 Research article
Research article
Nutritional Practice-Related Alteration of Mesenteric Tissue Oxygenation in Fully Enteral-Fed Very and Extremely Preterm Neonates
Anna Petrova* Ezra Chefitz and Rajeev Mehta
Department of Pediatrics, Rutgers-Robert Wood Johnson Medical School, USA
Anna Petrova, Department of Pediatrics, Rutgers Robert Wood Johnson Medical School, One Robert Wood Johnson Place, New Brunswick, New Jersey, United States of America
Received Date:May 31, 2024; Published Date:June 14, 2024
Abstract
Objective: The role of full enteral feeding (FEF) methods in preterm infants who are at risk for inadequate regulatory mechanisms supporting nutritional requirements for intestinal oxygen supplies is not well understood. In this study, we aimed to determine how the FEF method affects mesenteric tissue oxygenation parameters in fully enterally fed very preterm neonates. We also assessed whether the changes in mesenteric tissue oxygenation during and after feeding in these infants are influenced by hemodynamic and feeding-associated parameters.
Methods: We conducted a study to examine the effects of different feeding methods (gavage feeding for 30 or 60 minutes, and bottle feeding for 30 minutes) on mesenteric tissue oxygenation (rSO2-%M) and fractional tissue oxygen extraction (FTOE-M) in 30 premature infants born at 24-32 weeks of gestational age. We used near-infrared spectroscopy (NIRS) to measure rSO2-%M values before, during, and after the feeds, and linked these values to oxygen saturation (SpO2%) to calculate FTOE-M. We used the prior-to-feeding measurements as a reference point for the estimation of the changes in collected parameters rSO2%-M, FTOE-M, SpO2%, HR (beats/min), and RR (breaths/min) during and after feeding in each infant and overall, in each feeding group.
Results: During feeding, infants showed a consistent decrease in rSO2-%M and an increase in FTOE-M, which lasted for 30 minutes after a 30-minute feeding via bottle/gavage and for 60 minutes after a 60-minute gavage feeding. The changes in rSO2%-M and FTOE-M during feeding were exacerbated by higher respiratory rates and the use of preterm formula, but not feeding rate, gestational age, systemic oxygenation, or heart rate.
Conclusion: Regardless of the method, providing full enteral feeding to very or highly preterm neonates is associated with decreased oxygen levels and increased oxygen consumption in the mesenteric tissue. Higher respiratory rates and the use of preterm formula worsen the effect of feeding on mesenteric tissue oxygen levels.
Keywords:Mesenteric tissue oxygenation; very/extremely premature infants; near-infrared spectroscopy; full enteral feeding
Introduction
There is compelling evidence that full enteral feeding (FEF) in very/extremely preterm neonates could predispose them to intestinal ischemia due to inadequacies in the regulatory mechanisms for maintaining oxygen required for nutrient absorption [1-4]. The attainment of FEF in these infants is associated with an increased risk for necrotizing enterocolitis (NEC) [5-8], where intestinal ischemia is the primary underlying factor for the development of this prematurity-related devastating inflammatory bowel disease [9,10-12]. For that reason, optimizing the FEF strategies is a priority in neonatal medicine [13].
Near-infrared spectroscopy (NIRS) technology has been used to assess the role of different methods of enteral feeding in the alteration of intestinal oxygenation in immature infants. Studies have reported inconsistent findings showing increased splanchnic oxygenation after a 10-minute bolus [14,15] and either decreased [14] or no change [15] after 3 hours of continuous feeding. Sirota GL et al. [16] identified lower splanchnic oxygenation in preterm infants after 5 hours of continuous feeding than after 20-minute bolus feeding [16]. A randomized study did not show any difference in the NIRS-detected splanchnic tissue oxygenation after a 10-minute bolus feed as opposed to 3 hours of continuous feeding [17]. There is still a need for a better understanding of the role of different methods of FEF in the oxygenation of mesenteric tissue oxygenation in very preterm infants.
In the present study, we determined if and to what extent the FEF method, such as bottle or gavage feeds over 30 minutes or gavage over 60 minutes, altered the mesenteric tissue oxygenation parameters in fully enterally fed very preterm neonates. Additionally, we assessed whether the hemodynamic and feedingassociated parameters influence the changes in the mesenteric tissue oxygenation during and after feeding in the studied infants. Our study could contribute to the existing knowledge about feeding methods associated with deoxygenation of mesenteric tissue in immature infants at risk for NEC.
Material and Methods
For this study, we used a convenience sample of infants born at 32 or fewer weeks of gestational age to assess the changes in mesenteric tissue oxygenation parameters during and after FEF at a target volume of 140 to 160 mL/kg/day via bottle or gavage over 30 versus 60 minutes via an infusion pump. Infants with congenital anomalies, mechanical ventilation, diagnosis of NEC, sepsis, hemodynamically significant patent ductus arteriosus (PDA), or intraventricular hemorrhage (IVH) grade III-IV were excluded. Rutgers Robert Wood Johnson Medical School’s Institutional Review Board (IRB) approved the study, which required parental informed consent for participation. Participation of infants in this study did not influence their routine care. The feeding methods assigned by the practicing neonatologist included nasogastric tube feeding via gavage over approximately 30 minutes or with an infusion pump over 60 minutes, or bottle feeds with paced sucking for an average of 30 minutes.
We used the INVOS 5100B NIRS monitor and neonatal sensors (Somanetics Corporation, Michigan, USA) to record the rSO2- %M, which reflects a combination of intravascular oxygenated/ deoxygenated venous, arterial, and capillary hemoglobin (Hb) in approximately a 75:20:5 ratio [18-20]. The sensor was applied to the skin surface in the midline just below the umbilicus and secured with bio-occlusive dressing to prevent sensor movement/ detachment and artifacts during the feeding. The INVOS system utilizes light‐emitting diodes (wavelength 730 and 810 nm). It uses the modified Beer-Lambert law to measure tissue oxygen saturation and eliminate the contribution of superficial tissue by utilizing the principle of spatial resolution (depth of photon penetration proportional to the source-detector separation). The neonatal sensor, which is approved for use in patients <5 kg, has source-detector distances of 3 and 4 cm. The maximum reading is 95% and the minimum is 15% [21].
We started rSO2-%M monitoring 30 minutes before the infant was due to be fed (T0) and continued during the feeding (T1) and post-feeding for at least 60 minutes (T2). The rSO2-%M values were recorded at 1-minute intervals. The measurements of the oxygen saturation (SpO2%), heart rate (HR beats/min), and respiratory rate (RR breaths/min)] were obtained using the GE DASH 4000 (GE Healthcare, Waukesha, WI, USA) and logged at 1-minute intervals corresponding to the timing of the NIRS measurements. The timetable of the NIRS and vital sign monitoring was collected for each study participant. The DASH 4000 monitor displays newborn SpO2% ranges from 50% to 100% and HR from 30 to 200 beats/ min. The rSO2-%M and SpO2% were used to calculate the fractional tissue oxygen extraction in the mesenteric tissue (FTOE-M) as follows: [SpO2% - rSO2%-M] × 100/SpO2% [22,23]. During this study, the target oxygen saturation (SpO2%) for preterm infants receiving oxygen in the unit was 88–94%.
We used the electronic health records to collect the following variables: gender, gestational age and weight at birth (weeks, grams), age and weight at the time of NIRS monitoring (day of life [DOL, days], postmenstrual age [PMA, weeks], weight [grams]. The type of procedures and diagnosis documented within 24 hours of the NIRS monitoring such as hematocrit [Hct %], blood transfusion, use of oxygen through a nasal cannula or continuous positive airway pressure [CPAP], caffeine, and any neonatal morbidities were also collected. We obtained feeding data, including the type of feeding (mother’s own milk [MOM] or preterm formula [PF]) and volume of feeding per feed from the bedside nursing flow sheet, and calculated the rate of feeding per minute (mL/min).
All statistical analyses were conducted using STATISTICA 13.2 (TIBCO Software Inc., Palo Alto, CA, USA). We compared the demographic, clinical, and feeding characteristics in the three feeding groups using chi-square for categorical and the analysis of variance (ANOVA) followed by the Tukey honest significant difference test for the continuous variables. We used the prior-tofeeding measurements as a reference point for the estimation of the changes in rSO2%-M, FTOE-M, SpO2%, HR (beats/min), and RR (breaths/min) during and after feeding in each infant and overall, in each feeding group. The Kruskal-Wallis ANOVA and Median Test for three independent groups and the Mann-Whitney U Test for two independent groups were used to analyze the nonparametric distributed continuous variables. To obtain a better understanding of the duration of postprandial changes in the mesenteric tissue oxygenation, the rSO2%-M (T2) and FTOE-M (T2) were analyzed in two periods for approximately 30 minutes each (T2/1 and T2/2). We also calculated the percent change of rSO2%-M and FTOE-M during and after the feeding as compared to T0. The percent change represents the absolute difference of rSO2%-M (T2) and FTOE-M at T0 to that during (T1) and after (T2/1 and T2/2) the feeding divided by the T0 values multiplied by 100 [24]. We constructed regression models to control the changes in the mesenteric tissue oxygenation parameters recorded during the feeding for systemic oxygen saturation, respiratory and heart rates, and relevant clinical data. We used stepwise linear regression models to select the large effect size factors [25] to predict the degree of percent change of rSO2%-M and FTOE-M during the feeding and in the postprandial stage.
Statistical findings are presented as mean with +/- standard deviation (SD), median with inter-quartile range (IQR) if required, to analyze the non-parametric data and regression coefficient (β) with a 95% Confidence Interval (95%CI). The statistical significance of the comparison measurements (T0-T1, T0-T2/1, T0- T2/2) obtained by the NIRS and GE DASH 4000 monitoring at oneminute intervals were defined at a P value of <0.001. A p-value of <0.05 was considered statistically significant for the comparison of the clinical, and feeding parameters, and group-based comparison of the percent change of rSO2%-M and FTOE-M.
Results
Thirty preterm infants born with a gestational age of 32 or fewer weeks were studied. The birth gestational age of the infants fed with 30-minute gavage was 26-32 weeks (n=10), 60-minute gavage was 24-27 weeks (n=10), and via bottle was 30-32 weeks (n=10). Infants in Group 2 were more likely to have been diagnosed with IVH, and PDA and had received a blood transfusion, mechanical ventilation/CPAP, and caffeine (Table 1). MOM was the primary nutrition for 70% of the infants in each feeding group. As shown in Table 1, the volume and rate of feed delivery (mL/min), were lower in the infants fed via 60-minute gavage as compared to those fed with 30 minutes of gavage or bottle. We recorded a decrease of the rSO2%-M and an increase of the FTOE-M during the feeding, which continued after the feeding for up to 30 minutes in infants fed via 30 minutes of gavage or bottle and up to 60 minutes in infants fed via 60 minutes of gavage (Table 2). However, the feeding groups were comparable for the percent change of rSO2%-M and FTOE-M during the feeding and at two points in the postprandial stage (Table 3). As shown in Table 2, there were changes in the simultaneously recorded vital signs, including an increase of HR and RR during the feeding in the infants fed with 30 and 60 minutes of gavage, and a decrease of the SpO2% in infants fed via a bottle. The postprandial levels of HR, RR, and SpO2% were comparable to the baseline in each infant feeding group.
Table 1:Feeding method-based comparison of infants’ clinical and feeding characteristics.
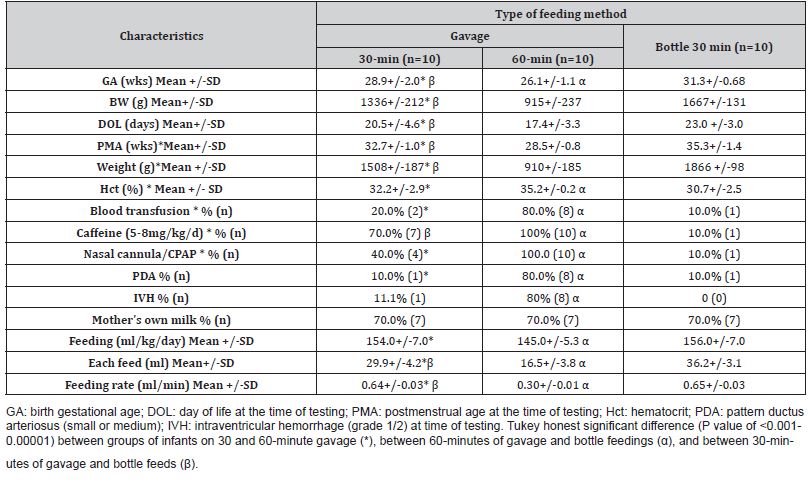
Table 2:Characteristic of changes in tested parameters from before to during and after feeding within each feeding group.
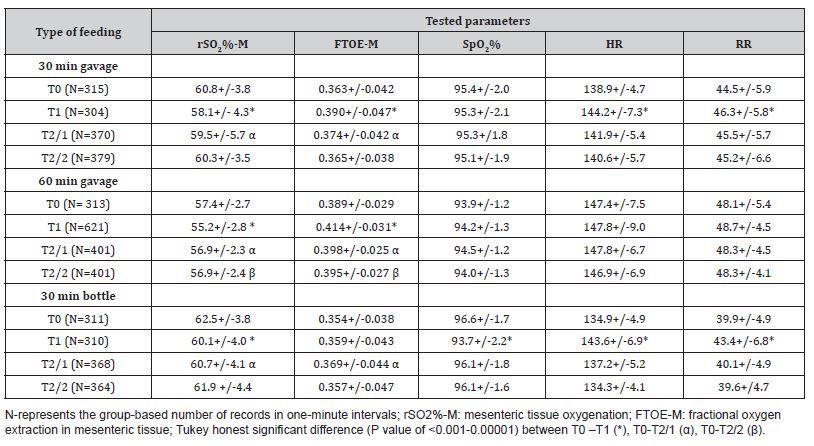
Table 3:Feeding method-based comparison of percent change of rSO2%-M and FTOE-M during and after feeding *
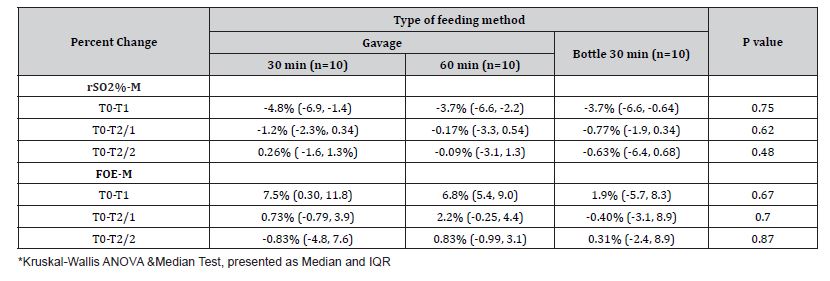
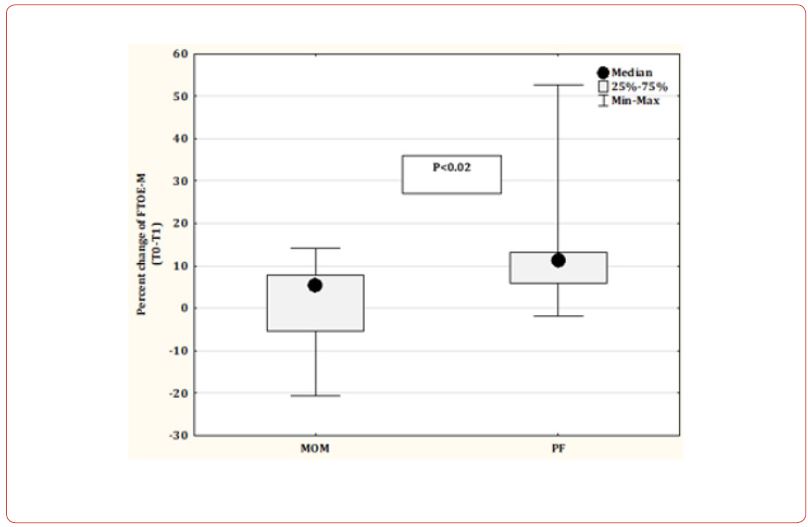
We constructed stepwise linear regression models (Table 4) where the percent change of rSO2%-M and FTOE-M were dependent variables. Predictive variables in Model 1 included the HR, RR, and SpO2% recorded before the feeding, Model 2 included the HR, RR, and SpO2% recorded during the feeding, and Model 3 included the GA (weeks), feeding rate (mL/min), type of feeding (MOM vs. PF), and the method of feeding (gavage vs. bottle). As shown in Table 4, Model 1 indicates the effect of the higher RR recorded at the T0 point on the prediction of a greater percent reduction of rSO2%-M. Furthermore, Models 1 and 2 revealed the association of higher RR at T0 and T1 points with a greater risk for the percent increase of FTOE-M. Except for the type of feeding (PF vs. MOM), none of the factors included in Model 3, were predictive of the magnitude of change in the mesenteric tissue oxygenation parameters (Table 4). Feeding with PF predicted a higher level of rSO2%-M reduction and an increase of FTOE-M as compared to feeding with MOM. Figure 1 presents a subgroup analysis showing a significantly higher median percent reduction of rSO2%-M and a higher percent increase of the FTOE-M in the infants fed with PF compared to those receiving MOM.
Table 4:Factors predicting the percent change of rSO2%-M and FTOE-M during feeding.
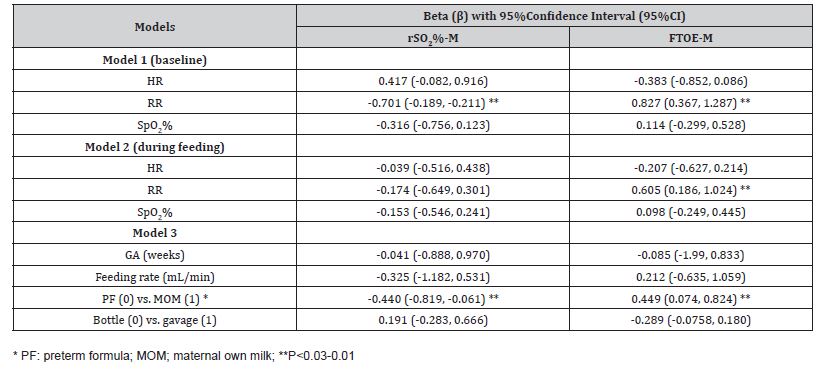
Figure 1: Mother’s own milk (MOM) versus preterm formula (PF) in percent change of rSO2%-M and FTOE-M level during feeding.

Discussion
The main findings from our study were recording a similar level of rSO2%-M reduction and FTOE-M increase during feeding that sustained for 30 minutes after a bottle or 30-minute gavage feeding but extended up to the end of the observation in the infants fed via 60-minute gavage. Constructed regression models revealed the association of increased RR and use of preterm formula with worsening changes of rSO2%-M and FTOE-M during the feeding, irrespective of the method and rate of feeding, gestational age, heart rate, and oxygen saturation.
Our study results correspond with the existing evidence that in neonates, oxygen demand during and after feeding is reliant on increased oxygen extraction [1,26]. Extreme prematurityassociated risk for the maximization of the baseline level of oxygen extraction [1,27] could predispose to the prolongation of the postprandial alteration of mesenteric tissue oxygenation in studied infants fed via gavage for 60 minutes. Moreover, the lack of HR acceleration during and after feeding in these infants reflects the insufficiency of the sympathetic system to mediate the increase of cardiac output to support the adequacy of the during-nutrition mesenteric tissue oxygenation [28,29]. Like the study by Poets et al. [30], we too recorded systemic desaturation during bottle feeding, which did not affect the degree of the mesenteric tissue oxygenation and extraction and did not extend to the postprandial stage. The study by Massa-Buck et al. [31] supports our findings, presenting a negative correlation of RR with oxygenation in the cerebral and somatic tissue in neonates born at 22 to 42 weeks of gestation. Some reports discuss the role of MOM, showing no impact of a 20-minute bolus feed with MOM but a persistent reduction of mesenteric tissue oxygenation in very preterm infants fed with the preterm formula [32]. Besides, exclusive feeding with human milk decreases the odds of developing NEC by 77% [5]. We could not assess the role of MOM in the risk reduction for NEC, but our data support the advantage of MOM over the preterm formula regarding the possible prevention of hypoxic injury to the mesenteric tissue. However, it is unclear why human milk is linked to a lower incidence of NEC or why some infants fed exclusively human milk develop NEC [2]. A recent study by Özkan H et al [33] did not find any worsening of oxygenation in the mesenteric tissue after the first bolus feeding but reported reduced oxygenation prior to feeding in very preterm infants who developed NEC at age 12.3±5.3 days as compared to infants who did not. Other investigators reported that decreased mesenteric tissue oxygenation and increased oxygen extraction within twenty-four hours after the onset of NEC symptoms predicted the progression of NEC to bowel perforation or death [34].
To date, no study has identified and validated the level of deoxygenation in the mesenteric tissue before the onset of NEC [35]. We could not assess the clinical value of the alteration in the mesenteric tissue oxygenation recorded during and after feeding because none of the enrolled infants developed NEC. Moreover, NIRS monitoring conducted for one feeding period/epoch is insufficient to make any clinical recommendations. However, identifying the factors that increase the risk for deoxygenation in mesenteric tissue could be crucial for further understanding the pathogenesis of NEC and developing an appropriate intervention to reduce the occurrence and severity of NEC.
We want to acknowledge several limitations of our study, including the use of a non-random sample, which increases the risk of selection bias, and the small number of participants even though the pre-post design permits the use of fewer participants as compared to a group-based comparison [36]. Furthermore, the observational design did not allow for the identification of the causal inference of the feeding methods on the tested parameters.
Conclusion
This study identified quantitatively similar reduction of oxygenation and increased oxygen extraction in the mesenteric tissue during feeding via bottle or gavage for 30 minutes or gavage over 60 minutes in the studied very/highly preterm neonates. Higher respiratory rates and the use of preterm formula are factors that worsen the magnitude of alterations in mesenteric tissue oxygenation during the feeding, regardless of the feeding method, gestational age, heart rate, and systemic oxygen saturation. We speculate that selective and continuous NIRS monitoring could be beneficial for the early recognition of abnormal intestinal perfusion in extremely preterm-born infants, especially those who are exclusively formula-fed.
Funding
This research received no external funding.
Acknowledgment
We acknowledge the assistance of Mayur Bhatt, MD, and Molly Nadelson, MD in participation of collection of NIRS measurements.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- Dotinga BM, Mintzer JP, Moore JE, Hulscher JBF, Bos AF, et al. (2020) Maturation of Intestinal Oxygenation: A Review of Mechanisms and Clinical Implications for Preterm Neonates. Front Pediatr 8: 354.
- Shulhan J, Dicken B, Hartling L, Larsen BM (2017) Current Knowledge of Necrotizing Enterocolitis in Preterm Infants and the Impact of Different Types of Enteral Nutrition Products. Adv Nutr 8(1): 80-91.
- Krimmel GA, Baker R, Yanowitz TD (2009) Blood transfusion alters the superior mesenteric artery blood flow velocity response to feeding in premature infants. Am J Perinatol 26(2): 99-105.
- Hunter CJ, Upperman JS, Ford HR, Camerini V (2008) Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr Res 63(2): 117-123.
- Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, et al (2010) An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 156(4): 562-567.e1.
- Walsh V, Brown JVE, Copperthwaite BR, Oddie SJ, McGuire W, et al. (2020) Early full enteral feeding for preterm or low birth weight infants. Cochrane Database Syst Rev 12(12): CD01354.
- Berti E, Puglia M, Perugi S, Gagliardi L, Bosi C, et al (2018) Feeding practices in very preterm and very low birth weight infants in an area where a network of human milk banks is in place. Front Pediatr 6: 387.
- Kuik SJ, van Zoonen AGJF, Bos AF, Van Braeckel KNJA, Hulscher JBF, et al. (2019) The effect of enteral bolus feeding on regional intestinal oxygen saturation in preterm infants is age-dependent: a longitudinal observational study. BMC Pediatr 19(1): 404.
- Claud EC (2009) Neonatal necrotizing enterocolitis -inflammation and intestinal immaturity (2009) Antiinflamm Antiallergy Agents Med Chem pp. 248-259.
- Palleri E, Wackernagel D, Wester T, Bartocci M (2020) Low splanchnic oxygenation and risk for necrotizing enterocolitis in extremely preterm newborns. J Pediatr Gastroenterol Nutr 71(3): 401-406.
- Caplan MS (2014) Necrotizing enterocolitis in preterm infants is related to enteral feeding, but the mechanisms remain uncertain and have changed over time. Curr Pediatr Rep 2: 241-247.
- Alsaied A, Islam N, Thalib L (2020) Global incidence of necrotizing enterocolitis: a systematic review and Meta-analysis. BMC Pediatr 20(1): 344.
- Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, et al. (2009) Role of human milk in extremely low birth weight infants' risk of necrotizing enterocolitis or death. J Perinatol 29(1): 57-62.
- Corvaglia L, Martini S, Battistini B, Rucci P, Aceti A, et al. (2014) Bolus vs. continuous feeding: effects on splanchnic and cerebral tissue oxygenation in healthy preterm infants. Pediatr Res 76(1): 81-85.
- Dani C, Pratesi S, Barp J, Bertini G, Gozzini E, et al. (2013) Near-infrared spectroscopy measurements of splanchnic tissue oxygenation during continuous versus intermittent feeding method in preterm infants. J Pediatr Gastroenterol Nutr 56(6): 652-656.
- Sirota GL, Litmanovitz I, Vider C, Arnon S, Moore SS, et al. (2022) Regional splanchnic oxygenation during continuous versus bolus feeding among stable preterm infants. Children (Basel) 9(5): 691.
- Bozzetti V, Paterlini G, De Lorenzo P, Gazzolo D, Valsecchi MG, et al. (2016) Impact of continuous vs bolus feeding on splanchnic perfusion in very low birth weight infants: A randomized trial. J Pediatr 176: 86-92.e2.
- Kleinman CS, Seri I, Victor S, Weindling MA (2008) Near-infrared spectroscopy and its use for the assessment of tissue perfusion in the neonates, in Kleinman CS, Seri I (eds). Hemodynamics and Cardiology. Elsevier Health Sciences: Philadelphia PA pp. 111-130.
- Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bülow J, et al. (2001) Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports 11(4): 213-222.
- Franceschini MA, Boas DA, Zourabian A, Diamond SG, Nadgir S, et al. (2002) Near-infrared spiroximetry: noninvasive measurements of venous saturation in piglets and human subjects. J Appl Physiol 92(1): 372-384.
- Gomez A, Sainbhi AS, Froese L, Batson C, Alizadeh A, et al. (2021) Near infrared spectroscopy for high-temporal resolution cerebral physiome characterization in TBI: A narrative review of techniques, applications, and future directions. Front Pharmacol 12: 719501.
- Wardle SP, Yoxall CW, Weindling AM (2000) Determinants of cerebral fractional oxygen extraction using near infrared spectroscopy in preterm neonates. J Cereb Blood Flow Metab 20(2): 272-279.
- Owen-Reece H, Smith M, Elwell CE, Goldstone JC (1999) Near infrared spectroscopy. Br J Anaesth 82(3): 418-426.
- Vickers AJ (2001) The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol 1: 6.
- Bozzetti V, Paterlini G, Meroni V, DeLorenzo P, Gazzolo D, et al. (2012) Evaluation of splanchnic oximetry, Doppler flow velocimetry in the superior mesenteric artery and feeding tolerance in very low birth weight IUGR and non-IUGR infants receiving bolus versus continuous enteral nutrition. BMC Pediatr12: 106.
- Eldelstone DI, Holzman IR (1981) Gastrointestinal tract O2 uptake and regional blood flows during digestion in conscious newborn lambs. Am J Physiol 241(4): G289-293.
- Zhang JH, Guan RL, Pan PP, Lu WN, Zhang HY, et al. (2021) Changing trend of abdominal regional oxygen saturation in very/extremely low birth weight infants in the early postnatal stage: a prospective study. Zhongguo Dang Dai Er Ke Za Zhi 23(10): 1015-1020.
- Martinussen M, Brubakk AM, Vik T, Yao AC (1996) Mesenteric blood flow velocity and its relation to transitional circulatory adaptation in appropriate for gestational age preterm infants. Pediatr Res 39(2): 275-280.
- Lipsitz LA, Ryan SM, Parker JA, Freeman R, Wei JY, et al. (1993) Hemodynamic and autonomic nervous system responses to mixed meal ingestion in healthy young and old subjects and dysautonomic patients with postprandial hypotension. Circulation 87(2): 391-400.
- Poets CF, Langner MU, Bohnhorst B (1997) Effects of bottle feeding and two different methods of gavage feeding on oxygenation and breathing patterns in preterm infants. Acta Paediatr 86(4): 419-423.
- Massa-Buck B, Amendola V, McCloskey R, Rais-Bahrami K (2017) Significant correlation between regional tissue oxygen saturation and vital signs of critically ill infants. Front Pediatr 5: 276.
- Dani C, Coviello C, Montano S, Remaschi G, Petrolini C, et al. (2021) Effect on splanchnic oxygenation of breast milk, fortified breast milk, and formula milk in preterm infants. Pediatr Res 89(1): 171-174.
- Özkan H, Çetinkaya M, Dorum BA, Köksal N (2021) Mesenteric tissue oxygenation status on the development of necrotizing enterocolitis. Turk J Pediatr 63(5): 811-817.
- Schat TE, Schurink M, van der Laan ME, Hulscher JB, Hulzebos CV, et al. (2016) Near-infrared spectroscopy to predict the course of necrotizing enterocolitis. PLoS One 11(5): e0154710.
- Howarth C, Banerjee J, Leung T, Aladangady N (2022) Could Near Infrared Spectroscopy (NIRS) be the new weapon in our fight against necrotising enterocolitis? Front Pediatr 10: 1024566.
- Guo Y, Logan HL, Glueck DH, Muller KE (2013) Selecting a sample size for studies with repeated measures. BMC Med Res Methodol 13: 100.
-
Anna Petrova* Ezra Chefitz and Rajeev Mehta. Nutritional Practice-Related Alteration of Mesenteric Tissue Oxygenation in Fully Enteral-Fed Very and Extremely Preterm Neonates. Glob J of Ped & Neonatol Car. 4(5): 2024. GJPNC.MS.ID.000600.
Mesenteric tissue oxygenation, Very/extremely premature infants, Near-infrared spectroscopy, Tissue oxygenation, Bottle and bolus feeding, Hemodynamically, Nasogastric tube, Persistent reduction
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






