 Review Article
Review Article
Eutectic Electrolytes for High-Safety Lithium Metal Batteries
Junming Wang1*, Qianqian Liu2, Jieyu Chang2, Jinhua Hu2 and Zhijiang Su1
1National Institute of Clean-and-Low-Carbon Energy Beijing 102211, China
2Key Laboratory of Electronic Materials and Devices of Tianjin School of Electronics and Information Engineering Hebei University of Technology, Tianjin 300131, China
Junming Wang, 1National Institute of Clean-and-Low-Carbon Energy Beijing 102211, China.
Received Date:October 01, 2024; Published Date:December 17, 2024
Abstract
Lithium metal batteries (LMBs), which are considered potential candidates for high-energy-density batteries, still face poor cycle stability and safety hazards caused by the growth of lithium dendrites and the unstable solid electrolyte interphases (SEI). Eutectic electrolytes offer a promising solution to enhance the safety and cycle life of LMBs due to their non-flammability, wide electrochemical window, high ionic conductivity, and costeffectiveness.This review aims to comprehensively introduce the concept, applications and crucial challenges of eutectic electrolytes in LMBs.
Keywords:Lithium metal batteries; safety issues; Eutectic electrolytes
Introduction
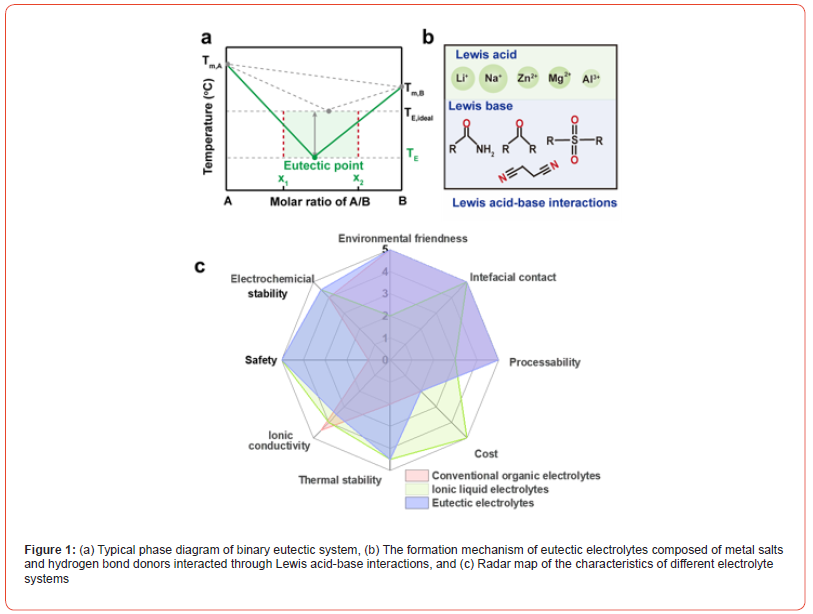
LMBs with lithium metal as anode are expected to achieve high energy densities of up to 500 Wh kg-1. However, commercial electrolyte systems are incompatible with lithium anodes with high reactivity between lithium metal and electrolytes. Moreover, the high volatility, strong flammability, and poor thermal stability pose safety threats to LMBs. Therefore, electrolyte systems hold a vital role in ensuring both the electrochemical performance and safety of LMBs. Developing intrinsically safe electrolyte systems with high interfacial stability have been a research hotspot in LMBs recently. Non-flammable electrolyte systems such as solidstate electrolytes, (localized) high concentration electrolytes, ionic liquid (IL) electrolytes and eutectic electrolytes have been proposed to improve the safety and reliability of LMBs [1]. Among them, eutectic electrolytes have attracted widespread attention due to their advantages such as low cost, low vapor pressure, nonflammability, wide electrochemical window, and high thermal/ chemical stability (Figure 1).
Fundamentals of Eutectic Electrolytes
Eutectic electrolytes are fundamentally originated from deep eutectic solvents (DESs), which are formed by two or more components through hydrogen bonding, Brønsted/Lewis acidbase, and van der Waals interactions, with the melting point (Tm) significantly lower than those of a single component and ideal mixtures (Figure 1a) [2-4]. When DESs consisting of metal salts and Lewis-base solvents, utilized as electrolytes in metal batteries are designated as eutectic electrolytes (Figure 1b). Typically, the cations in metal salts such as Li+, Zn2+ and Al3+ acting as Lewis acid interact with the solvents incorporating Lewis base such as amide, nitrile and sulfone, forming eutectic electrolytes. The robust intermolecular interactions in eutectic electrolytes endow them with low Tm, low vapor pressure, outperformed thermal/chemical stability, wide liquid range and non-flammability as analogs of the physical properties of IL electrolytes. Furthermore, eutectic electrolytes offer advantages over IL electrolytes, including a higher ion transport number, simpler preparation processes, lower costs, and enhanced environmental friendliness (Figure 1c). The unique characteristics of eutectic electrolytes present broad prospects in high-safety, large-scale applications of LMBs [4].
Eutectic Electrolytes Driving Safe Lmbs
Historical development of eutectic electrolytes
Since the pioneering work of DESs first reported in 2003 based on choline chloride (ChCl) [3] eutectic electrolytes for lithium batteries have been studied in the following years. Early research mainly focused on the amide-based eutectic electrolytes [5]. In amides, the C=O and N-H polar groups coordinate with Li+ and anions from lithium salts, respectively, weakening the hydrogen bonds within amides and the ionic bonds in lithium salts, ultimately resulting in a significant decrease in the melting points. For instance, the Tm of LiTFSI-NMAc (N-methylacetamide) eutectic electrolyte is as low as -72℃, with the room-temperature ionic conductivity of 1.35 mS cm⁻¹ [6]. Subsequently, sulfonamide,7 nitrile8, and imidazole9 based eutectic electrolytes through Li-O or Li-N interactions were successively proposed. In terms of Li salt, LiTFSI, due to its large anion size and high charge delocalization properties, is preferred for use in eutectic electrolytes, which exhibited higher ionic conductivity and lower viscosity compared to eutectic electrolytes formed with LiNO3 and LiPF6 [6].
In recent years, following the revival of LMBs, eutectic electrolytes have been of high interest as they offer enhanced safety for large-scale LMBs. Eutectic electrolytes demonstrated intrinsic safety superiority over conventional organic electrolytes during burning test. Moreover, eutectic electrolytes exhibited enhanced safety performance in the safety tests of practical LMBs such as nail penetration, cutting and thermal shock tests, further proving the utilization of eutectic electrolytes is an effective strategy to address the safety issues in LMBs [7].
Electrode/electrolyte interphase improvement
Eutectic electrolytes generally exhibit high oxidation stability,
with electrochemical windows over 5V. However, the instability of
the Lewis-base solvents such amide and nitrile towards Li metal
anode is a main concern. This is due to the high polarization of
Lewis-base solvents, leading to high reactivity with Li metal and
low coulombic efficiency. To enhance the compatibility between
eutectic electrolytes and lithium metal anodes, several strategies
have been proposed as follows.
(1) Adding SEI-forming additives such as LiODFB 8 and LiNO3
10, which preferentially form a stable SEI film on Li metal. For
example, Cui group added small amount of LiODFB to LiTFSIsuccinonitrile
(SN). The ODFB and TFSI anions participated to
form a stable SEI film and promoted the stability of Li metal and
stable cycling of LiCoO2/Li batteries [8-10].
(2) Regulating the solvation structure of eutectic electrolytes.
by solvent This could be achieved by choosing less-reactive
solvents and leveraging the competitive solvation of different
solvents. For example, novel solvents such as butadiene sulfone
[11] and dimethylmalononitrile [12] have been proposed to
improve the interfacial stability. In addition, Zhang’s group
improved the interfacial stability of LiTFSI-SN- butyrolactam
(BL) ternary eutectic electrolyte by leveraging the preferential
coordination of BL toward Li+, resulting in the formation of
stable solid electrolyte interphase films [13].
(3) Forming eutectic polymer electrolytes. Eutectic polymer
electrolytes are generally prepared by adding eutectic
electrolytes as plasticizers into solid polymer matrix. The
polymer matrix interacts with the solvents or Li+ ions, reducing
the free solvent molecules, promoting ion transport and even
changing the Li+ solvation environment, which provides a
new strategy for enhancing the interfacial stability [9,14]. For
example, incorporating LiTFSI-1,2-dimethylimidazole (DMIm)
eutectic electrolyte into PVDF polymers could change the Li+
coordination environment and mitigate the side reactions
between DMIm and Li metal, thus improved the cycling
performance of Li/LFP cells [9]. Moreover, eutectic polymer
electrolytes could avoid the safety risk of electrolyte leakage,
further enhance the safety of LMBs.
Summary and Outlook
In summary, the development of eutectic electrolytes in highsafety LMBs is still in its infancy and demonstrating tremendous potential. Challenges and opportunities still persist within eutectic electrolytes. The lower ionic conductivity and higher viscosity of eutectic electrolytes compared with conventional organic electrolytes makes ion transport more sluggish. At the same time, eutectic electrolytes still face challenges in achieving satisfactory compatibility with Li metal. Therefore, developing novel electrolyte formulations that possess both high ionic conductivity and interphase stability undoubtedly deserves further study. For example, screening and designing of less reactive solvents and multi-component systems by theoretical calculations and machine-learning techniques holds great promise to this challenge. Moreover, it is necessary to explore the interactions between eutectic electrolytes and interphase stability, as well as their impact on the safety performance, for better electrolyte design. Gaining insights into the fundamental mechanisms governing the interplay between eutectic electrolytes and interphase stability, alongside their consequential effects on safety performance, is imperative for better electrolyte design.
Acknowledgements
The authors acknowledged the funding support from Natural Science Foundation of Hebei Province (B2022202063) and Scientific Research Plan Project of Tianjin Education Commission (2023KJ294)
Conflict of Interest
No Conflict of interest.
References
- QQ Liu, L G (2023) Wang Fundamentals of Electrolyte Design for Wide-Temperature Lithium Metal Batteries. Adv. Energy Mater 13: 2301742.
- EL Smith, Abbott AP, Ryder KS (2014) Deep Eutectic Solvents (DESs) and Their Applications. Chem Rev 114: 11060-11082.
- Andrew P Abbott, Glen Capper, David L Davies, Raymond K Rasheeda, Tambyrajaha V (2003) Novel solvent properties of choline chloride/urea mixtures. Chem Commun, 70-71.
- L S Geng, Wang X P, Han K, Hu P, Zhou L, et al. (2022) Eutectic Electrolytes in Advanced Metal-Ion Batteries. ACS Energy Lett 7: 247-260.
- Y S Hu, Li H, Huang X J, Chen L (2004) Novel room temperature molten salt electrolyte based on LiTFSI and acetamide for lithium batterie. Electrochem commun 6: 28–32.
- A Boisset, Menne S, Jacquemin J, Balducci A, Anouti M (2013) Deep eutectic solvents based on -methylacetamide and a lithium salt as suitable electrolytes for lithium-ion batteries. Phys Chem Chem Phys 15: 20054-20063.
- O E Geiculescu, DesMarteau D D, Creager S E, Haik O, Hirshberg D, et al. (2016) Novel binary deep eutectic electrolytes for rechargeable Li-ion batteries based on mixtures of alkyl sulfonamides and lithium perfluoroalkylsulfonimide salts. J Power Sources 307: 519-525.
- Z L Hu, Xian F, Guo Z Y, Lu C L, Du X F, et al. (2020) Non-flammable nitrile deep eutectic electrolyte enables high voltage lithium metal batteries. Chem Mater 32: 3405–3413.
- P. Pei, Li Y. J, Ou T, Liang X. C, Yang Y, Jia E. N, Tan Y, J. G. S. Li−N Interaction Induced Deep Eutectic Gel Polymer Electrolyte for High Performance Lithium-Metal Batteries. Angew. Chem. Int Ed 61: e202205075.
- YH Liang, Wu W B, Li D P, Wu H, Gao C C, et al. (2022) Highly Stable Lithium Metal Batteries by Regulating the Lithium Nitrate Chemistry with a Modified Eutectic Electrolyte. Adv Energy Mater 12: 2202493.
- TK Zhou, Lei C J, Li J Y, Wang H J, Liu T T, et al. (2024) Butadiene Sulfone Based Binary Deep Eutectic Electrolyte for High Performance Lithium Metal Batteries. Angew. Chem Int Ed: e202408728.
- L Zhao, Xu A, Cheng Y, Xu H T, Xu L, Mai L Q (2022) A Highly Stable and Non-Flammable Deep Eutectic Electrolyte for High-Performance Lithium Metal Batteries. Angew. Chem Int Ed 696: e202411224.
- W B Wu, Liang Y H, Li D P, Bo Y Y, Wu D, et al. (2022) A Competitive Solvation of Ternary Eutectic Electrolytes Tailoring the Electrode/Electrolyte Interphase for Lithium Metal Batteries. ACS Nano 16: 14558-14568.
- H Zhang, Zhou LX, Du X F, Zhang JJ, Tian S W, et al. (2022) Cyanoethyl cellulose-based eutectogel electrolyte enabling high voltage-tolerant and ion-conductive solid-state lithium metal batteries. Carbon Energy 4: 1093-1106.
-
Junming Wang*, Qianqian Liu, Jieyu Chang, Jinhua Hu and Zhijiang Su. Eutectic Electrolytes for High-Safety Lithium Metal Batteries. Insi in Chem & Biochem. 3(2): 2024. ICBC. MS.ID.000558.
-
Biochemical, Nutritional Value, Balanites, Aegyptiaca, Laloub, Seed Oil, Biochemistry, protein, Physicochemical, chloroform, benzene, diethyl.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






