-doped zirconate–cerate–based proton conductor, zirconate- based proton conductor, cerate-based proton conductor, and hybrid-doped zirconate–cerate–based proton conductor [35].
Advantages
1. Higher partial pressure of hydrogen and steam [36].
2. Lower concentrations are overpotential at the anode [37].
3. Low operating temperature [38].
4. High ionic conductivity [39].
5. Lower energy loss in H+ transport [40–42].
6. Transference of the H+ is lower with increased temperature
[43].
7. High Nernst potential [44].
8. Better performance by ceramic electrolyte perovskite materials
[39].
9. At the cathode, water is formed to avoid fuel dilution [45].
Disadvantages
1. The performance of SOFC is affected by the choice of electrolyte,
as each electrolyte has distinct ohmic losses as a specific
operating condition [46].
2. Higher concentrations of overpotential at the cathode and
higher density of current [47].
3. The electrolyte exhibits inadequate chemical stability when
exposed to CO2 and water vapor [48,49].
4. The electrolyte’s sintering ability could improve, resulting in
low, dense electrolytes [50].
Photocatalytic Reactions
Water Splitting
The process of creating hydrogen through photocatalysis states that semiconductors FeS2 and zirconate are stimulated by visible light and ultraviolet, respectively, when heterostructure zirconate– FeS2 photocatalyst is exposed to simulated solar irradiation. The transfer of the electrons to their conduction band from the valance band is experienced by semiconductors. Accessible to execute the reduction of water to increase the number of electrons, electrons from FeS2 are injected into the zirconate conduction band. Water oxidation occurs in FeS2, where holes from the zirconate valence band are moved. This reduces charge recombination and boosts photocatalytic activity by achieving the redox reactions at distinct locations. Hydrogen growth rates from water splitting can be attained by the photocatalysts created in this work under simulated irradiation [51].
Degradation of Pollutants
The mechanism of photocatalytic degradation is typically the formation of corresponding charge carriers in CB and VB of the photocatalytic material, which drives photocatalytic degradation. As a result, OH and the active oxygen moieties O2 - are created through the photocatalyst’s holes, and electrons mineralize polluting molecules. While determining the photocatalytic mechanism at Ce4+/3+: MgZrO3 NCs, Electron Spin Resonance (ESR) and Radical scavenger experiments studies were considered. These studies unequivocally demonstrated that MO degradation was caused by the participation of both OH and O2-. The n-type semiconductor materials are abundantly evident from the slope data. About the Silver-Silver chloride (Ag-AgCl) electrode, the respective flat band potentials of Ce4+/3+: MgZrO3, MgZrO3, and ZrO2 are -1.23, -1.32, and − 1.71 V. The comparable values against the reversible hydrogen electrodes RHE are -0.62, -0.71, and -1.1, in that order. For n-type semiconductors, on the other hand, the data will be 0.1 V further in a negative direction since their corresponding conduction band edges can be assumed to be −0.72, −0.81, and − 1.2 V.
By replacing the band gap values determined through the Kubelka-Munk function with values of 4.18V, 3.71 V, and 1.89 V, valence band locations were calculated using this data. Degradation of amoxicillin (AMO) degrading mechanism has been postulated, depending on the findings of ESR investigations, band energy estimates, and scavenger in figure10. The picture displays the band edge positions for each pristine MgZrO3 and ZrO2. The band edge places of the composites Ce4+/3+: MgZrO3 NCs have changed due to adding cerium dopant to MgZrO3. The position of the valence band shifted to the conduction band, and the higher positive value showed a modest alteration when compared to their pristine counterparts. The realignment converts the UV-sensitive pristine resources to solar light-sensitive Ce4+/3+: MgZrO3 NCs, with a band gap of 3.09 eV. When exposed to visible light, the electrons in the composite move to the conduction band from the valence band. As a result, the holes and the electrons accumulate in VB and CB of Ce4+/3+. The photoluminescence (PL) analysis shows that increased electropositive of zirconia has reduced photo-electron recombination by the spatial rearrangement and MgZrO3 NCs material. Notably, a composite of CB energy was more favorable compared to the H2O/ηOH standard potential and more damaging than the O2/ ηO2 standard potential. As a result, the VB and CB of Ce4+/3+: MgZrO3 NCs will favorably generate ·O2 and ·OH. These highly reactive species explain the material’s improved photocatalytic activity. Table 3 compares the strength of Ce4+/3+: MgZrO3 NCs’ strength with materials documented for pollutant degradation [21].
Table 3:Comparison of the strength of Ce4+/3+: MgZrO3 NCs with materials documented for pollutant degradation.
Applications of Zirconates in Photocatalysis
Over the years, the use of perovskite compounds lead-free zirconate titanate (PZT) in electronics due to the need for lead-free substitutes for PZT, there is currently a resurgence of interest in creating calcium and barium zirconate titanate [1]. In the environmental, tribological, photoactivity, refrigeration, hydrophobic coating, hydrophobic coating, thermal barrier coating, and corrosion protection are the applications of eight Rare Earth Oxides REOs (R2O3, R = Sm, Er, Yb, Eu, Gd, Y, Ce, and Dy). Employing rare earth oxides as doping agents or nonstoichiometric chemicals is praiseworthy for improving the system performance of particular applications [24].
Hydrogen Production from Methane
Furthermore, the hydrogen used in conventional synthetic ammonia is typically obtained from reforming methane, which carries a hidden risk for greenhouse pressure and methane transportation. By contrasting various deep learning techniques, it is discovered that only nitrogen adsorption data can fully forecast the ammonia yield using a convolutional neural network based on graphs. Water photolysis is typically utilized as a hydrogen source to produce photosynthetic ammonia, which minimizes adverse environmental effects and enhances conversion safety [27].
Use in the Removal of Contaminants
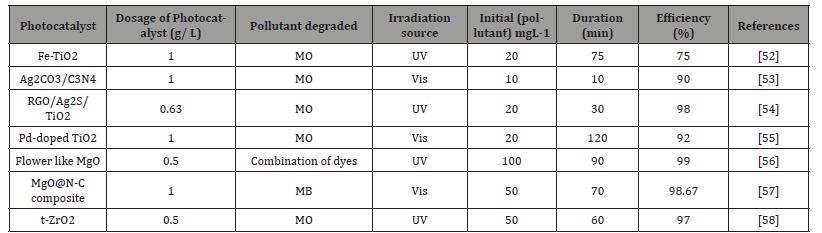
The main application for mixed-metal oxides of lanthanide (Ln) and tin (Sn) has been the removal of dyes. They have not been used to remove many contaminants, including dangerous heavy ions, highly poisonous gases, drug contamination, complex organic compounds, and hazardous and refractory pollutants [28]. Nanoscale mixed-metal oxides such as lanthanide (Ln) and tin (Sn) are used for photocatalytic destruction of unwanted pollutants. Table 4: photocatalytic degradation of undesirable contaminants utilizing nanoscale mixed-metal oxides of tin (Sn) and lanthanide (Ln).
Table 4:photocatalytic degradation of undesirable contaminants utilizing nanoscale mixed-metal oxides of tin (Sn) and lanthanide (Ln).
Table 5:
In High-Temperature Fuel Cells, Re2Zr2O7 as Solid Electrolytes
Rare earth zirconium oxides (Re2Zr2O7) of the pyrochlore type are used in high-temperature fuel cells as solid electrolytes, oxidation catalysts, fluorescence center hosts, and thermal barrier coatings. They are also used in petrol turbines and diesel engines as potential hosts for radioactive wastes and surplus actinides [6].
Used in Water Treatment
Zirconates are employed in the treatment of organic contaminants in water. CZO nanoparticles were employed in the photodegradation of organic pollutants, including methyl thorium chloride (MB) and ammonium purpurate (MR). Cobalt Zirconate (Co2ZrO5) is a new photocatalyst that exhibits photocatalytic activity under Ultraviolet-visible (UV–vis) light for the degradation of MB [10]. Calcium zirconate (CaZrO3) is used for hydrogen sensing, luminescence, multilayer capacitors, and catalytic processes [12].
For Organic Dye Photodecomposition
Zirconium oxide and zirconia photocatalysts for organic dye photodecomposition operate in visible and UV light [6]. Research has explored the application of lead zirconate titanate (PZT) and its composite ferroelectric materials in treating wastewater with organic dyes. We have reviewed the latest research publications by different scholars on PZT and its application in the photocatalytic breakdown of organic dyes. Because of its ferroelectric characteristics, PZT has essential uses in nonvolatile storage, ultrasonic sensors, actuators, and infrared detectors.
For Wastewater Treatment
Ln2Zr2O7 can be used in solid electrolytes, luminescence center host material, photocatalysts, nuclear waste storage, oxygen sensors, thermal barrier coating (TBC), and other applications. Applications for photocatalysis have been reported using Ln2Zr2O7 nanostructures. Heterogeneous photocatalysis is a noteworthy chemical catalysis used for wastewater treatment [18].
Applications of Catalysis of Rare Earth Oxides (REOs) PZO-Highly Temperature-Sensitive Quantities
Maintaining and controlling the temperature of the material is crucial for applying PZO in functional devices, as evidenced by the fact that PZO pyroelectric and electrocaloric efficiencies are both extremely sensitive to temperature [72]. Colorimetry using a smartphone has shown promise in evaluating dye degradation under piezo catalysis. The values acquired using a spectrophotometer and smartphone-based colorimetry nearly matched each other. These findings demonstrate the usefulness of smartphone-based colorimetry for catalytic research [73].
Photocatalytic Substances as a Powerful Approach for Antimicrobial Applications
Recently, photocatalytic materials have gained prominence as a significant antibacterial solution. As photocatalysis involves light interacting with semiconductors to inactivate bacteria and break down pollutants, creating surfaces coated with photocatalysts for antimicrobial purposes is increasingly gaining significance. Antimicrobial disinfection encompasses the destruction of proteins through reactive oxygen species generated by the bacteria’s photocatalytic activity, the impact of generated metal ions on proteins, and the harm to bacterial cell membranes resulting from the interaction between nanomaterials and bacteria. Semiconductor nanoparticles have been produced recently for antibacterial applications and photocatalytic destruction of organic contaminants. Because of their small particle size, ease of manufacture, affordability, Ultraviolet protection, antimicrobial activity, photocatalytic self-cleaning, stability, and hydrophobicity, widely utilized diverse photocatalytic antimicrobial nanomaterials have gained much attention [74].
Hydrogen Gas Generation
Nowadays, functional nanostructures are used in nano-catalysis to produce hydrogen. Due to their unique qualities for this application, two zirconium-based dielectric oxides, BaZrO3 and SrZrO3, have garnered a lot of attention in wireless communication devices, pyroelectric and piezoelectric sensors, heterogeneous catalysts, multilayer ceramic capacitors (MLCC), and refractories [20]. Water splitting technology can produce hydrogen with the best energy resources, delivering electricity with sustainable energy. Electrocatalysis and photocatalysis are the methods most commonly employed to accomplish renewable energy-driven water splitting. Most hydrogen is currently produced by thermal processes such as gasification, reforming, and thermochemical techniques. BaZrO3 or BZO electrolyte material is used in electrochemical hydrogen devices, including solid oxide fuel cells (SOFCs), protonic ceramic fuel cells (PCFCs), hydrogen sensors, and pumps. The central section of the review provides an overview of recent studies looking into these applications. It offers a thorough understanding of how different aspects of the doping, sintering aid, synthesis process, and environments affect operating conditions, the morphology, performance, and composition of BaZrO3 or BZO electrolyte materials and composition [75].
In particular, current research on doped eight R2O3 inorganic
materials (R = Sm, Eu, Ce, Dy, Gd, Er, Yb, and Y) has shown remarkable
results in the following types, summarized in this review article
by M. K. Hossain et al. (2022).
a. Refrigeration
b. Catalysis
c. Environmental
d. Thermal barrier coating
e. Corrosion protection
f. Tribological
g. Hydrophobic coating
h. Photoactivity
Conclusion
A thorough investigation into incredibly infrequent earth zirconates has revealed many synthesis techniques and varied uses in a wide range of scientific and technological fields. Thanks to synthesis techniques, which include liquid approaches, sol-gel, hydrothermal, and reverse coprecipitation, researchers now have a wide range of compositions and architectures. These materials can be used in a variety of applications. Research has been done on the electrical characteristics of rare earth zirconates, which highlights their potential for use in humidity sensors, among other sensing applications. The work emphasizes how light rare earth zirconates, especially those with lanthanide elements on the right side of the rare earth series, exhibit high sensitivities and quick response and recovery times.
The study explores the use of zirconates, including their function as high-temperature fuel cells when employed as solid electrolytes, the generation of hydrogen from methane, and the elimination of impurities by photocatalytic processes in various applications. Further applications of zirconate adaptability include water splitting, coating to improve functionality in multiple industries, photodecomposition of organic dyes, and water treatment. Although these applications have great potential, obstacles remain, including the need to create flexible ceramic fiber membranes, comprehend the chemical compatibility of the electrolytes used in electrochemical cells, and resolve restrictions related to specific nanoparticles. The goal of ongoing research is to overcome these obstacles and develop the field of zirconates even further.
Future zirconate developments are expected to significantly impact environmentally sustainable chemistry and energy production, particularly when combined with other materials. The wide range of applications highlights zirconates’ versatile potential and establishes them as significant materials for use in various scientific and technological fields. Zirconates are positioned to have an environmental technology as long as they continue their journey of discovery and invention.
Contribution of Authors
Maryam Haider: writing-original draft; visualization, investigation, validation, software, Aki Raza Kashif: visualization, investigation, Ali Naqi: formal analysis, Muhammad Usama Younas: writing-original, draft methodology, investigation, formal analysis, data curation, conceptualization, supervision, Farasat Haider: validation, investigation, Talal Akthar: validation.
Funding Information
This research was not funded by any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare no conflict of interest.
References
- J Munir, MK Iftikhar, MI Jamil, MU Din, T Alshahrani, et al. (2023) Physical properties of elastically and thermodynamically stable magnetic AcXO3 (X= Cr, Fe) perovskite oxides: a DFT investigation. Phys Scr 98: 65513.
- JM Herrmann (2010) Fundamentals and misconceptions in photocatalysis. J Photochem Photobiol A Chem 216: 85-93.
- R Shwetharani, HR Chandan, M Sakar, GR Balakrishna, KR Reddy, et al. (2020) Photocatalytic semiconductor thin films for hydrogen production and environmental applications. Int J Hydrogen Energy 45: 18289-18308.
- S Zhong, Y Xi, S Wu, Q Liu, L Zhao, et al. (2020) Hybrid cocatalysts in semiconductor-based photocatalysis and photoelectrocatalysis. J Mater Chem A 8: 14863-14894.
- WS Koe, JW Lee, WC Chong, YL Pang, LC Sim (2020) An overview of photocatalytic degradation: photocatalysts, mechanisms, and development of photocatalytic membrane. Environ Sci Pollut Res 27: 2522-2565.
- RP Singh, SK Warkhade, RS Das, GS Gaikwad, S Prasad, et al. (2022) Cationic surfactant controlled synthesis of Ag2ZrO3 and Ag/Ag2ZrO3: An efficient visible light active photocatalysts, Inorg. Chem. Commun. 145: 110035.
- BT Son, NV Long, NTN Hang (2021) The development of biomass-derived carbon-based photocatalysts for the visible-light-driven photodegradation of pollutants: a comprehensive review. RSC Adv 11: 30574-30596.
- S Zinatloo-Ajabshir, M Salavati-Niasari, A Sobhani, Z Zinatloo-Ajabshir (2018) Rare earth zirconate nanostructures: recent development on preparation and photocatalytic applications. J Alloys Compd 767: 1164-1185.
- NR Hendekhale, A Mohammad-Khah (2020) A novel synthesis of Co2ZrO5 and m-ZrO2 nanoparticles by sono-precipitation and hydrothermal methods and their application in UV/Visible-photocatalytic studies. J Environ Chem Eng 8: 104065.
- KK Rao, T Banu, M Vithal, G Swamy, KR Kumar (2002) Preparation and characterization of bulk and nano particles of La2Zr2O7 and Nd2Zr2O7 by sol–gel method. Mater Lett 54: 205-210.
- D Liu, F Zhou, L Qu, Y Wang, B Xu, et al. (2022) A novel porous La2Zr2O7 ceramic aerogels with ultralow thermal conductivity and photocatalytic property. J Am Ceram Soc 105: 2772-2782.
- M Rashid, RB Behram, F Aziz, A Mahmood, NA Kattan, et al. (2020) Optoelectronic pressure dependent study of alkaline earth based zirconates AZrO 3 (A= Ca, Ba, Sr) using ab-initio calculations. Eur Phys J B 93: 1-9.
- U Farooq, F Naz, R Phul, NA Pandit, SK Jain, et al. (2020) Development of heterostructured ferroelectric SrZrO3/CdS photocatalysts with enhanced surface area and photocatalytic activity. J Nanosci Nanotechnol 20: 3770-3779.
- LS Cavalcante, AZ Simoes, JC Sczancoski, VM Longo, R Erlo, et al. (2007) SrZrO3 powders obtained by chemical method: synthesis, characterization and optical absorption behaviour. Solid State Sci 9: 1020-1027.
- A Satapathy, SK Dash, SK Rout, S Parida (2023) Barium zirconate—A simple perovskite with multidimensional applications, in: Perovskite Met. Oxides Elsevier pp. 231-252.
- X Chen, J Wang, C Huang, S Zhang, H Zhang, et al. (2015) Barium zirconate: a new photocatalyst for converting CO 2 into hydrocarbons under UV irradiation. Catal Sci Technol 5: 1758-1763.
- K Yuan, H Li, X Jin, C Li, X Wang, et al. (2022) Electrospun flexible calcium zirconate fiber membrane with excellent thermal stability and alkali resistance. Ceram Int 48: 12408-12414.
- F Alharthi, R Wahab, S Manoharadas, BF Alrayes, N Ahmad (2021) Effect of Preparation Method and Ni2+ Substitution on the Structural, Thermal, and Optical Properties of Nanocrystalline Lanthanum Zirconate Pyrochlore. Crystals 11: 1463.
- Y Wang, YJ Jin, T Wei, ZG Wang, G Cao, et al. (2022) Size disorder: A descriptor for predicting the single-or dual-phase formation in multi-component rare earth zirconates. J Alloys Compd 918: 165636.
- M Ubaidullah, M Fazil, T Ahmad (2022) Short review on fabrication, structural and dielectric characterization of zirconium based oxide nanoparticles. Mater Sci Eng 6: 152-156.
- KS Prashanth, MS Raghu, FA Alharthi, S Sreenivasa, VSA Devi, et al. (2021) Solar light sensitive hybrid Ce4+/3+ doped perovskite magnesium zirconate nano cubes for photocatalytic hydrogen evolution and organic pollutant degradation in water. J Environ Chem Eng 9: 105364.
- RS Das, SK Warkhade, A Kumar, GS Gaikwad, AV Wankhade (2020) Graphitic carbon nitride@ silver zirconate nanocomposite (gC3N4@ Ag2ZrO3): a Type-II heterojunction for an effective visible light photocatalysis and bacterial photo-inactivation. J Alloys Compd 846: 155770.
- S Zinatloo-Ajabshir, N Ghasemian, M Mousavi-Kamazani, M Salavati-Niasari (2021) Effect of zirconia on improving NOx reduction efficiency of Nd2Zr2O7 nanostructure fabricated by a new, facile and green sonochemical approach. Ultrason. Sonochem 71: 105376.
- MK Hossain, MHK Rubel, MA Akbar, MH Ahmed, N Haque, et al. (2022) A review on recent applications and future prospects of rare earth oxides in corrosion and thermal barrier coatings, catalysts, tribological, and environmental sectors. Ceram Int 48: 32588-32612.
- X Luo, L Luo, X Zhao, H Cai, S Duan, et al. (2022) Single-phase rare-earth high-entropy zirconates with superior thermal and mechanical properties. J Eur Ceram Soc 42: 2391-2399.
- I Petrila, K Popa, F Tudorache (2016) Microstructure, electrical and humidity sensing properties of light rare earths zirconates. Sensors Actuators A Phys 247: 156-161.
- Y Zhou, X Wang, X Huang, H Deng, Y Hu, et al. (2022) Predicting the photosynthetic ammonia on nanoporous cobalt zirconate via graph convolutional neural networks. Mol Catal 529: 112565.
- S Zinatloo-Ajabshir, M Mousavi-Kamazani (2021) Recent advances in nanostructured Sn− Ln mixed-metal oxides as sunlight-activated nanophotocatalyst for high-efficient removal of environmental pollutants. Ceram Int 47: 23702-23724.
- C Liu, J Zhang, S Deng, P Wang, Y He (2016) Direct preparation of La2Zr2O7 microspheres by cathode plasma electrolysis. J Colloid Interface Sci 474: 146-150.
- Y Tong, J Zhu, L Lu, X Wang, X Yang (2008) Preparation and characterization of Ln2Zr2O7 (Ln= La and Nd) nanocrystals and their photocatalytic properties. J Alloys Compd 465: 280-284.
- BS Surendra, R Gagan, GN Soundarya (2020) Thermal barrier and photocatalytic properties of La2Zr2O7 NPs synthesized by a Neem extract assisted combustion method. Appl Surf Sci Adv 1: 100017.
- AM Ferrari-Lima, AC Ueda, EA Bergamo, RG Marques, EA V Ferri, et al. (2017) Perovskite-type titanate zirconate as photocatalyst for textile wastewater treatment. Environ Sci Pollut Res 24: 12529-12537.
- PP Khirade, A V Raut, RC Alange, WS Barde, AR Chavan (2021) Structural, electrical and dielectric investigations of cerium doped barium zirconate (BaZrO3) nano-ceramics produced via green synthesis: Probable candidate for solid oxide fuel cells and microwave applications. Phys B Condens Matter 613: 412948.
- K Li, B Peng, T Peng (2016) Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. Acs Catal 6: 7485-7527.
- NLRM Rashid, AA Samat, AA Jais, MR Somalu, A Muchtar, et al. (2019) Review on zirconate-cerate-based electrolytes for proton-conducting solid oxide fuel cell. Ceram Int 45: 6605-6615.
- AK Demin, PE Tsiakaras, VA Sobyanin, SY Hramova (2002) Thermodynamic analysis of a methane fed SOFC system based on a protonic conductor. Solid State Ionics 152: 555-560.
- S Hossain, AM Abdalla, SNB Jamain, JH Zaini, AK Azad (2017) A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew Sustain Energy Rev 79: 750-764.
- E Fabbri, L Bi, H Tanaka, D Pergolesi, E Traversa (2011) Chemically stable Pr and Y co‐doped barium zirconate electrolytes with high proton conductivity for intermediate‐temperature solid oxide fuel cells. Adv Funct Mater 21: 158-166.
- Z Tao, L Yan, J Qiao, B Wang, L Zhang, et al. (2015) A review of advanced proton-conducting materials for hydrogen separation. Prog Mater Sci 74: 1–50.
- A Demin, P Tsiakaras (2001) Thermodynamic analysis of a hydrogen fed solid oxide fuel cell based on a proton conductor. Int J Hydrogen Energy 26: 1103-1108.
- S Assabumrungrat, W Sangtongkitcharoen, N Laosiripojana, A Arpornwichanop, S Charojrochkul, et al. (2005) Effects of electrolyte type and flow pattern on performance of methanol-fuelled solid oxide fuel cells. J Power Sources 148: 18-23.
- W Jamsak, S Assabumrungrat, PL Douglas, N Laosiripojana, R Suwanwarangkul, et al. (2007) Performance of ethanol-fuelled solid oxide fuel cells: Proton and oxygen ion conductors. Chem Eng J 133: 187-194.
- B Singh, S Ghosh, S Aich, B Roy (2017) Low temperature solid oxide electrolytes (LT-SOE): A review. J Power Sources 339: 103-135.
- M Ni, MKH Leung, DYC Leung (2007) Mathematical modelling of proton‐conducting solid oxide fuel cells and comparison with oxygen‐ion‐conducting counterpart. Fuel Cells 7: 269-278.
- SAM Ali, M Anwar, AM Abdalla, MR Somalu, A. Muchtar (2017) Ce0. 80Sm0. 10Ba0. 05Er0. 05O2-δ multi-doped ceria electrolyte for intermediate temperature solid oxide fuel cells. Ceram Int 43: 1265-1271.
- MN Khan, CD Savaniu, AK Azad, P Hing, JTS Irvine (2017) Wet chemical synthesis and characterisation of Ba0. 5Sr0. 5Ce0. 6Zr0. 2Gd0. 1Y0. 1O3− δ proton conductor. Solid State Ionics 303: 52-57.
- F Zhao, A V Virkar (2005) Dependence of polarization in anode-supported solid oxide fuel cells on various cell parameters. J Power Sources 141: 79-95.
- Y Guo, Y Lin, R Ran, Z Shao (2009) Zirconium doping effect on the performance of proton-conducting BaZryCe0. 8− yY0. 2O3− δ (0.0≤ y≤ 0.8) for fuel cell applications. J Power Sources 193: 400-407.
- N Zakowsky, S Williamson, JTS Irvine (2005) Elaboration of CO2 tolerance limits of BaCe0. 9Y0. 1O3–δ electrolytes for fuel cells and other applications. Solid State Ionics 176: 3019-3026.
- MD Gonçalves, PS Maram, A Navrotsky, R Muccillo (2016) Effect of synthesis atmosphere on the proton conductivity of Y-doped barium zirconate solid electrolytes. Ceram Int 42: 13689-13696.
- AM Huerta-Flores, JM Mora-Hernández, LM Torres-Martínez, E Moctezuma, D Sánchez-Martínez, et al. (2018) Extended visible light harvesting and boosted charge carrier dynamics in heterostructured zirconate–FeS 2 photocatalysts for efficient solar water splitting. J Mater Sci Mater Electron 29: 18957-18970.
- T Tianzhong, Z Jinlong, T Baozhu, C Feng, H Dannong (2008) Preparation of Fe {sup 3+}-doped TiO {sub 2} catalysts by controlled hydrolysis of titanium alkoxide and study on their photocatalytic activity for methyl orange degradation, J. Hazard. Mater. 155.
- J Chen, J Zhong, J Li, S Huang, W Hu, et al. (2017) Synthesis and characterization of novel Ag2CO3/g-C3N4 composite photocatalysts with excellent solar photocatalytic activity and mechanism insight. Mol Catal 435: 91-98.
- X Liu, Z Wang, Y Wu, Z Liang, Y Guo, et al. (2019) Integrating the Z-scheme heterojunction into a novel Ag2O@ rGO@ reduced TiO2 photocatalyst: Broadened light absorption and accelerated charge separation co-mediated highly efficient UV/visible/NIR light photocatalysis. J Colloid Interface Sci 538: 689-698.
- CH Nguyen, CC Fu, RS Juang (2018) Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J Clean Prod 202: 413-427.
- Y Zheng, L Cao, G Xing, Z Bai, J Huang, et al. (2019) Microscale flower-like magnesium oxide for highly efficient photocatalytic degradation of organic dyes in aqueous solution. RSC Adv 9: 7338-7348.
- X Zheng, K Wang, Z Huang, Y Liu, J Wen, et al. (2019) MgO nanosheets with N-doped carbon coating for the efficient visible-light photocatalysis. J Ind Eng Chem 76: 288-295.
- CV Reddy, B Babu, IN Reddy, J Shim (2018) Synthesis and characterization of pure tetragonal ZrO2 nanoparticles with enhanced photocatalytic activity. Ceram Int 44: 6940-6948.
- AM Al-Hamdi, M Sillanpää, J Dutta (2015) Gadolinium doped tin dioxide nanoparticles: an efficient visible light active photocatalyst. J Rare Earths 33: 1275-1283.
- LP Singh, MN Luwang, SK Srivastava (2014) Luminescence and photocatalytic studies of Sm 3+ ion doped SnO 2 nanoparticles. New J Chem 38: 115-121.
- EL Foletto, S Battiston, GC Collazzo, MM Bassaco, MA Mazutti (2012) Degradation of leather dye using CeO 2–SnO 2 nanocomposite as photocatalyst under sunlight. Water Air Soil Pollut 223: 5773-5779.
- AM Al-Hamdi, M Sillanpää, J Dutta (2014) Photocatalytic degradation of phenol in aqueous solution by rare earth-doped SnO 2 nanoparticles. J Mater Sci 49: 5151-5159.
- WU Shide, LI Chao, W Wei, W Huanxin, Z Youqi, et al. (2010) Synthesis and photocatalytic property of Ce-doped SnO2. J Rare Earths 28: 168-170.
- AM Al-Hamdi, M Sillanpää, J Dutta (2016) Intermediate formation during photodegradation of phenol using lanthanum doped tin dioxide nanoparticles. Res Chem Intermed 42: 3055-3069.
- K Li, H Wang, H Yan (2006) Hydrothermal preparation and photocatalytic properties of Y2Sn2O7 nanocrystals. J Mol Catal A Chem 249: 65-70.
- J Zeng, H Wang, Y Zhang, MK Zhu, H Yan (2007) Hydrothermal synthesis and photocatalytic properties of pyrochlore La2Sn2O7 nanocubes. J Phys Chem C 111: 11879-11887.
- MA Farrukh, I Muneer, KM Butt, S Batool, N Fakhar (2016) Effect of dielectric constant of solvents on the particle size and bandgap of La/SnO2‐TiO2 nanoparticles and their catalytic properties. J Chinese Chem Soc 63: 952-959.
- HS Arif, G Murtaza, H Hanif, HS Ali, M Yaseen, et al. (2017) Effect of La on structural and photocatalytic activity of SnO2 nanoparticles under UV irradiation. J Environ Chem Eng 5: 3844-3851.
- MS Morassaei, S Zinatloo-Ajabshir, M Salavati-Niasari (2016) Simple salt-assisted combustion synthesis of Nd 2 Sn 2 O 7–SnO 2 nanocomposites with different amino acids as fuel: an efficient photocatalyst for the degradation of methyl orange dye. J Mater Sci Mater Electron 27: 11698-11706.
- W Fan, J Hu, J Huang, X Wu, S Lin, et al. (2015) Electronic structure and photocatalytic activities of (Bi2− δYδ) Sn2O7 solid solution. Appl Surf Sci 357: 2364-2371.
- MS Morassaei, S Zinatloo-Ajabshir, M Salavati-Niasari (2016) New facile synthesis, structural and photocatalytic studies of NdOCl-Nd2Sn2O7-SnO2 nanocomposites. J Mol Liq 220: 902-909.
- K Aryana, JA Tomko, R Gao, ER Hoglund, T Mimura, et al. (2022) Observation of solid-state bidirectional thermal conductivity switching in antiferroelectric lead zirconate (PbZrO3). Nat Commun 13: 1573.
- VP Singh, A Susaniya, SC Jain, R Vaish (2021) Characterization of the piezoelectric lead zirconate titanate catalyzed degradation of rhodamine B and methylene blue dyes by smartphone-based colorimetry. Instrum Sci Technol 50: 95-104.
- K Atacan, N Güy, M Özacar (2022) Recent advances in photocatalytic coatings for antimicrobial surfaces. Curr Opin Chem Eng 36: 100777.
- MK Hossain, R Chanda, A El-Denglawey, T Emrose, MT Rahman, et al. (2021) Recent progress in barium zirconate proton conductors for electrochemical hydrogen device applications: A review. Ceram Int 47: 23725-23748.
-
Maryam Haider, Ali Raza Kashif*, Ali Naqi, Muhammad Usama Younas, Adil Usman, etc.all. Recent Advancements in Photocatalysis of Zirconates: an Approach to Minimize Environmental Toxicity. Insi in Chem & Biochem. 3(2): 2022. ICBC. MS.ID.000560.
-
Biochemical, Nutritional Value, Balanites, Aegyptiaca, Laloub, Seed Oil, Biochemistry, protein, Physicochemical, chloroform, benzene, diethyl.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






