 Research Article
Research Article
The Impact of Bi-hemispheric iTBS Stimulation of the IPL and IFG on Social Reciprocity in ASD
Mitra Assadi MD1*, Suzanne Bauer PsyD2, Esq, Reza Koiler PhD3, Ryan Ally MD4, Richard Fischer MD5 and Rodney Scott MD, PhD6
1Professor of Neurology, Thomas Jefferson University Medical School, United States
2Christiana Care Hospital, Department of Psychiatry, Center for Autism, United States
3Neuromechanix inc, Delaware, United States
4Christiana Care Hospital, Department of Psychiatry, United States
5Christiana Care Hospital, Clinical Assistant Professor of Neurology, Thomas Jefferson University Medical School, United States
6Professor of Neurology and Pediatrics, Thomas Jefferson University Medical School, AI DuPont Children’s Hospital, Chairman, United State
Mitra Assadi, MD, Department of neurology, Thomas Jefferson University, Wilmington Delaware, United States
Received Date: July 31, 2025; Published Date: August 06, 2025
Abstract
ASD encompasses a wide range of limitations in reciprocal, social and communicative milestones. Given the significant limitations of the existing pharmacological interventions, use of non-invasive brain stimulation such as repetitive Transcranial Magnetic Stimulation (rTMS) has generated hope for mitigating ASD-related behaviors. Some experts attribute the limitations in social cognition in ASD to abnormal mirror neuron function or modulation. Mirror neurons are concentrated in the inferior frontal gyrus (IFG) and the inferior parietal lobule (IPL). Leveraging the hemispheric dependent function of mirror neurons, we aimed to investigate the impact of rTMS stimulation of the bilateral IFG and IPL on social reciprocity in ASD. We used the Childhood Autism Rating Scale, 2nd edition, high functioning (CARS2) as the outcome measure. The assessment was completed at baseline, in 3 weeks (phase I), 6 weeks (phase II) and 3 weeks after completion of TMS (phase III). A total of 6 ASD subjects (3 girls and 3 boys) with an average age of 14.8 years were recruited. Statistical analysis demonstrated a decrease in the total CARS2 scores commensurate with an improvement in social reciprocity/communication when comparing the baseline values to phase I (p<0.001), phase II (p<0.001), and phase III (p<0.001). This small experiment serves as a proof of principle for the novel approach of iTBS targeting the bilateral IPL/IFG as a therapeutic intervention in ASD.
Keywords: iTBS; Mirror neurons; IPL; IFG; Social reciprocity
Introduction
ASD encompasses a wide range of limitations in reciprocal, social and communicative milestones, as well as restrictive/ repetitive behaviors, leading to significant life-time challenges [1,2]. ASD is a neurodevelopmental disorder with staggering clinical, social and financial burdens [3,4]. Attempts on understanding the underlying pathophysiology in ASD have led to two major schoolsof thought: one focuses on the abnormalities of the mirror neurons and other cortical areas involved in reciprocity, while the other proposes a more widespread altered neuronal organization and dynamics. Those advocating for the former theory attribute the limitations in social cognition in ASD to abnormal mirror neuron function or modulation [5-11]. Mirror neurons are concentrated in the inferior frontal gyrus (IFG) and the inferior parietal lobule (IPL) [6]. Additional cortical regions involved in abstract social cognition include the medial prefrontal cortex (mPFC), temporal-parietal junction (TPJ) and posterior cingulate gyrus (pCG) [12-14] which all together comprise a “social reciprocity network” [15].
Given the significant limitations of the existing pharmacological interventions, use of non-invasive brain stimulation such as repetitive Transcranial Magnetic Stimulation (rTMS) [16] has generated hope for mitigating ASD-related behaviors. rTMS is a promising tool in neuropsychiatry [17,18] and has demonstrated safety in children [19,20]. The metanalyses of the existing research suggest a modest improvement in some of the behavioral measures post rTMS in ASD [21,22]. While the protocols varied considerably, many used low frequency stimulation on the dorsolateral prefrontal cortex (DLPFC). This narrow focus of targeting DLPFC may preclude investigating other potential targets [23] such as the mirror neuron regions.
While the above rTMS based research studies have produced novel insights and yielded modest improvements in social relatedness and repetitive behaviors, we hypothesized that targeting the mirror neuron regions may provide additional insights and symptomatic improvement in ASD. There are very few studies using IPL or IFG as research/therapeutic targets [24-29]. Moreover, the existing body of literature attests to the hemispheric dependent function of the mirror neurons [30-32]. Leveraging this concept, we aimed to investigate the impact of rTMS stimulation of the bilateral IFG and IPL on social reciprocity in ASD.
This work followed our previous experiments of rTMS targeting the mirror neuron regions in ASD. Our feasibility study on 4 patients suggested that rTMS applied to the left-IPL may ameliorate social cognition. [33] More recently, we conducted a study applying 10 sessions of rTMS to the right or left IPL in subjects with ASD (DDD 604994, NCT 05371912) which demonstrated an improvement in social awareness [34].
Materials and Methods
This pilot project was funded by the Delaware Health Science Alliance (NCT 06807684) and conducted as a single-center, singleblind, sham controlled study to compare 2 parallel groups, active and sham at Christiana Care Health System in Delaware. The research protocol was approved by the Internal Review Board and the patients/parents signed the consent form to participate in this study.
A 3-week comparative study (phase I, control versus sham) was followed by an open-label 3-week period (phase II) where subjects in the sham group were switched to active, and the active group continued to receive active treatment. The patients were randomized for participation in the two treatment arms. Each patient received a total of 18 sessions (3/week for 6 weeks) of active rTMS or sham on the bilateral IPL/IFG, using Magstim® Horizon®. We delivered intermittent theta burst stimulation (iTBS) 2400 stimulations/session equally divided between the right and left IPL and IFG. In our experience, the threshold for adequate cortical stimulation in this age group is between 48-52% of the maximum stimulator output. Therefore, for consistency and to assure applying a super-threshold stimulation, we utilized 55% of the maximum stimulator output for all patients [35].
For targeting the IPL and IFG we used the standard 10/20 electrode placement system via a commercial EEG cap. The IPL localizes to the P3/4 [36] and the IFG to F5/6 [23] electrodes. During the sham phase of the study, we implemented a standard shamming technique which consists of rotating the coil by 90 degrees [37]. The patients/families and the sub-investigator performing and interpreting the neuropsychological assessments remained blinded regarding the randomization for the 2 study arms.
Outcome measures
Childhood Autism Rating Scale, 2nd edition, high functioning (CARS2, HF): This is a 15-item scale, designed for ASD patients’ level 1 and 2, 6-18 years old, implemented by a neuropsychologist to obtain quantitative behavioral measures based on patient’s performance, as well as questionnaires soliciting parental input [38]. The testing was completed at the end of phase I and II and 3 weeks after completion of TMS (phase III).
SRS-2: The Social Responsiveness Scale – Second Edition (SRS- 2) is a 65-item, caregiver-rated scale assessing social interaction and communication deficits [39]. The parents were asked to complete and submit the questionnaire electronically at the above intervals.
Data analysis
The CARS2 total scores across the study period were examined using a longitudinal Generalized Estimating Equation (GEE) model to account for within-subject correlation of repeated measures at four time points (Baseline, Phase I, Phae II, Phase III).
CARS2 total scores were modeled as a continuous outcome using a Gaussian distribution with an identity link. Fixed effects included treatment group, timepoint, and group by time interaction. The active/active group was the reference group. An exchangeable working correlation structure was specified as our data set was too small for stable AR(1) GEE.
To address small-sample bias in variance estimation, standard errors were obtained using a delete one cluster jackknife (Mancl & DeRouen 2001). All tests were two sided with statistical significance set at p<0.05.
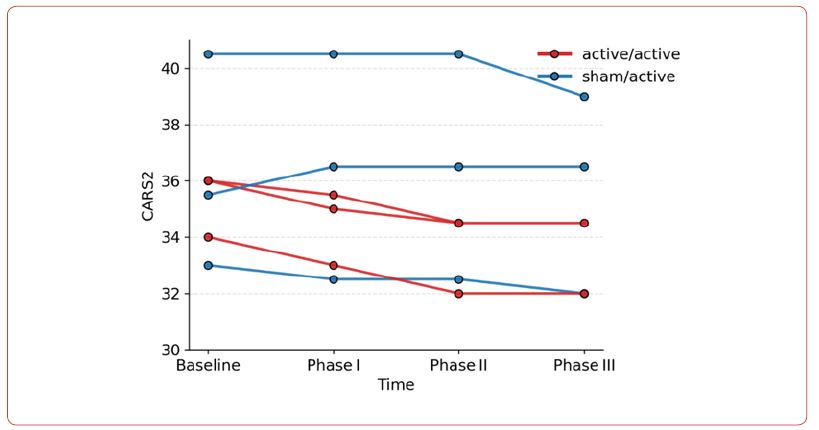
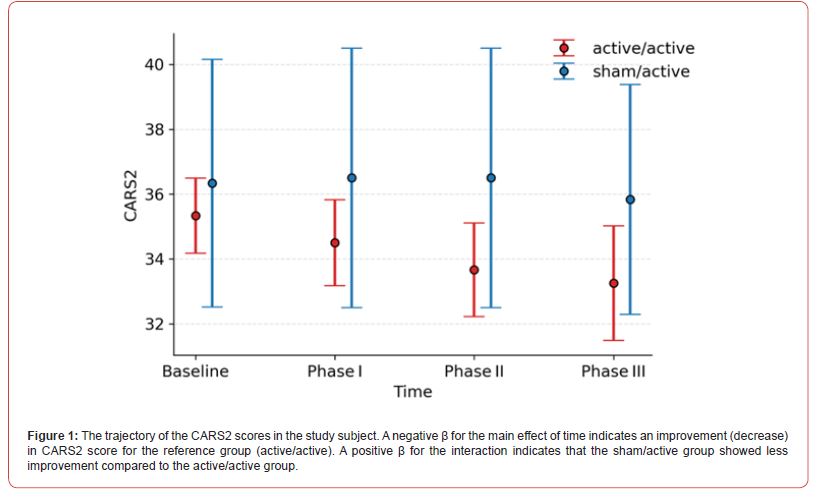
Results
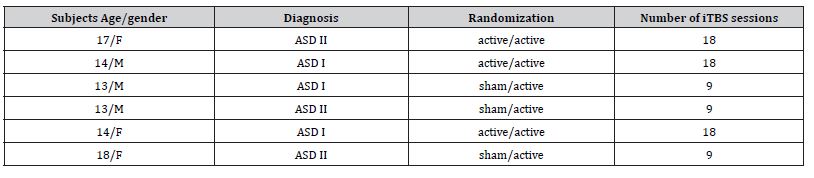
A total of 6 ASD subjects (3 girls and 3 boys) with an average age of 14.8 years were recruited between October 2024 to June 2025 and completed the study (table 2). None of the subjects experienced any adverse effects.
Table 1:Inclusion/exclusion criteria.
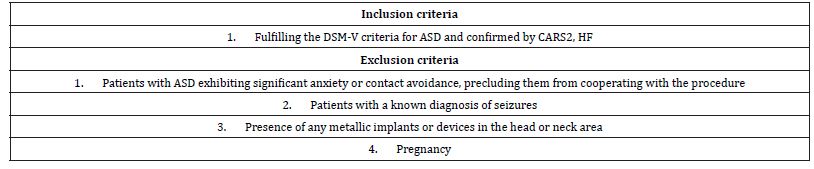
Table 2:Study subjects.
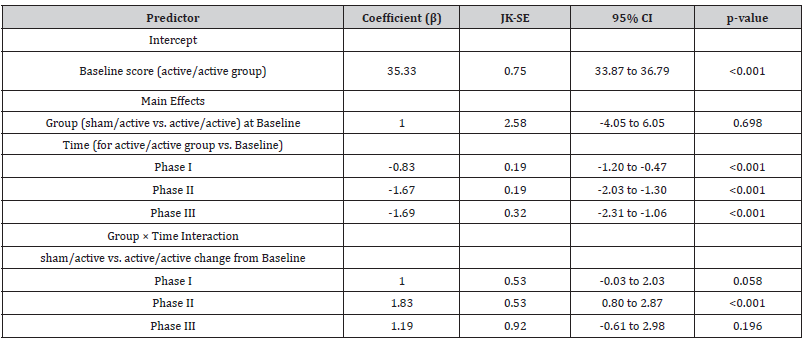
The GEE analysis demonstrated a significant main effect of time, indicating an overall reduction in CARS2 scores. In the active/ active group, the mean score decreased by 0.83 points at Phase I ((JK-SE = 0.19, p < 0.001), 1.67 points at Phase II (JK-SE = 0.19, p < 0.001), and 1.69 points at Phase III (JK-SE = 0.32, p < 0.001) relative to the baseline. There was a significant group by time interaction. Positive interaction coefficients indicate less improvement in the sham/active group.
At Phase I, the between-group difference approached significance (β = 1.00, JK-SE = 0.53, p = 0.058). In Phase II, the difference was significant; the sham/active group showed 1.83 points less reduction in scores (β = 1.83, JK-SE = 0.53, p < 0.001). In Phase III interaction was not significant (β = 1.19, JK-SE = 0.92, p = 0.196).
The parental reports using the SRS2 was uploaded by the parents at the end of each phase. Unfortunately, 50% of the data points were not completed and as such a statistical analysis was not possible. Moreover, given the small sample size, performing a statistical analysis on the different components of CARS2 was not possible.
Table 3:CARS2 mean and SD.
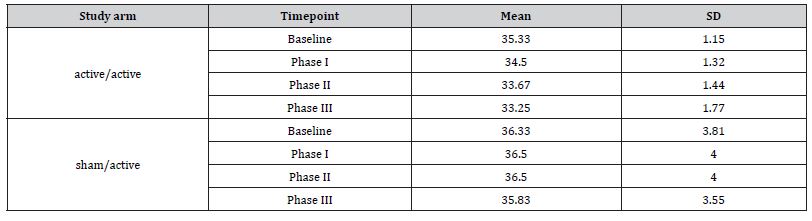
Table 4:Jackknife GEE Results for CARS2.
Discussion
We have recruited 6 subjects with ASD and administered iTBS stimulation of the bilateral IPL/IFG and demonstrated a decrease in the total CARS2 scores commensurate with an improvement in social reciprocity/communication. CARS2 is a semi quantitative assessment which evaluates social reciprocity, emotional responses as well as verbal and nonverbal communication. While we acknowledge the small sample size which limits the generalizability of our findings, we propose that conceptually speaking, targeting the bilateral “mirror neuron regions” such as IPL and IFG may be considered as a novel approach in designing rTMS treatment trials for ASD.
Despite the wealth of literature testifying to the role of mirror neurons in ASD and social cognition, [5-8] there are very few studies using IPL or IFG as research/therapeutic targets [24-29].
The hemispheric dependent function of mirror neurons is well recognized. While the left IFG is activated during observation of stimuli without an emotional component, the right IFG responds to the stimuli with an emotional component and hence contributes to experiencing empathy. Moreover, both the left IFG and IPL are involved in processing the verbal components of the social interactions and have a crucial role in the communication difficulties that characterize ASD. On the other hand, the right IPL is involved in multi-sensory integration/nonverbal communication. As such, while both the right and left IFG and IPL contribute to social cognition, they each orchestrate different elements of reciprocity [31,30,40]. We propose that leveraging the hemispheric dependent function of the IPL and IFG, such as bi-hemispheric stimulation of these areas, may allow for optimizing neurostimulation strategies.
Bi-hemispheric iTBS application in ASD is a novel approach and has been rarely attempted. Ni et al have spearheaded this line of work by conducting 2 separate studies implementing iTBS stimulation of the bilateral superior temporal sulcus in children and adults with ASD. While in their first experiment statistical significance was not reached, they demonstrated superiority of 8 weeks of intervention compared to 4 weeks [41]. In a more recent study of 13 adults with ASD they proposed potential therapeutic efficacy of this approach [42].
In our study, the subjects in the active/active arm had slightly lower CARS2 scores at baseline indicating a milder phenotype, which may have also contributed to the better outcome compared to the sham/active group. This finding is consistent with the previous observations reporting better treatment results produced by rTMS in individuals who are less severely affected [41]. The mechanisms of action of rTMS remain an area of significant investigation [43,44]. Our results have demonstrated longevity of the rTMS effects being sustained 3 weeks after the termination of the intervention, possibly attributable to an increased level of brain-derived neurotrophic factor leading to neuronal plasticity [45-47].
Conclusion
This small experiment serves as a proof of principle for the novel approach of iTBS targeting the bilateral IPL/IFG as a therapeutic intervention in ASD. Future studies with larger number of subjects are necessary to solidify this concept and further refine the approach. With continued investigation of the effects of rTMS and the neurobiological underpinnings of ASD, the future developments may eventually allow highly individualized neurostimulation approaches to treat ASD patients [48,49].
Acknowledgement
The authors acknowledge the contributions made by Stephanie Roth, Medical Librarian, and Esther Connor, REEG technician.
Conflicts of Interest
None of the authors have any conflicts to disclose.
References
- American psychiatric association, diagnostic and statistical manual of mentalmanual.
- M Fakhoury (2015) Autistic spectrum disorders: A review of clinical features, theories and diagnosis. Int J Dev Neurosci 43: 70-77.
- Center for Disease Control (2020) Community Report on Autism, Autism and Developmental Disabilities Monitoring (ADDM) Network.
- J P Leigh, J Du (2015) Brief Report: Forecasting the Economic Burden of Autism in 2015 and 2025 in the United States. Journal of Autism and Developmental Disorders 45(12): 4135-4139.
- M Dapretto (2006) Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience 9(1): 28-30.
- G Rizzolatti, M Fabbri-Destro, L Cattaneo (2009) Mirror neurons and their clinical relevance. Nature Clinical Practice Neurology 5(1): 24-34.
- J H G Williams, A Whiten, T Suddendorf, D I Perrett (2001) Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Reviews 25(4): 287-295.
- G Rizzolatti, M Fabbri-Destro (2010) Mirror neurons: from discovery to autism. Exp Brain Res 200(3-4): 223-237.
- L Yates, H Hobson (2020) Continuing to look in the mirror: A review of neuroscientific evidence for the broken mirror hypothesis, EP-M model and STORM model of autism spectrum conditions. Autism 24(8): 1945-1959.
- Y Wang, A F de C Hamilton (2012) Social top-down response modulation (STORM): a model of the control of mimicry in social interaction. Front Hum Neurosci 6: 153.
- A F de C Hamilton (2008) Emulation and Mimicry for Social Interaction: A Theoretical Approach to Imitation in Autism. Q J Exp Psychol (Hove) 61(1): 101-115.
- I Fishman, C L Keown, A J Lincoln, J A Pineda, R A Müller (2014) Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry 71(7): 751-760.
- R K Kana, L E Libero, M S Moore (2011) Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys Life Rev 8(4): 410-437.
- A F de C Hamilton, R M Brindley, U Frith (2007) Imitation and action understanding in autistic spectrum disorders: how valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia 45(8): 1859-1868.
- F B Kayarian, A Jannati, A Rotenberg, E Santarnecchi (2020) Targeting Gamma-Related Pathophysiology in Autism Spectrum Disorder Using Transcranial Electrical Stimulation: Opportunities and Challenges. Autism Res 13(7): 1051-1071.
- J P Lefaucheur (2014) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clinical Neurophysiology 125(11): 2150-2206.
- A Demirtas-Tatlidede, A M Vahabzadeh-Hagh, A Pascual-Leone (2013) Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology 64: 566-578.
- L M Oberman, A Rotenberg, A Pascual-Leone (2015) Use of transcranial magnetic stimulation in autism spectrum disorders. J Autism Dev Disord 45(2): 524-536.
- C Krishnan, L Santos, M D Peterson, M Ehinger (2015) Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul 8(1): 76-87.
- C H Allen, B M Kluger, I Buard (2017) Safety of Transcranial Magnetic Stimulation in Children: A Systematic Review of the Literature. Pediatr Neurol 68: 3-17.
- J B Barahona-Corrêa, A Velosa, A Chainho, R Lopes, A J Oliveira-Maia (2018) Repetitive Transcranial Magnetic Stimulation for Treatment of Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Frontiers in Integrative Neuroscience 12: 27.
- J R Smith (2022) Treatment Response of Transcranial Magnetic Stimulation in Intellectually Capable Youth and Young Adults with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Neuropsychol Rev 33(4): 834-855.
- Y Huang (2020) Potential Locations for Noninvasive Brain Stimulation in Treating Autism Spectrum Disorders-A Functional Connectivity Study. Front Psychiatry 11: 388.
- Y Yang, H Wang, Q Xue, Z Huang, Y Wang (2019) High-Frequency Repetitive Transcranial Magnetic Stimulation Applied to the Parietal Cortex for Low-Functioning Children with Autism Spectrum Disorder: A Case Series. Front Psychiatry 10: 293.
- J N Kang, J J Song, M F Casanova, E M Sokhadze, XL Li (2019) Effects of repetitive transcranial magnetic stimulation on children with low-function autism. CNS Neuroscience & Therapeutics 25(11): 1254-1261.
- L Jahangard (2019) Does rTMS on brain areas of mirror neurons lead to higher improvements on symptom severity and empathy compared to the rTMS standard procedure? - Results from a double-blind interventional study in individuals with major depressive disorders. J Affect Disord 257: 527-535.
- I Puzzo, N R Cooper, S Cantarella, P B Fitzgerald, R Russo (2013) The effect of rTMS over the inferior parietal lobule on EEG sensorimotor reactivity differs according to self-reported traits of autism in typically developing individuals. Brain Res 1541: 33-41.
- M C Keuken (2011) The role of the left inferior frontal gyrus in social perception: an rTMS study. Brain Res 1383: 196-205
- J Kaokhieo (2023) Effects of repetitive transcranial magnetic stimulation combined with action-observation-execution on social interaction and communication in autism spectrum disorder: Feasibility study. Brain Res 1804: 148258.
- M M Y Chan, Y M Y Han (2020) Differential mirror neuron system (MNS) activation during action observation with and without social-emotional components in autism: a meta-analysis of neuroimaging studies. Mol Autism 11(1): 72.
- Y Cheng, K H Chou, Y T Fan, C P Lin (2011) ANS: aberrant neurodevelopment of the social cognition network in adolescents with autism spectrum disorders. PLoS One 6(4): e18905.
- R K Kana (2017) Neural networks underlying language and social cognition during self-other processing in autism spectrum disorders. Neuropsychologia 102: 116-123.
- M Assadi, J Dave, P Leone, N Redjal, A Curtin (2020) Enhancement of behavioral and linguistic outcome measures in autism spectrum disorder through neuro-navigated transcranial magnetic stimulation: A pilot study. J Clin Neurosci 74: 151-154.
- M Assadi, R Koiler, T Harrison-Goldman, R Fischer, A Curtin (2023) The effect of repetitive transcranial magnetic stimulation on social cognition in autism spectrum disorder: preliminary analysis of a pilot clinical trial. Brain Network and Modulation 2(4): 73.
- P H Donaldson, N J Rinehart, P G Enticott (2015) Noninvasive stimulation of the temporoparietal junction: A systematic review. Neurosci Biobehav Rev 55: 547-572.
- U Herwig, P Satrapi, C Schönfeldt-Lecuona (2003) Using the International 10-20 EEG System for Positioning of Transcranial Magnetic Stimulation. Brain Topography 16(2): 95-99.
- S H Lisanby, D Gutman, B Luber, C Schroeder, H A Sackeim (2001) Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biological Psychiatry 49(5): 460-463.
- S Shaffer, J Fuentes (2014) On or Off the ‘Spectrum’? The Complexity of Screening and Diagnosing Autism Spectrum Disorder (ASD), JAACAP Connect 1: 2.
- J N Constantino (2013) Social Responsiveness Scale,” in Encyclopedia of Autism Spectrum Disorders, New York pp. 2919-2929.
- Q Chen, F E Garcea, R A Jacobs, B Z Mahon (2018) Abstract Representations of Object-Directed Action in the Left Inferior Parietal Lobule. Cereb Cortex 28(6): 2162-2174.
- H C Ni (2021) Intermittent theta burst stimulation over the posterior superior temporal sulcus for children with autism spectrum disorder: A 4-week randomized blinded controlled trial followed by another 4-week open-label intervention. Autism 25(5): 1279-1294.
- H C Ni, Hsiang Yuan Lin 2, Yi Lung Chen 3, June Hung 4, Chen Te Wu, et al. (2022) 5-day multi-session intermittent theta burst stimulation over bilateral posterior superior temporal sulci in adults with autism-a pilot study. Biomed J 45(4): 696-707.
- M S George, J J Taylor, E B Short (2013) The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry 26(1): 13-18.
- A Valero-Cabré, J L Amengual, C Stengel, A Pascual-Leone, O A Coubard (2017) Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev 83: 381-404.
- H Y Wang (2011) Repetitive transcranial magnetic stimulation enhances BDNF-TrkB signaling in both brain and lymphocyte. J Neurosci 31(30): 11044-11054.
- B Cheeran (2008) A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol 586(23): 5717-5725.
- A Jannati (2020) Continuous Theta-Burst Stimulation in Children with High-Functioning Autism Spectrum Disorder and Typically Developing Children. Front Integr Neurosci 14: 13.
- C Zrenner, D Desideri, P Belardinelli, U Ziemann (2018) Real-time EEG-defined excitability states determine efficacy of TMS-induced plasticity in human motor cortex. Brain Stimul 11(2): 374-389.
- B Zrenner, Christoph Zrenner, Pedro Caldana Gordon, Paolo Belardinelli, Eric J McDermott, et al. (2020) Brain oscillation-synchronized stimulation of the left dorsolateral prefrontal cortex in depression using real-time EEG-triggered TMS. Brain Stimul 13(1): 197-205.
-
Mitra Assadi MD*, Suzanne Bauer PsyD, Esq, Reza Koiler PhD, Ryan Ally MD, Richard Fischer MD and Rodney Scott MD, PhD. The Impact of Bi-hemispheric iTBS Stimulation of the IPL and IFG on Social Reciprocity in ASD. Glob J of Ped & Neonatol Car. 5(4): 2025. GJPNC.MS.ID.000620.
iTBS, Mirror neurons, IPL, IFG, Social reciprocity, Pathophysiology, Single-blind, Protocol, Neuropsychologist
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






