 Case Report
Case Report
Impact Of Surfactant Therapy on Respiratory Distress Syndrome in Nigerian Neonates: A Report of Two Cases
Oloyede IP1*, Ebi U2, Ekpo FE3 and Ekanem EE4
1Honorary Consultant Department of Paediatrics, University of Uyo Teaching Hospital Uyo, Nigeria
2Department of Anaesthesia, University of Uyo Teaching Hospital, Nigeria
3Medical Officer, Ibom specialist Hospital, Nigeria
4Department of Paediatrics, University of Calabar Teaching Hospital, Calabar, Nigeria
Iso Precious Oloyede, Honorary Consultant Department of Paediatrics, University of Uyo Teaching Hospital Uyo, Akwa Ibom State, Nigeria.
Received Date: July 23, 2021; Published Date: August 18, 2021
Abstract
Introduction: Lack of capacity to manage respiratory distress syndrome (RDS) has been cited by several workers as a major cause of neonatal mortality in Nigeria. Continuous positive airway pressure and surfactant administration have been identified as the main stay of treatment. The difficulty in sourcing surfactant and paucity of facilities for CPAP ventilation are major challenges in our region. Two cases illustrating improved access to the modality of treatment in our environment is presented.
Case summary: We present a case report of two preterm neonates who were referred to our facility with a history of prematurity, respiratory distress and low oxygen saturation on pulse oximetry. The children were treated with intra- tracheal bovine lipid extract surfactant (BLES) sourced at a relatively cheap cost, and CPAP. They improved rapidly and were discharged home within two weeks of admission with no noticeable sequelae.
Conclusion/Recommendation: Surfactant administration and CPAP ventilation are the optimal treatment for RDS and are now available in our environment at a relatively affordable cost. We therefore recommend the provision of CPAP in all facilities where babies are delivered, subsidization of the cost of surfactants and training of more health-workers on surfactant administration.
Keywords: Respiratory distress syndrome; Surfactant; CPAP; Nigeria
Introduction
Respiratory distress syndrome (RDS) is the commonest respiratory disorder of premature infants. Its incidence is directly proportional to the degree of prematurity [1]. The incidence of RDS in Nigeria is largely unknown, however approximately 400,000 children neonates are diagnosed of RDS yearly in the United States of America [2]. Some Nigerian studies have reported RDS as a major cause of death in premature neonates [3-5]. All these studies reported unavailability of surfactants within the country as a major factor in neonatal mortality from RDS. However, a more recent study by Okonkwo et al, while corroborating the limited use of surfactant replacement therapy (SRT) in Nigerian health facilities, has pointed to the improved survival of pre-terms who had RDS especially when given with adequate respiratory support [6]. The severity of RDS varies widely from the need for low supplemental oxygen to lethal respiratory failure even with surfactant therapy and mechanical ventilation. The presentation of RDS results from a surfactant deficient lung that is prone to collapse leading to ventilation-perfusion mismatch, severe hypoxia and lung injury when spontaneous or mechanical ventilation is initiated [7]. The diagnosis of RDS is based on initial clinical symptoms, chest radiograph picture consistent with the disease, the clinical course and response to surfactant therapy [1]. Treatment modalities including use of antenatal steroids, supplemental oxygen, surfactant treatment and Continuous Positive airway pressure (CPAP) ventilation in high resource countries, have led to a marked reduction of mortality in preterm babies with RDS [8,9]. In low and middle income countries (LMIC) a combination of host factors like sepsis, birth asphyxia, necrotizing enterocolitis, pulmonary hypoplasia potentiates the course of RDS leading to higher mortality from the disease [10]. Absence of skilled personnel, able to administer surfactant and CPAP add to the picture [2].
We therefore report the first two cases of preterm babies treated in a quaternary hospital in Uyo Akwa Ibom State, Nigeria for RDS using relatively low priced and relatively easily sourced surfactant and mechanical ventilation. We hope that this report will add to the body of knowledge needed by policy makers to deliberate on the need to prioritize scarce resources and consider the inclusion of C-PAP and surfactants as essential interventions for new-borns with the aim of reducing childhood mortality due to prematurity in general and RDS in particular.
Case Report
Baby EE, a 32 hour male infant was referred from a private clinic with complaints of premature birth and respiratory distress of 2 hours after delivery. Baby was delivered at ?36 weeks gestational age (GA) to a 33 year old blood group O+ve booked mother via elective Caesarean section (ELCS). The ELCS was secondary to type three placentae praevia. The mother had a first episode of per vaginal bleed at six months GA and was admitted for a two day bed rest. A second episode occurred about three days post discharge for which she was readmitted for a three week period and later discharged. She received two doses of antenatal steroids and was booked for an ELCS at 36 weeks. Mother went for the scheduled ELCS and baby was delivered with cord round the neck. The baby developed respiratory distress about two hours post-delivery and had a single episode of high grade fever (t=38.9). The baby was transferred to the incubator and managed with locally improvised bubble C-PAP, supplemental oxygen, intravenous ceftriaxone and gentamicin. Due to worsening symptoms the baby was referred to our facility.
Physical examination on admission revealed severe respiratory distress, tachypnea and dyspneoa. He was plethoric with mild to moderate jaundice. A ballard score done corresponded to 34 weeks GA. His anthropometry were as follows: weight – 2.6kg, length- 50cm, occipitofrontal circumference – 33cm. His vital signs were as follows Temperature = 37.6°c, SPO2 =82%, Respiratory rate (RR)= 96 breaths/min, Heart rate (HR)= 192 beats/min. Significant systemic examination findings were vesicular breath sounds with bilateral coarse crepitations; the first and second heart sound were heard with a gallop rhythm and the liver was tipped. The chest radiograph on admission showed the typical bilateral ground glass appearance (Figure 1).
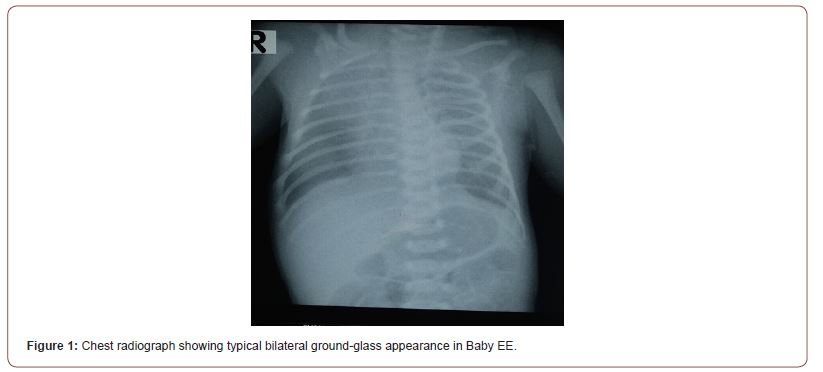
A diagnosis of RDS in a preterm neonate, congenital pneumonia complicated with congestive heart failure and neonatal jaundice was made. The infant was given 270mg of bovine lipid extract surfactant (BLES )from Thompson and Grace pharmaceuticals via the endotracheal route and ventilator driven Nasal C-PAP was commenced at 60% oxygen and 6cm H2O PEEP. Other supportive treatment included intravenous 8% D/15th saline, IV frusemide 4mg stat, Iv ceftazidime and IV genticin. In addition, child was nursed in a thermo-neutral environment under phototherapy light, commenced on nil per oris, as required suctioning and continuous vital signs monitoring. A second dose of endotracheal BLES was given 48 hours after the first dose with an accelerated improvement in the child’s condition. The infant’s RR (96 to 58 breaths/min), HR (196 to 144 bpm) and SPO2 (82 to 96-99%) gradually came back to normal by the fourth day on admission. Oral feeds were established by the fifth day of admission. The CPAP oxygen concentration and PEEP was gradually reduced and discontinued by the fifth day on admission. The baby was discharged home on day 10 of admission after completion of antibiotics. He has been regular on follow-up and is doing very well with no evidence of chronic lung disease.
Baby OU, a seven hour old female neonate was referred from a private clinic on account of difficulty in breathing, fast breathing and dropping SPO2 in spite of intranasal oxygen. She was delivered at 33 weeks of gestation via emergency CS on account of placenta praevia major with antepartum hemorrhage to a 33 year old Para 2 lady. Her Apgar scores were 7 and 8 at one and five minutes respectively. Her birthweight was 2kg. Her mother received two doses of intramuscular dexamethasone at 31 weeks of gestation.
Physical examination on admission revealed a severely cyanosed female neonate, with grunting respiration, severely dyspneic and tachypneic. Her SPO2 was 50%, RR= 100breaths/min, HR= 180 bts per minute. Her breath sounds were broncho-vessicular and only the normal heart sounds were heard, and no heart murmur was present. A working diagnosis of Preterm low birthweight with RDS and Presumed sepsis was made. The baby was immediately intubated and giving 270mg of BLES surfactant and then placed on a ventilator driven intranasal C-PAP using 40% oxygen and a PEEP of 6cm H2O. Intravenous (IV) Ceftazidime and Genticin were commenced but these were later changed to IV meropenem and metronidazole at 72 hours of life when child developed some temperature instability and desaturation. Within 30mins of post surfactant administration and CPAP commencement, the grunting had stopped, RR had dropped to 72breaths/min and the dyspneoa had improved significantly. A second dose of surfactant was given at 72 hours of life after which child improved dramatically and C-PAP was discontinued on the 4th day of life. Child was discharged on the 14th day of life after completing intravenous antibiotics and is doing well.
Discussion
Our diagnosis of RDS was made clinically on the basis of increased work of breathing with reduced saturation on pulse oximetry and in one case was confirmed by the typical ground glass appearance on chest radiograph. [1,11]. The commencement of nasal CPAP with oxygen concentrations of <100% and CPAP pressures of 6cm H2O in our patients were based on the fact that the use of non-invasive CPAP have been found to be superior to the use of heated, humidified oxygen delivered by high-flow nasal cannula (HFNC) and has less risk of bronchopulmonary dysplasia (BPD) compared to invasive ventilation in spontaneously breathing babies [12,13]. The rapid improvement of our patients after 72 hours of life with a discontinuation of CPAP by the fourth day of life is in keeping with reports that early administration of surfactant may decrease the need for prolonged ventilatory support with the avoidance of barotrauma. It could also be due to the synergistic effect of antenatal steroids and surfactant [11]. The use of two doses of BLES, which is an animal derived surfactant has been shown to reduce air leaks such as pneumothorax and pulmonary interstitial emphysema [14].
The main thrust of this paper is to demonstrate the availability of surfactant in Nigeria. Surfactant hitherto has been largely unavailable in Nigeria and this scenario contributed to the high mortality among preterm neonates in the country [2,6]. Cost, rather than care, has been the major hindrance to SRT, because even in facilities with skilled personnel for surfactant administration, this service is subject to availability and affordability of surfactant in an environment where out-of-pocket expenses is the main modality of health financing [6,15]. In 2019 BLES was launched in Nigeria by a company with the collaboration of Nigeria Society of Neonatal Medicine (NISONM). With the launch of BLES and after meetings with stakeholders coordinated by NISONM, the surfactant was made available at the equivalent of one hundred dollars ($100.00) a vial, which is a third of the cost as at 2018 [6,16] NISONM has since then in collaboration with this company, trained more than 200 infant caregivers in surfactant administration and CPAP [6,16,17]. The availability of some of these trained personnel in our centre facilitated the care of both infants.
Conclusion
We have discussed two cases of out-born preterm neonates with RDS whose rapid improvement was as a result of the use of CPAP and the early administration of intra-tracheal surfactants. We therefore recommend the provision of C-PAP in all health facilities that take deliveries, continuing subsidization of the cost of surfactant and upscaling of training of skilled healthcare workers on endotracheal intubation and delivery of surfactants, as surfactant is currently more available in the country.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Kamath BD, Macguire ER, McClure EM, Goldenberg RE, Jobe AH (2011) Neonatal mortality from respiratory distress syndrome: Lessons for low-resource countries. Pediatrics 127(6): 1139-1146.
- Kumar P, Sandesh Kiran PS (2004) Changing trends in the management of respiratory distress syndrome (RDS). Indian J Pediatr 71(1): 49-54.
- Ugochukwu EF, Ezechukwu CC, Agbata CC, Ezumba I (2002) Preterm Admissions in a Special Baby Care Unit: The Nnewi Experience. Nigerian Journal of Paediatrics 29(2): 75-79.
- Ezechukwu CC, Ugochukwu EE, Egbuonu I, Chukwuka JO (2004) Risk factors for neonatal mortality in a regional tertiary Hospital in Nigeria. Nig J Clin Prac 7(2): 50-52.
- Okechukwu AA, Achonwa A (2009) Morbidity and Mortality Patterns of Admissions in a Special Baby Care Unit of University of Abuja Teaching Hospital, Gwagwalada, Nigeria. Niger J Clin Pract 12(4): 389-394.
- Okonkwo IR, Okolo AA (2018) The scope and extent of exogenous surfactant utilization in Nigerian health care facilities: benefits of its regular use to outcome of premature babies. J Matern Fetal Neonatal Med 33(8): 1276-1281.
- Rodriguez R (2003) Management of respiratory distress syndrome: an update. Respir Care 48(3): 279-286.
- Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, et al. (2002) Members of the Vermont Oxford Network. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Paediatrics 110(1 Pt 1): 143-151.
- Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, et al. (2007) Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 196(2): 147.
- Lawn JE, Kerber K, Enweronu-Laryea C, Massee Bateman O (2009) Newborn survival in low resource settings: are we delivering? BJOG 116(suppl 1): 49-59.
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, et al. (2019) European Consensus Guidelines on the Management of Respiratory Distress Syndrome – 2019 Update. Neonatol 115(4): 432-450.
- Roberts CT, Owen LS, Manley BJ, Frøisland DH, Donath SM, et al. (2016) Nasal high-flow therapy for primary respiratory support in preterm infants. N Engl J Med 375(12): 1142-1151.
- Schmölzer GM, Kumar M, Pichler G, Aziz K, O’Reilly M, et al. (2013) Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and metaanalysis. BMJ 347: f5980.
- Singh N, Halliday HL, Stevens TP, Suresh G, Soll R, et al. (2015) Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev (12): CD010249.
- Adebami OJ, Joel-Medewase VI, Agelebe E, Ayeni TO, Kayode OV, et al. (2017) Determinants of outcome in newborns with respiratory distress in Osogbo, Nigeria. Int J Res Med Sci 5(4): 1487-1493.
- Okonkwo IR, Okolo AA, Abdulmhien- Iyoha B (2016) Scope of Neonatal care services in major Nigerian Hospitals. Nig J Paed 43(1): 8-13.
- Okonkwo IR, Dhaache AI, Okolo AA (2020) Uptake of surfactant replacement therapy (INSURE and MIST) after the introduction of Bovine Lipid Extract surfactant in Nigeria. Annual General and scientific meeting of the paediatric association of Nigeria, Kano Nigeria, pp. 21-24.
-
Oloyede IP, Ebi U, Ekpo FE, Ekanem EE. Impact Of Surfactant Therapy on Respiratory Distress Syndrome in Nigerian Neonates: A Report of Two Cases. Glob J of Ped & Neonatol Car. 3(5): 2021. GJPNC.MS.ID.000572.
Respiratory distress syndrome, Surfactant, CPAP, Nigeria, Neonates, Neonatal mortality, Infants, Ventilation, Breathing
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






