 Research Article
Research Article
Ganoderic Acids in Ganoderma Lucidum-Mediated PD-1 Reduction and Immunoregulation
Gan Wang1*, Fatima Chihn1, Zhenhao Li2, Jianlong Zhou2 and Xiaoxin S Xu1
1Institute of Environmental Health Sciences, Wayne State University, China
2Shouxiangu Pharmaceutical LLC., Wuyi, Zhejiang Province, P. R. China
Gan Wang, Institute of Environmental Health Sciences, Wayne State University, 540 E Canfield Road, Detroit, MI 48201, China.
Received Date:January 18, 2024; Published Date:January 26, 2024
Abstract
Ganoderma lucidum (G. lucidum), also called Lingzhi (China) or Reishi, (Japan and Korea) is a medicinal fungus that has been used in many Asian countries for centuries to treat various illness and to promote healthy life. Enhancing immune response is one of the well-known benefits of G. lucidum consumption. The results of our previous study demonstrated that treatment of cultured human B lymphocytes with extract prepared from spores of G. lucidum (GLE) caused a significant reduction of PD-1 protein in the cultured B lymphocytes. Many bioactive compounds, including polysaccharides and triterpenoids, are contained in both fruit body and spores of G. lucidum. In this work, we investigated the role of purified ganoderic acids, including ganoderic acid A (GAA), ganoderic acid B (GAB), ganoderic acid D (GAD), and ganoderic acid F (GAF), in reducing PD- 1 protein in cultured cell systems. Using both GM02248 human B-lymphocytes and Jurkat human T-lymphoma cells, the results of our immunoblotting studies demonstrated that treatment with these individual ganoderic acids caused significant reductions of PD-1 protein in both GM02248 and Jurkat cells. The results of our immunofluorescence (IF)-based microscopy study further confirmed the effects of individual ganoderic acids in reducing PD-1 protein in Jurkat cells. The results of our cell proliferation studies revealed that both GM02248 and Jurkat cells were well tolerant to these individual ganoderic acids and no significant change in the rate of cell proliferation was observed in these cells with the presence of individual ganoderic acids at concentrations as high as 100M. In addition, the results of our real time PCR studies indicated that treatment with these individual ganoderic acids did not cause significant decrease in transcription of the pdcd-1 gene in either GM02248 or Jurkat cells. These results suggest that ganoderic acids play a key role in G. lucidum-mediated PD-1 protein reduction. Considering the important function of PD-1 protein in modulating immune response and in treating many diseases, especially in cancer treatment, these results suggest that ganoderic acids could be developed as novel immunomodulating drugs for treatment of cancer and many other diseases.
Introduction
Ganoderma lucidum (G. lucidum), also known as Lingzhi or Reishi, is a medicinal fungus that has been used for centuries in China and other Asian countries (e.g., Korea and Japan) to treat various illness, including chronic bronchitis, inflammation, hyperlipidemia, hypertension, neurasthenia, hepatitis, and leukopenia [1-7]. G. lucidum has also been used to improve health and to promote longevity [2,4,7,8]. One of the well-known benefits of G. lucidum consumption is its ability to improve immune response [9,10]. The underlying mechanism, however, is not fully understood. Determining the molecular mechanism of G. lucidum-mediated immune modulation, and identifying its responsible compounds, therefore, would be important in understanding the G. lucidum-mediated immune response and developing G. lucidum-based therapeutics for treatment of many disease conditions.
Many important bioactive compounds are contained in the fruit body and spores of G. lucidum [1-7]. Triterpenoids and polysaccharides are two of the most important pharmacological compounds carried in G. lucidum. There are over 150 different triterpenoid compounds identified in G. lucidum [1,4,7]. Recent studies suggest that triterpenoids of G. lucidum possess activities against cancer cell proliferation/metastasis [11-15] whereas the polysaccharides of G. lucidum function as immunomodulators [16-19]. The results of our previous studies demonstrated that extract prepared from the spores of G. lucidum (GLE) caused significant reduction of PD-1 protein, an important immunomodulator that plays a critical role in immune response [20-24] and in cancer treatment [25-32], in cultured human B-lymphocytes [33]. In this work, we studied the role of purified triterpenoids in G. lucidum-mediated PD-1 protein reduction. Using purified ganoderic acids, including ganoderic acid A (GAA), ganoderic acid B (GAB), ganoderic acid D (GAD), and ganoderic acid F (GAF), the results of our immunoblotting studies demonstrated that treatments of GM02248 human B-lymphocytes and Jurkat human T-lymphoma cells with these ganoderic acids caused significant reduction of the PD-1 protein in these cells. The results of our immunofluorescence (IF) microscopy studies confirmed the effects of purified GAF on reducing PD-1 protein in Jurkat cells. The results of our cell toxicity studies revealed that both GM02248 and Jurkat cells were well tolerant to the presence of purified ganoderic acids, with no significant difference in the rate of cell proliferation was observed when these cells were treated with individual ganoderic acids at concentrations as high as 100 μM. The results of our reverse transcription-based qPCR (real time PCR) study indicated that the presence of purified ganoderic acids (100 μM) did not inhibit transcription of the pdcd-1 gene in these cells, suggesting that a transcriptional inhibition of PD-1 gene was not a mechanism for these ganoderic acids-mediated PD-1 protein reductions. Taking together, these results strongly suggest that ganoderic acids play a key role in G. lucidum-mediated PD-1 protein reduction and ganoderic acids could be developed as novel immunomodulating drugs for treatment of cancer and many other immune-related diseases via targeting the PD-1 protein of immune cells.
Materials and Methods
Cells, chemicals, and oligonucleotides used in this study. The GM02248 human B- lymphocytes were purchased from the Coriell Institute for Medical Research (Camden, NJ). The Jurkat human T-lymphoma cell line was purchased from American Type Culture Collection (ATCC) (Manassas, VA). Both GM02248 and Jurkat cells were maintained in RPMI1640 medium supplemented with 10mM HEPES (pH7.6) and 10% fetal bovine serum (FBS) at 37oC with 5% CO2. The phorbol 12-myristate 13-acetate (PMA) was purchased from Sigma Aldrich Inc. (St. Louis, MO). The ionomycin (Io) was purchased from Cayman Chemical Company (Ann Arbor, MI). The ganoderic acid A (GAA) and ganoderic acid D (GAD) were purchased from Sigma Aldrich Inc. The ganoderic acid B (GAB) and ganoderic acid F (GAF) were purchased from the Cayman Chemical Company. The PMA was prepared as 10mg/ml stock in dimethyl sulfoxide (DMSO) and stored at the -80oC freezer. The Io was purchased as 10mM ethanol stock and stored at the -80oC freezer. The individual ganoderic acids were prepared as 100mM stock in 95% ethanol and stored at the -80oC freezer. The oligonucleotides used in this study were synthesized by Midland Certified Reagent Company (Midland, TX) and the sequences of these oligonucleotides were listed in Table 1.
Table 1:Primers Used in the Real Time PCR Study.
Cell proliferation assay
Both GM02248 and Jurkat cells were diluted in the cell growth medium to a density of ~5x105 cells/ml in T-25 flasks. Some cells were treated with individual ganoderic acids at indicated concentrations (0, 10 μM, 50μM, or 100 μM) by adding the ganoderic acid stock solution directly into the cultured cells. All cells were cultured in cell culture incubator at 37oC with 5% CO2. The cell density was determined for each treatment at the time points 0 hr, 24 hrs, 48 hrs, 72 hrs, and 96 hrs following the ganoderic acid treatment using an Invitrogen’s Countess automated cell counter. The cell growth curve was established for both GM02248 and Jurkat cells using the cell density data.
PD-1 induction and ganoderic acid treatment
Both cultured GM02248 and Jurkat cells were treated with PMA (50ng/ml) and Ionomycin (1 μM) for 24 hours to induce expression of PD- 1 protein. Some of the PD-1 inducing cells were then treated with individual ganoderic acid (100 μM) for 48 hours. Both untreated and ganoderic acid-treated cells were harvested. Some of the harvested cells were lysed in RIPA cell lysis buffer and the cell lysates were used in immunoblotting assay to determine the levels of desired target proteins. The remaining cells were used for isolation of total RNA using a RNeasy Mini Kit (Qiagen Inc. Valencia, CA). The RNA samples were used in a reverse transcription-based qPCR (real time PCR) assay to determine the mRNA levels of desired target genes.
Immunoblotting assay
Cell lysates prepared from both untreated and ganoderic acidtreated GM02248 and Jurkat cells were used in immunoblotting assays to determine the levels of desired target proteins in each cell lysate. The immunoblotting assay was performed using SDSPAGE with 10% gel and 15 μg total protein/lane was used. After transferring the proteins to polyvinylidene difluoride (PVDF) membranes, the membranes were first hybridized with a primary antibody that targeted individual target proteins and then hybridized with an ECL- conjugated second antibody that targeted the primary antibody. The primary antibodies used in this study included goat anti-human PD-1 antibody (AF1086, R &D System), mouse anti-human CD4 antibody (MT-310, Santa Cruz Biotechnology), mouse anti- human CDK-7 antibody (C-4, Santa Cruz Biotechnology), mouse anti-human CCL5 (RANTES) antibody (C-12, Santa Cruz Biotechnology), mouse anti-human XPC antibody (D-10, Santa Cruz Biotechnology), mouse anti- human μ -Actin antibody (C-2, Santa Cruz Biotechnology), and mouse anti-human GAPDH antibody (6C5, Santa Cruz Biotechnology). The protein level of μ -Actin was used as a protein loading control of GM02248 cells and the protein level of GAPDH was used as protein loading control of Jurkat cells. Quantification of the protein band was conducted using ImageJ software (https://imagej.nih.gov/ij/).
Immunofluorescence (IF) microscopy assay
For IF microscopy study, both untreated and GAF-treated Jurkat human T-lymphoma cells were fixed in 1xPBS containing 4% formaldehyde for 15 minutes at room temperature. After incubating the cells in a blocking buffer (1X PBS, 5% normal serum, 0.3% Triton X-100) for 30 minutes at room temperature to block any unspecific binding, the Jurkat cells were then incubated in flow cytometry staining buffer (1xPBS, 1% bovine serum albumin, and 0.1% sodium azide) containing APC-labeled mouse anti-human PD-1 antibody (eBioJ105, Invitrogen) for 1 hour at room temperature. The nuclei of the Jurkat cells were counterstained with a DAPI reagent. The PD-1+ cells were visualized by a Zeiss LSM 780 Fluorescence microscope using red light (630nm), and the nuclei of cells were visualized using blue light (480nm). A total of 100-250 cells were counted for each sample, and the percentage of PD-1+ cells were determined for each treatment.
Reverse transcription-based qPCR (Real-time PCR) assay
Total RNA isolated from both untreated and treated GM02248 and Jurkat cells were used in the real-time PCR assay. A reverse transcription reaction was used to generate cDNA from the RNA samples using a High- Capacity cDNA Reverse transcription Kit (Applied Biosystems Inc, Foster City, CA). The cDNAs were then used as templates in a qPCR protocol to determine the mRNA levels of several genes, including pdcd-1, CCL5, IL-6, and TNF, using a Power Sybr Green PCR Master Mix (Applied Biosystems Inc.) with primers designed to bind to the coding region sequences of these genes. The mRNA level of β-actin gene was also determined from each RNA sample and used as an internal control for the real-time PCR study. The mRNA level of β-actin gene in the untreated GM02248 and Jurkat cells was used as a standard, and the levels of pdcd-1, CCL5, IL-6, and TNF mRNAs in each RNA sample were calculated as fold change to that of the untreated GM02248 and Jurkat cells, respectively. Triplicates were used for each sample in the real-time PCR study, and 3 independent experiments were conducted for the real-time PCR study. The mRNA level of each target gene was calculated as mean fold + standard deviation (S.D.) to that of the GM02248 or Jurkat cells. Statistical analysis was used to determine if any significant difference in the level of mRNA exists for tested gene between untreated and treated cells for GM02248 and Jurkat cells (*p ≤0.05; **p ≤ 0.01).
Data Analysis
All data were presented as mean + SD. Statistically significant differences were determined by a GraphPad Prism software (La Jolla, CA) using a student’s t test with 95% confidence interval (CI). The data were obtained from at least 3 independent experiments.
Results
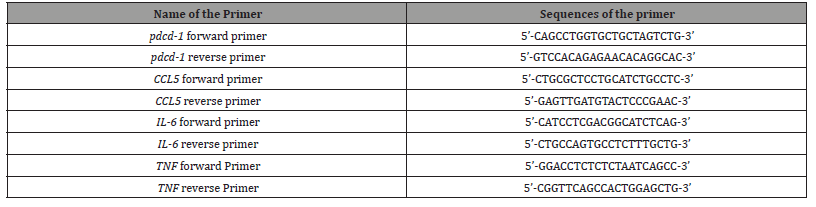
The effects of ganoderic acids on GM02248 and Jurkat cell proliferation
To study the role of ganoderic acids in G. lucidum-mediated PD-1 protein reduction, we first determined the effects of purified ganoderic acids on cell proliferation. Both GM02248 and Jurkat cells were treated with individual ganoderic acids at indicated concentrations (0, 10 μM, 50 μM, and 100 μM) by adding the ganoderic acid stock solution directly into the cell growth medium. The cells were cultured in the cell culture incubator at 37oC with 5% CO2 supplement. The cell density was determined for both GM02248 and Jurkat cells in each treatment 24 hrs, 48 hrs, 72hrs, and 96 hrs after initiation of the ganoderic acid treatment. The cell growth curve was established for both GM02248 and Jurkat cells at each concentration using the cell density data (Figure 1). The results of our cell proliferation studies revealed that both GM02248 and Jurkat cells were well-tolerance to these purified individual ganoderic acids and no significant difference in cell proliferation was observed with the presence of individual ganoderic acids at concentrations as high as 100 μM (Figure 1).
The effects of ganoderic acids on reducing PD-1 protein in GM02248 and Jurkat cells
The results of our recent study have demonstrated the effect of extract prepared from spores of G. lucidum (GLE) on reducing PD-1 protein in the cultured human B-lymphocytes [33]. The GLE used in that study was prepared as an ethanol extract, which contained many different bioactive compounds of G. lucidum. To identify the compounds that are responsible for the GLE-mediated PD-1 protein reduction, we further determined the effects of ganoderic acids on reducing PD-1 protein in the cultured GM02248 and Jurkat cells using purified ganoderic acid A (GAA), ganoderic acid B (GAB), ganoderic acid D (GAD), and ganoderic acid F (GAF). Both GM02248 and Jurkat cells were first treated with PMA/ionomycin to induce expression of PD-1 protein. The PD-1 expressing cells were then treated with individual ganoderic acids at 100 μ M for 48 hours. Both untreated and ganoderic acid-treated cells were harvested and the cell lysates were analyzed by immunoblotting assay to determine the protein level of PD-1 in each cell lysate (Figure 2). In addition, the protein levels of several other proteins, including CCL5, CD4, CPK7, XPC, GAPDH, and μ -Actin were also determined from the GM02248 or Jurkat cell lysates (Figure 2).
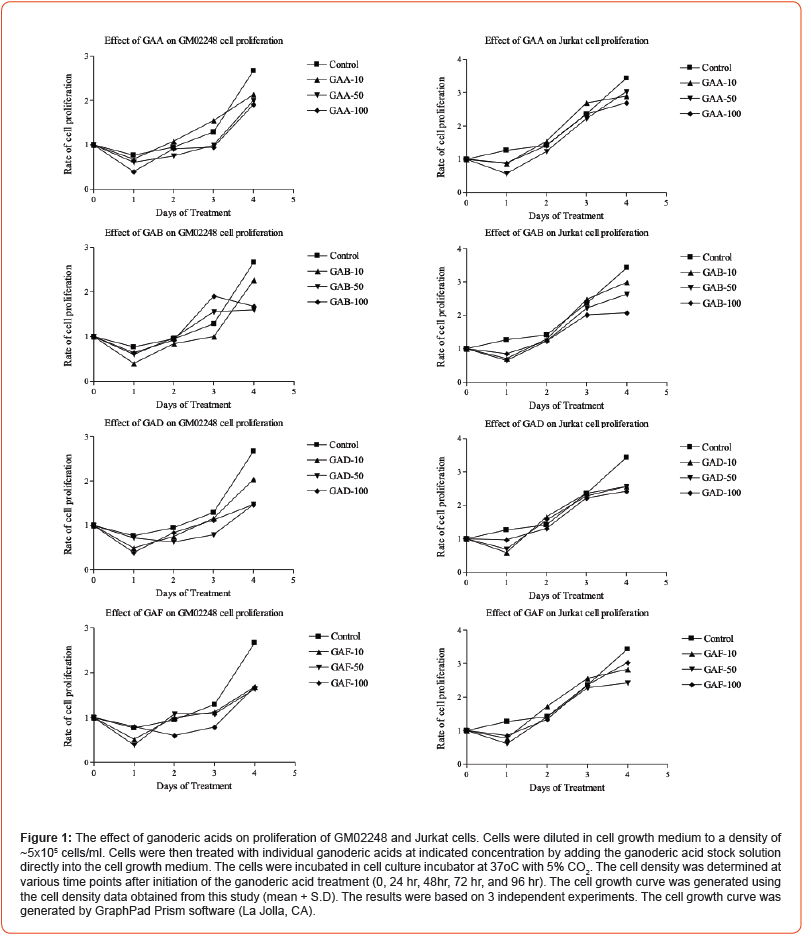
The protein level of μ-Actin and GAPDH were used as protein loading controls of GM02248 and Jurkat cells respectively for our immunoblotting studies. The results of our immunoblotting study indicated that the PMA/Ionomycin treatment caused 11.5 folds increase of PD-1 protein in GM02248 cells when compared to that of the untreated GM02248 cells (Figure 2). In the presence of GAA, GAB, GAD, and GAF, however, the levels of PD-1 protein in the PMA/ ionomycin-treated GM02248 cells were reduced to only 94%, 100%, 80%, and 68% of the untreated GM02248 cells, respectively (Figure 2). Similarly, the PMA/Ionomycin treatment increased the PD-1 protein level by 5.5 folds in the Jurkat cells (Figure 2). In the presence of GAA, GAB, and GAF, however, the PD-1 protein level in the PMA/Ionomycin-treated Jurkat cells were reduced to 20%, 26%, and 59% of the untreated Jurkat cells (Figure 2). As controls, the levels of other tested proteins, including CCL5, CD4, CDK7, and XPC, were not significantly affected by the presence of ganoderic acid in GM02248 or Jurkat cells (Figure 2).
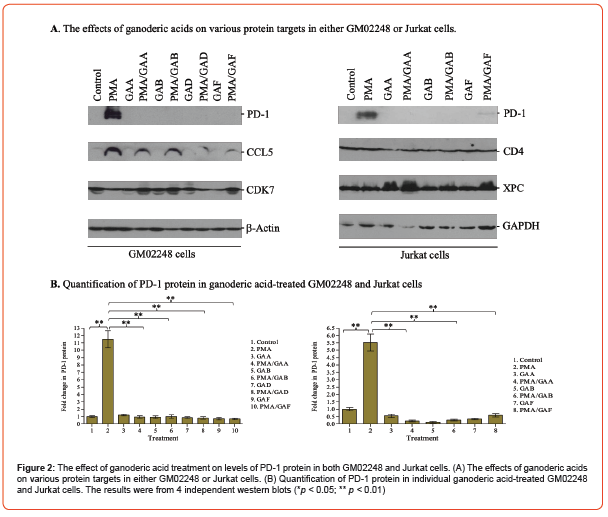
To confirm the results obtained from our immunoblotting study, we also performed an immunofluorescence (IF)-based microscopy study to determine the percentage of PD-1+ cells in both untreated and ganoderic acid-treated Jurkat cells. The Jurkat cells were treated with individual ganoderic acids (100 μM) in the presence or absence of PMA/ionomycin for 48 hours. Both untreated and ganoderic acid-treated Jurkat cells were then fixed by formaldehyde, stained with an APC-labeled PD-1 antibody, and counter- stained with a DAPI reagent. The percentage of PD- 1+ cells were determined for each treatment (Figure 3 and Table 2). The results of our IF-based microscopy study revealed that 21.2% of untreated Jurkat cells expressed low levels of PD-1 protein (Figure 3 and Table 2).
Table 2:The effect of ganoderic acid treatment on PD-1+ cells in the cultured Jurkat cells.
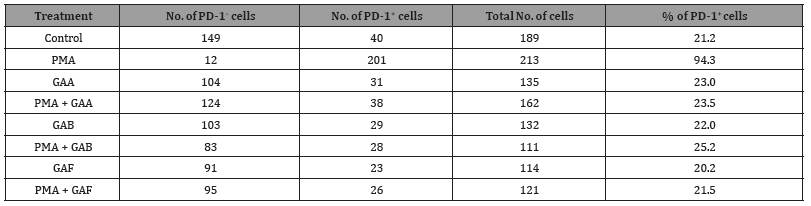
(The number of PD-1+ and PD-1- cells were counted from 3 views/treatment).
The PMA/Ionomycin treatment increased the PD-1+ cells to 94.3% (Figure 3 and Table 2). Treatment with 100 μM GAA, GAB, or GAF, however, decreased the percentage of PD-1+ cells to 23.5%, 25.2%, and 21.5% respectively for the PMA/Ionomycin- treated Jurkat cells (Figure 3 and Table 2). The presence of purified individual ganoderic acids has insignificant effect to the percentage of PD-1+ Jurkat cells (23% in the GAA-treated Jurkat cells, 22.0% in the GAB-treated Jurkat cells, and 20.2% in the GAF-treated Jurkat cells vs 21.2% in the untreated Jurkat cells) (Figure 3 and Table 2). This result, in combination with our immunoblotting results, clearly demonstrated that the presences of these purified ganoderic acids caused significant reduction of the PD-1 protein in both GM02248 and Jurkat cells, suggesting the key role of ganoderic acids in G. lucidum- mediated PD-1 protein reduction.
The effects of ganoderic acids on transcriptions of immune-responsive genes in GM02248 and Jurkat cells
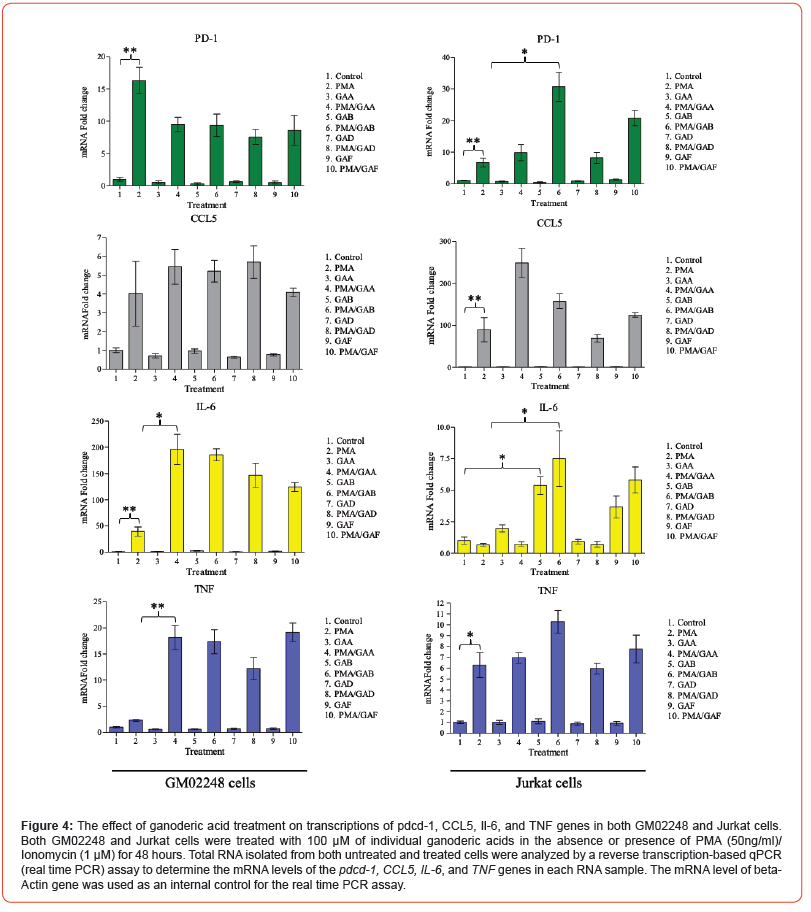
The results of our immunoblotting and IF-based microscopy studies have demonstrated the effects of purified ganoderic acids on reducing PD-1 protein in both GM02248 and Jurkat cells. To determine if a transcriptional inhibition played a role for the purified ganoderic acid-mediated PD-1 protein reduction, we further determined the mRNA levels of pdcd-1 gene from the RNA samples isolated from these cells using a reverse transcription-based qPCR (real- time PCR) study. As controls, several immune responsive genes, including CCL5, IL-6, and TNF, were also determined from these RNA samples by the real time PCR assay. Total RNA isolated from both untreated and ganoderic acid-treated GM02248 and Jurkat cells were analyzed by the real time PCR assay to determine the mRNA levels of pdcd-1, CCL5, IL-6, and TNF genes (Figure 4). The results of our real time PCR study revealed that the PMA/Ionomycin treatment increased transcription of the pdcd-1 mRNA to 16.3 folds in GM02248 cells (Figure 4).
In the presence of 100mM GAA, GAB, GAD, and GAF, the pdcd-1 mRNA levels in the PMA/Ionomycin-treated GM02248 cells were 9.5 folds, 9.4 folds, 7.6 folds, and 8.6 folds to that of the untreated GM02248 cells, respectively (Figure 4). The treatment with GAA, GAB, GAD, or GAF alone did not cause meaningful change in the pdcd-1 mRNA level of GM02248 cells (Figure 4). Similarly, the PMA/Ionomycin treatment increased the pdcd-1 mRNA level by 6.6 folds in Jurkat cells (Figure 4); in the presence of 100 μM GAA, GAB, GAD, and GAF, however, the pdcd-1 mRNA levels in the PMA/Ionomycin- treated Jurkat cells were increased to 9.9 folds, 30.7 folds, 8.2 folds, and 20.8 folds, respectively (Figure 4). The presence of GAA, GAB, GAD, or GAF also did not cause meaningful change in the pdcd-1 mRNA level in the Jurkat cells (Figure 4). The presence of the purified ganoderic acids also caused increases in transcriptions some but not all of the tested immune related genes (Figure 4). For example, the presence of GAA, GAB, GAD, and GAF clearly caused some more increases in the mRNA levels of IL-6 and TNF genes in the GM02248 cells (Figure 4). In the Jurkat cells, however, only the presence of GAB and GAF but not GAA or GAD caused more increases in the mRNA level of IL-6 gene (Figure 4). This result suggests that a transcriptional inhibition is not a mechanism for ganoderic acid-mediated PD-1 protein reduction in these cells. In addition, this result also suggests that different ganoderic acid might have different effect in transcriptions of certain immune related genes.
Discussion
Our previous studies demonstrated the effect of extract prepared from the spores of G. lucidum (GLE) on reducing PD-1 protein of cultured human B-lymphocytes [33]. Given the important roles of PD-1 protein in modulating immune response [21-25] and in cancer immunotherapy [26-32], it would be necessary to identify the bioactive compounds that are responsible for G. lucidum- mediated PD-1 protein reduction so that G. lucidum-based therapeutics can be developed for treatment of cancer and other immune-related diseases. In this study, we focused on determining the role of purified ganoderic acids, including GAA, GAB, GAD, and GAF, in reducing PD-1 protein of cultured human B and T-lymphocytes. The results of our immunoblotting study clearly demonstrated the effects of these purified ganoderic acids on reducing PD-1 protein in both GM02248 human B lymphocytes and Jurkat human lymphoma cells. The results of our IF-based microscopy study further revealed that the presence of GAF reduced the percentage of PD-1+ Jurkat cells. In addition, the results of our cell proliferation studies indicated that the presence of ganoderic acids did not significantly affect the rate of cell proliferation at concentrations as high as 100 μ M. These results suggest that ganoderic acids play a key role in G. lucidum-mediated PD-1 protein reduction in these cells and contribute significantly to G. lucidum-mediated immune response. Considering the important function of PD-1 protein in immunomodulation [21-25] and in treatment of many diseases [26-32], these results suggest that ganoderic acids of G. lucidum could be developed as novel immunomodulating drugs for treatment of cancer and many other diseases via targeting the PD-1 protein of immune cells.
The results of our immunoblotting studies revealed that the presence of the tested ganoderic acids, including GAA, GAB, GAD, and GAF, reduced the level of PD-1 protein in the cultured GM02248 and Jurkat cells (Figure 2). Therefore, it is highly likely that the triterpenoids carried in G. lucidum play a key role in G. lucidum-mediated PD-1 protein reduction as observed in our previous study [33]. There are over 150 triterpenoids identified in the G. lucidum and many of them possess very different chemical structures [1,4,5,7]. The ganoderic acids used in this study possess similar chemical structures. Although the results obtained from this work demonstrated the effect of these ganoderic acids in reducing PD-1 protein in the cultured human cell models, it is unclear if other triterpenoids with different chemical structures could also to reduce the PD-1 protein in these cells. It would be necessary to determine the effects of those triterpenoids on reducing PD-1 protein in immune cells so that the mechanisms of G. lucidum-mediated immunomodulation and immunoresponse could be better understood and the G. lucidum-based therapeutics can be developed for more elective treatment of cancer and many other diseases. The results obtained from our studies clearly suggest that the ganoderic acids of G. lucidum play a key role in G. lucidum-mediated PD-1 reduction. However, it is well known that many different compounds, including polysaccharides and peptidoglycans, are also contained in the G. lucidum [1-7]. Works of others have indicated the modulation effects of polysaccharides in modulating immune response [16-19]. The results of our study, however, indicated that the presence of polysaccharides did not cause reduction of PD-1 protein in the cultured human B or T cells (data not provided). Therefore, the polysaccharides of G. lucidum must use a different mechanism to modulate immune response. In fact, works of others suggested that the polysaccharide might modulate immune response through the generation of reactive oxygen species (ROS), the secretion of cytokines, cell proliferation, or the phagocytic activity of macrophages [51]. Therefore, it is likely that the immune response observed in the G. lucidum is a combined effects of all these bioactive compounds through different mechanisms and targets.
The results of our real time PCR studies indicated that the presence of purified individual ganoderic acids did not inhibit transcription of the pdcd-1 gene in these cell models; however, the results of our immunoblotting studies clearly demonstrated the effect of ganoderic acids in reducing the level of PD-1 protein in the cultured GM02248 and Jurkat cells. Therefore, a transcriptional inhibition of pdcd-1 gene is unlikely a mechanism for ganoderic acid-mediated PD- 1 protein reduction; therefore, other mechanisms, such as PD-1 protein degradation, must be involved in ganoderic acid-mediated PD-1 protein reduction in these cells. Further studying the underlying mechanism would be necessary in understanding the G. lucidum-mediated immune responses and in developing G. lucidum- based therapeutics for treatment of many diseases. Interestingly, the results of our real time PCR study suggest that different ganoderic acids might possess different effects in transcriptions of specific genes, especially the immune-related genes. Given the important roles of these immune-related proteins in treatment of cancer and other diseases, it would be important to determine the effects of individual ganoderic acids in transcriptions of important immune-related genes so that the ganoderic acids that can most effectively modulate immune response will be identified and used for treatment of cancer and other immune-related diseases.
Significant efforts have been devoted for novel therapies that target the PD-1/PD L-1 pathway for cancer treatment [26-32]. The antibody-based immunotherapies that target either PD-1 or PD L-1 have been developed and used successfully in the treatment of many cancer types [26- 32]. However, many adverse effects are associated with the antibody-based immunotherapies [36,37]. Ganoderic acids have some clear advantages over antibody-based immunotherapy: the size of ganoderic acid is small (~520 Da), the stability of ganoderic acid is very high, and the toxicity of ganoderic acid is very low [42-46]. If the effect of ganoderic acids in reducing PD-1 protein observed in our cell model system could be confirmed in animal model and in human study, ganoderic acids-based small molecule immunomodulating drugs could be easily developed and used in treatment of cancer and other immune-related diseases.
Conclusion
In conclusion, the results of our studies clearly demonstrated that the effects of purified ganoderic acids, including GAA, GAB, GAD, and GAF, in reducing PD-1 protein level in the cultured GM02248 and Jurkat cells model systems. Therefore, the ganoderic acids of G. lucidum play a key role in G. lucidum-mediated PD-1 reduction. Considering the important function of PD- 1 protein in modulating immune response and in cancer immunotherapy, and the clear advantages of ganoderic acids over antibody-based immunotherapy, our studies suggest that ganoderic acids of G. lucidum could be developed into novel small molecule immunomodulating drugs for treatment of cancer and many immune-related diseases.
Acknowledgement
We thank Ms. Lisa Mayernik and Ms. Acacia Farber-Krug of the Microscopy, Imaging, and Cytometry Resource Core of Wayne State University for their technical assist in IF-microscopy studies.
Author Contributions
GW designed and carried out most of the studies. GW also drafted the manuscript. FC was involved in western blotting studies. ZL, JZ, and XX were involved in the design of the study. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The authors declared no conflicts of interest regarding the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Performance of this work was facilitated by the Cell Culture Facility Core of the Institute of Environmental Health Sciences (IEHS), Wayne State University, and the Microscopy, Imaging, and Cytometry Resource Core of School of Medicine, Wayne State University. The Cell Culture Facility Core is supported, in part, by NIH Center Grant P30 ES020957 to the IEHS, Wayne State University. The Microscopy, Imaging, and Cytometry Resource Core is supported, in part, by NIH Center grant P30 CA22453 to the Karmanos Cancer Institute, Wayne State University, and the Perinatology Research Branch of the National Institute of Child Health and Development. This work was supported by both a grant from the Shouxiangu Pharmaceuticals LLC and an internal fund to GW.
References
- Ahmad R, Riaz M, Khan A, Aljamea A, Algheryafi M, et al. (2021) Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytother Res 35: 6030-6062.
- Seweryn E, Ziała A, Gamian A (2021) Health-Promoting of Polysaccharides Extracted from Ganoderma lucidum. Nutrients 13: 2725.
- Geng X, Zhong D, Su L, Lin Z, Yang B (2020) Preventive and therapeutic effect of Ganoderma lucidum on kidney injuries and diseases. Adv Pharmacol. 87:257-276.
- Soccol CR, Bissoqui LY, Rodrigues C, et al. (2016) Pharmacological properties of biocompounds from spores of the Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (Agaricomycetes): a review. Int J Med Mushrooms 18: 757-767.
- Sanodiya BS, Thakur GS, Baghel RK, Prasad GB, Bisen PS (2009) Ganoderma lucidum: a potent pharmacological macrofungus. Curr Pharm Biotechnol 10:717-742.
- Boh B, Berovic M, Zhang J, Zhi-Bin L (2007). Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol Annu Rev 13:265–301.
- Shiao MS. Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chem Rec. (2003) 3:172-180.
- Chan SW, Tomlinson B, Chan P, Lam CWK (2021) The beneficial effects of Ganoderma lucidum on cardiovascular and metabolic disease risk. Pharm Biol 59:1161-1171.
- Koo MH, Chae HJ, Lee JH, Suh SS, Youn UJ (2021) Anti-inflammatory lanostane triterpenoids from Ganoderma lucidum. Nat Prod Res 35: 4295-4302.
- Bhardwaj N, Katyal P, Sharma AK (2014) Suppression of inflammatory and allergic responses by pharmacologically potent fungus Ganoderma lucidum. Recent Pat Inflamm Allergy Drug Discov 8: 104-17.
- Liu G, Zeng T (2021) Sporoderm-Removed Ganoderma lucidum Spore Powder May Suppress the Proliferation, Migration, and Invasion of Esophageal Squamous Cell Carcinoma Cells Through PI3K/AKT/mTOR and Erk Pathway. Integr Cancer Ther 20: 15347354211062157.
- Xian H, Li J, Zhang Y, Li D, Zhu Y, et al. (2021) Antimetastatic Effects of Ganoderma lucidum Polysaccharide Peptide on B16-F10-luc-G5 Melanoma Mice with Sleep Fragmentation. Front Pharmacol 12: 650216.
- Acevedo-Díaz A, Ortiz-Soto G, Suárez-Arroyo IJ, Zayas-Santiago A, Martínez Montemayor MM (2019) Ganoderma lucidum Extract Reduces the Motility of Breast Cancer Cells Mediated by the RAC⁻Lamellipodin Axis. Nutrients 11(5):1116.
- Loganathan J, Jiang J, Smith A, Jedinak A, Thyagarajan-Sahu A, et al. (2014) The mushroom Ganoderma lucidum suppresses breast-to-lung cancer metastasis through the inhibition of pro-invasive genes. Int J Oncol 44(6).
- Weng CJ, Yen GC (2010) The in vitro and in vivo experimental evidences disclose the chemopreventive effects of Ganoderma lucidum on cancer invasion and metastasis. Clin Exp Metastasis 27(5): 361-9.
- Su J, Li D, Chen Q, Li M, Su L, et al. (2018) Anti-breast Cancer Enhancement of a Polysaccharide from Spore of Ganoderma lucidum with Paclitaxel: Suppression on Tumor Metabolism with Gut Microbiota Reshaping. Front Microbiol 9: 3099.
- Xu Z, Chen X, Zhong Z, Chen L, Wang Y (2011) Ganoderma lucidum polysaccharides: immunomodulation and potential anti-tumor activities. Am J Chin Med 39: 15-27.
- Li LF, Liu HB, Zhang QW, et al. (2018) Comprehensive comparison of polysaccharides from Ganoderma lucidum and G sinense: chemical, antitumor, immunomodulating and gut- microbiota modulatory properties. Sci Rep 8: 6172.
- Liang CJ, Lee CW, Sung HC, et al. (2014) Ganoderma lucidum polysaccharides reduce lipopolysaccharide-induced interleukin-1β expression in cultured smooth muscle cells and in thoracic aortas in mice. Evid Based Complement Alternat Med 2014: 305149.
- Shinohara T, Taniwaki M, Ishida Y, Kawaichi M, Honjo T (1994) Structure and chromosomal localization of the human PD-1 gene (PDCD1). Genomics 23: 704-706.
- Blank C, Mackensen A (2007) Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 56: 739-745.
- Yao S, Chen L (2014) PD-1 as an immune modulatory receptor. Cancer J 20: 262-264.
- Kamphorst AO, Ahmed R (2013) Manipulating the PD-1 pathway to improve immunity. Curr Opin Immunol 25:381-388.
- Peng W, Liu C, Xu C, et al. (2012) PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res 72: 5209-5218.
- Meng X, Liu X, Guo X, et al. (2018) FBXO38 mediates PD-1 ubiquitination and regulates anti- tumour immunity of T cells. Nature 564: 130-135.
- Xu-Monette ZY, Zhou J, Young KH (2018) PD-1 expression and clinical PD-1 blockade in B- cell lymphomas. Blood 131:68-83.
- Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R (2019) PD-1/PD-L1 immune checkpopint: Potential target for cancer therapy. J Cell Physiol 234(2): 1313-1325.
- Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T (2022) Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol. 37-50.
- Reck M, Remon J, Hellmann MD (2022) First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J Clin Oncol 40(6): 586-597.
- Topalian SL, Taube JM, Pardoll DM (2020) Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 367(6477): eaax0182.
- Constantinidou A, Alifieris C, Trafalis DT (2019) Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther 194: 84-106.
- Pawłowska A, Suszczyk D, Okła K, Barczyński B, Kotarski J, et al. (2019) Immunotherapies based on PD-1/PD-L1 pathway inhibitors in ovarian cancer treatment. Clin Exp Immunol 195: 334-344.
- Wang G, Wang L, Zhou J, Xu X (2019) The possible role of PD-1 protein in Ganoderma lucidum- mediated immunomodulation and cancer treatment. Integr Cancer Ther. 18:1534735419880275.
- Meng X, Liu X, Guo X, Jiang S, Chen T, et al. (2018) FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature 564(7734):130-135.
- Zhou XA, Zhou J, Zhao L, Yu G et al. (2020) KLHL22 maintains PD-1 homeostasis and prevents excessive T cell suppression, Proc. Natl. Acad. Sci. USA, 117: 28239–28250.
- Martins F, Sofiya L, Sykiotis GP, Lamine F, et al. (2019) Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 16(9): 563-580.
- Plachouri KM, Vryzaki E, Georgiou S (2019) Cutaneous Adverse Events of Immune Checkpoint Inhibitors: A Summarized Overview. Curr Drug Saf 14(1): 14-20.
- Marin-Acevedo JA, Kimbrough EO, Lou Y (2021) Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol 14(1):45.
- Skalniak L, Zak KM, Guzik K, et al. (2017) Small-molecule inhibitors of PD-1/PD-L1 immune checkpoint alleviate the PD-L1-induced exhaustion of T-cells. Oncotarget 8: 72167-72181.
- Guzik K, Zak KM, Grudnik P, et al. (2017) Small-molecule inhibitors of the programmed cell death-1/programmed death- ligand 1 (PD-1/PD-L1) interaction via transiently induced protein states and dimerization of PD-L1. J Med Chem 60: 5857-5867.
- Chen S, Song Z, Zhang A (2019) Small-molecule immuno-oncology therapy: advances, challenges and new directions. Curr Top Med Chem 19: 180-185.
- Ansari MHR, Khan W, Parveen R, Saher S, Ahmad S (2022) Pharmacokinetic, Metabolomic, and Stability Assessment of Ganoderic Acid H Based Triterpenoid Enriched Fraction of Ganoderma lucidum Karst. Metabolites 12(2):97.
- Cheng CR, Yang M, Guan SH, Wu XH, Pang XY, et al. (2013) Pharmacokinetics of ganoderic acid D and its main metabolite by liquid chromatography- tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 930:1-6.
- Cao FR, Xiao BX, Wang LS, Tao X, Yan MZ, et al. (2017) Plasma and brain pharmacokinetics of ganoderic acid A in rats determined by a developed UFLC-MS/MS method. J Chromatogr B Analyt Technol Biomed Life Sci 1052:19-26.
- Tawasri P, Ampasavate C, Tharatha S, Chiranthanut N, Teekachunhatean S (2016) Effect of Oral Coadministration of Ascorbic Acid with Ling Zhi Preparation on Pharmacokinetics of Ganoderic Acid A in Healthy Male Subjects: A Randomized Crossover Study. Biomed Res Int 2016: 2819862.
- Teekachunhatean S, Sadja S, Ampasavate C, Chiranthanut N, Rojanasthien N, et al. (2012) Pharmacokinetics of ganoderic acids a and f after oral administration of ling zhi preparation in healthy male volunteers. Evid Based Complement Alternat Med 2012: 780892.
- Chen Y, Xie MY, Gong XF (2007) Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J Food Eng 81: 162-170.
- Guo WL, Pan YY, Li L, Li TT, Liu B, Lv XC (2018) Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct 9: 3419-3431.
- Liu J, Kurashiki K, Shimizu K, Kondo R (2006) 5alpha-reductase inhibitory effect of triterpenoids isolated from Ganoderma lucidum. Biol Pharm Bull 29: 392-395.
- Lu QY, Jin YS, Zhang Q, et al. (2004) Ganoderma lucidum extracts inhibit growth and induce actin polymerization in bladder cancer cells in vitro. Cancer Lett 216: 9-20.
- Tong H, Mao D, Zhai M, Zhang Z, Sun G, et al. (2015) Macrophage activation induced by the polysaccharides isolated from the roots of Sanguisorba officinalis. Pharm Biol 53: 1511–1515.
-
Inass Omer Mohamed Malik, Mahdi Abd elmageed Mohammed Ali, Hatim MY Hamadnalla etc all... Biochemical Properties and Nutritional Value of Balanites Aegyptiaca (Laloub) Seed Oil. Insi in Chem & Biochem. 2(1): 2021. ICBC. MS.ID.000553.
-
Biochemical, Nutritional Value, Balanites, Aegyptiaca, Laloub, Seed Oil, Biochemistry, protein, Physicochemical, chloroform, benzene, diethyl.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






