 Research Article
Research Article
Hypertensive Disorders During Pregnancy, Fatal Effects on Mother and Baby-Prevention a Challenge in Low Resource Settings
Chhabra S*, Jaiswal S and Suman A
Department of Obstetrics Gynecology, Mahatma Gandhi Institute of Medical Sciences, India
Chhabra S, Department of Obstetrics Gynecology, Mahatma Gandhi Institute of Medical Sciences, Sevagram Wardha, Maharashtra, India.
Received Date: September 16, 2020; Published Date: October 08, 2020
Abstract
Background: Hypertensive disorders during pregnancy (HDsP), unpredictable, multifaceted disorders which can rapidly develop into dangerous eclampsia and /or life-threatening multiorgan dysfunction, are major causes of morbidity, mortality of mothers, babies.
Objective: Objective was to know status of maternal severe morbidity, mortality and perinatal mortality due to HDsP at a rural institute.
Materials and Methods: Present study was conducted in obstetrics gynecology of a rural institute of low resource region.
Results: There were 34089 obstetric cases admitted over 5 years of analysis period. Of them 4201 had HDsP, 12.32% of all cases. Severe morbidity due to HDsP occurred in 348 (8.28%) women who had HDsP. Of 348 cases with SM ,150 (3.57% of all HDsP) and 18% of all SM, had placental abruption, 63 (1.49% of all HDsP), 18.1% of SM had HELLP, 59 (1.40% of all HDsP), 16.95% of cases of SM had Pulmonary edema, 5 (0.11% of HDsP), 1.43% of all SM, had renal failure, and 71 (1.69% of all HDsP), 20.40% of SM had coagulation failure. Fifteen maternal deaths occurred due to HDsP, 0.35% of 4201 cases of HDsP, 4.31% of 348 cases of severe morbidity due to HDsP. Maternal deaths due to HDsP contributed to 24.6% of overall maternal mortality. Overall 153 perinatal deaths occurred in HDsP cases. contributing to 15.69% perinatal deaths in the same period.
Conclusion: In low resource region incidence of HDsP was high, severity and case fatality were also high. They contributed to a lot of maternal mortality and perinatal mortality.
Keywords: Hypertensive disorders; Pregnancy; Severe morbidity; Mortality; Perinatal mortality
Background
Hypertensive disorders during pregnancy (HDsP), unpredictable and multifaceted disorders which can rapidly develop into dangerous eclampsia and other life-threatening multiorgan dysfunction, are the major causes of morbidity and mortality of women and babies. Bhattacharya et al. [1] reported that the case fatality rates for eclampsia ranged between 0 to 1.8% in high-income countries, 17.7% in India and 2.3% in Uganda. In the UK, preeclampsia affected up to 6% of pregnant women and 2% of severe cases progress to eclampsia. Overall HDsP, which included, gestational hypertension (GH), preeclampsia (PE), and eclampsia (EC) have been reported to occur in 5.2-8.2%, 1.8-4.4% and 0.2-9.2% women respectively [2]. Others have also reported HDsP ranging from 1.6 to 10 cases per 10,000 deliveries in developed countries [3-6] and 6 to 157 cases per 10,000 deliveries in developing countries [7,8]. In India, the incidence of preeclampsia was reported to be 8-10% pregnancies. In a community based study in India hypertension during pregnancy was reported in 6% [9]. Another study revealed 7.8% incidence of HDsP [10]. It has been reported that the HDsP occur in 3 to 8% of pregnancies worldwide [11,12].
Objective
Objective was to know status of severe morbidity and mortality of mother and perinatal mortality due to HDsP at a rural institute in a low resource region, with plans for trying preventive research.
Materials and Methods
Present study was conducted in the department of obstetrics gynecology of a rural institute in a low resource region after approval of ethics committee of the institute. Records of cases of HDsP managed over five years were analyzed to get information about major maternal morbidities and mortality and perinatal mortality as base information for planned preventive interventional study.
Results
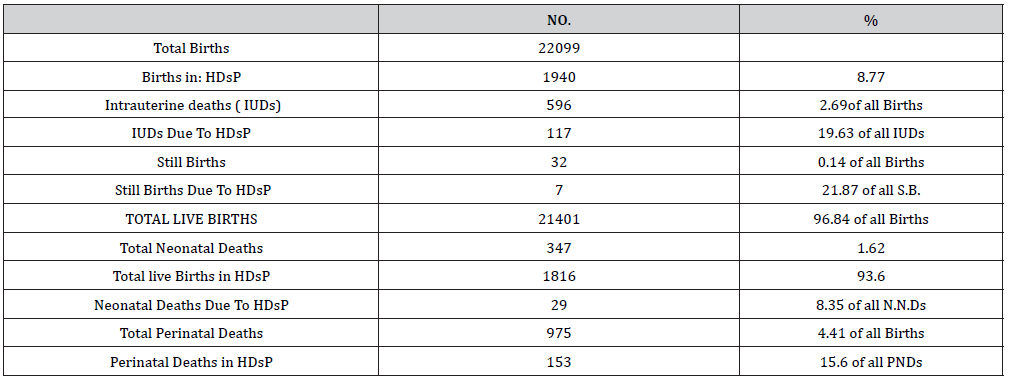
There were 34089 obstetric cases over 5 years of the analysis period. Of them 4201 women had HDsP, 12.32% of all obstetric cases. There were 2877 (68.48%) cases of GH, [2014 (44.9% of all HDsP) mild GH and 863 (20.5%) severe GH], 734 (17.44%) PE, [534 (12.71%) mild PE and 20 (4.76%) severe PE] and 304 (7.23%) Eclampsia (Table 1). Severe morbidity due to HDsP occurred in 348 (8.28%) women with HDsP. Overall 150 (3.57% of all HDsP) women had placental abruption, 104 (49.7%) of 209 primigravida with severe morbidity, 35 (36.8%) of 95 women with 1 or 2 births, 11 (23.4%) of 44 with more than 2 births. Of the 150 women, who had placental abruption, 50 (33%) were of 15-19 years age, 74 (49.3%) of 20-29 years, 12 (8%) of 30-39 years and 04 (2.6%) of 40-49 years. Total 63 (1.49% of all HDsP) women had Haemolysis, Elevated Liver, Enzyme and Low Platelets. (HELLP), 38 (18.1%) of 209 primigravida with severe morbidity, 15 (15.7%) of 95 with one or two births and 09 (1.9%) of 44with more than 2 births, 10(15.8%) women were of 15-19 years age, 30(44.6%) of 20-29 years, 15(23.8%) of 30-39 years and 08 (12.7%) of 40-49 years. A total of 59 women (1.40% of all HDsP) had pulmonary edema, 33 (15.7%) of 209 primigravida with severe morbidity, 15 (15.7%) of 95 with one or two births, 11 (25%) of 44 with more than 2 births, 37(62.7%) were of 20-29 years, 12(20.3%) of 30-39 years and 04(6.8%) of 40-49 years. Five (0.11% of all HDsP) had renal failure, 02(0.9%) out of 209 primigravida, 02 (2.10%) of 95 with one or two births and one (2.1%) of 44 with more than two births. One (1%) was an adolescent and 04(2.2%) were of 20-29 years. Seventy one (1.69% of all HDsP), women developed coagulation dysfunction , 30 (14.35%) of 209 primigravida, 26(27.3%) of 95 with one or two births and 15(31.9% of 44) with more than 2 births, 17(9.1%) of 35 were of 20-29 years, 10 (4.7%) of 30-39 years and 8 (3.8%) of 40-49 years (Table 2). More women with first pregnancy had complications. Fifteen maternal deaths occurred due to HDsP, 0.35% of all cases of HDsP (4201), and 4.31% of 348 cases of severe morbidity. Maternal deaths due to HDsP contributed to 24.6% of maternal mortality over the analysis period. HDsP cases contributed to 19.63% intrauterine deaths, 21.67% still births and 8.35% of all neonatal deaths, overall contributed to 15.69% Perinatal mortality during the analysis period (Table 3 & 4).
Table 1:Severity of hypertensive disorders.
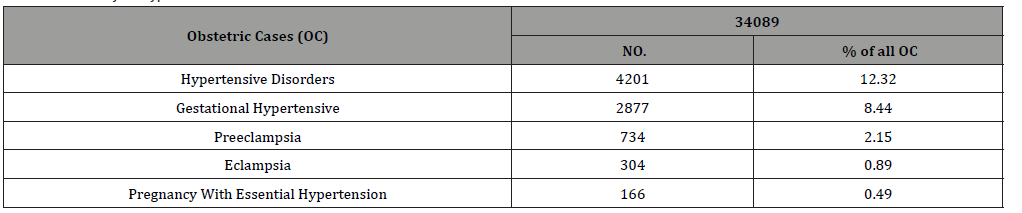
Table 2:Complications with hypertensive disorders during pregnancy.
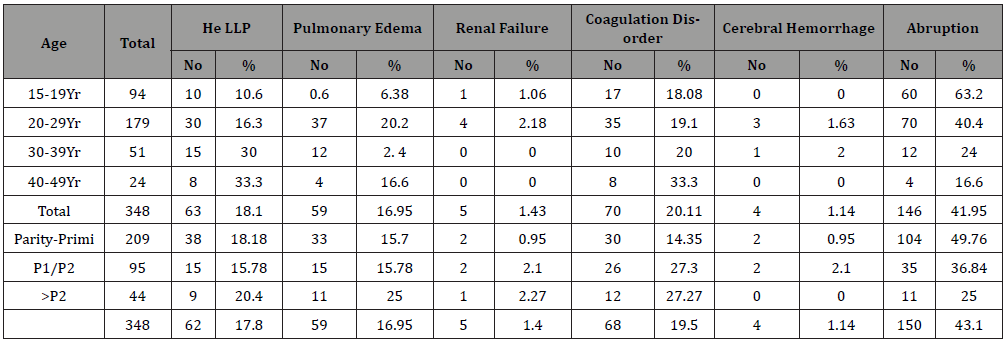
Table 3:Hypertensive disorders and mode of delivery.
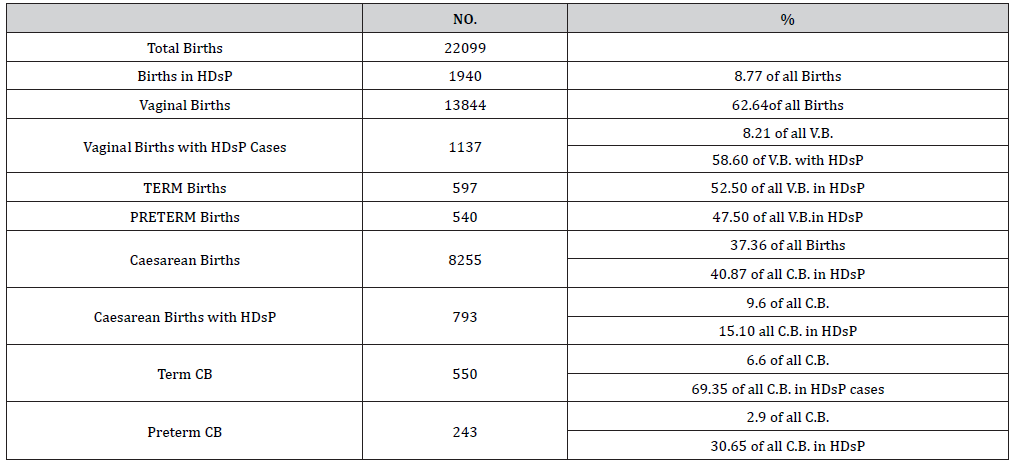
Table 4:Perinatal mortality due to hypertensive disorders.
Discussion
Severe complications associated with HDsP are probably among the most difficult to predict and prevent. Because of high burden of HDsP, delayed recognition, delay in accessing vital services and delay in accessing prompt and appropriate care, many women present with multi organ involvement leading to death. In a study [13], HDsP were responsible for severe obstetric complications during hospitalization for delivery and contributed to a relatively large proportion of hospitalization for severe obstetric complications. Lawn et al. [14] opined that 99% of such problems occurred in less developed countries. Poon et al. [15] reported that pre-eclampsia killed around 76000 women and 500000 babies every year. The only current available preeclampsia cure is delivery of the placenta, because placenta is believed to be responsible for proinflammatory substances which affected the maternal cardiovasculature apparatus responsible for the clinical picture [16]. Further complications in mother and baby also depend on the gestation at which it occurs, becomes obvious and further management. For very preterm pre-eclampsia (24- 34 weeks’ gestation), the current evidence suggested fetal health benefited by prolonging the pregnancy. But this in turn required close clinical surveillance of the woman [17]. A study revealed that early onset HDsP lead to more adverse perinatal outcome than late onset HDsP. However, in a study for pregnancy which could go to around 34 weeks of pregnancy, perinatal survival was similar to term cases [18]. More studies are required to investigate further. A randomized controlled trial revealed a non-significant reduction in maternal adverse outcome if late preterm pre-eclampsia cases were intervened before term [19]. Chappell et al. [20] suggested shared decision making in cases of late preterm pre-eclampsia (34-37 weeks’ gestation), offering initiation of delivery with the aim of reducing maternal morbidity and severe hypertension, balanced against the increased risk of neonatal unit admissions, without increasing newborn respiratory or other morbidities. Researchers reported that at the expense of increased neonatal unit admissions related to premature births, immediate planned delivery reduced maternal morbidity and severe hypertension compared with expectant management among women with late preterm pre-eclampsia but the under estimation of long-term neonatal morbidities remains. There was strong evidence to suggest that planned delivery reduced, severe hypertension and maternal morbidity compared to expectant management but with more neonatal unit admissions. However, others [21] reported that pregnancy prolongation in early-onset preeclampsia was associated with improved offspring outcome and survival. These effects did not appear to be deleterious to short-term maternal cardiovascular and metabolic function but were associated with a modest increase in risk of residual albuminuria, admissions because of prematurity but no greater neonatal morbidity. In a study the case fatality due to eclampsia ranged from 0 to 1.8% in high-income countries to 18% in middle-income countries like India, which reflected the gap in quality of care. No maternal death due to eclampsia occurred in a one-year period in the whole of the Sweden, and one hospital in India reported 11 eclampsia-related deaths [22], Jiang et al. [23] also did a study and reported gap between high-income countries and low-and middle-income countries. HDsP, seem to be systemic inflammatory disease that may lead to multi organ damage, be it liver, kidneys, lungs, and central nervous system with danger of renal failure, thrombocytopenia, disseminated intravascular coagulation, acute pulmonary edema, and future chronic hypertension and cerebro-vascular disorders, 3 to 25 times risk than normotensive women. So, the problems of diagnosis and management in low resource regions. In the present study, it was revealed that HDsP, occurred in 12.32% obstetric cases. Severe morbidity occurred in 348 (8.28%) of 4201 cases of HDsP. Of all the women with SM, 209 (60%) were primigravida, 95(27.2%) with one and two births and 44(12.64%) had more than 2 births. Total 94 (27%) women with SM were of 15-19 age, 179(51.4%) of 20-29 years age, 51(14.7%) of 30-39 years age and 24 (6.9%) of 40-49 years age. Out of total 348 cases with SM, 150 (3.57% of all HDsP) and 18% of all SM had placental abruption, 63 (1.49% of all HDsP) and 18.1% of all SM had HELLP. 59 (1.40% of all HDsP) , 16.95% of cases of SM had pulmonary edema, 5 (0.11% of HDsP) and 1.43% of all SM, had renal failure, 71 (1.69% of HDsP), 20.40% of SM had coagulation dysfunction. The placenta- related complications included placental insufficiency, placental abruption, fetal growth restriction, preterm births and intrauterine fetal deaths. In the present study 13.4% women had preterm births in HDsP cases, 231 (10%) women of GH, 80 (12%) of preeclampsia, and 132 (40%) of eclampsia. HDsP have been reported to be accounting for 12% of all maternal deaths globally [24]. In the present analysis HDsP contributed to 24.6% maternal mortality. Also, women with HDsP have more chances of preterm births, fetal growth restriction (FGR), low birth weight (LBW) and perinatal deaths. In the present analysis HDsP cases contributed to 19.63% intrauterine deaths, 21.67% still births and 8.35% of all neonatal deaths during the analysis period and HDsP cases. contributed to 15.69% perinatal deaths in same period. A previous study showed that the risk of neurodevelopmental delay and neonatal death decreased with increased gestational age, even in late preterm births [25]. Studies in low- and middleincome countries suggested that improving communications is essential. Early identification of pre-eclampsia and its appropriate management before the onset of eclampsia, it is recognized as a way to mitigate the worst outcome for mothers and newborns. Ridder et al. [26] reported that maternal cardiovascular function played a significant role in the pathophysiology of preeclampsia. The predisposition of women with cardiovascular dysfunction for developing preeclampsia, the development of cardiovascular dysfunction prior to disease onset, the predominance of cardiovascular signs/ biology at presentation , and the longterm cardiovascular health risks, all support the assertion that preeclampsia could be a primary cardiovascular disorder. Since HDsP continue to be a leading cause of maternal and Perinatal mortality and morbidity with direct estimated maternal deaths of about 41000 per year (14% of all maternal deaths), of whom 94% occurred in low- income countries [27] attempts continue to find modes of prediction and prevention. Peguero et al. [28] did a prospective cohort study to assess women with early-onset severe preeclampsia whether longitudinal changes in angiogenic factors improved the predictions of adverse outcome and reported that levels of placental growth factor [PIGF], soluble fms-like tyrosine kinase [sFIt-1] and s Fit- 1/PIGF ratio, added prognostic value of longitudinal changes of angiogenic factors in early- onset severe preeclampsia. Others [29] have proposed a new biomarker. Glycosylated fibronectin (GlyFn) for late onset HDsP too. Recently Guy et al. [30] have reported that first trimester combined screening for pre-eclampsia, maternal risk factors, blood pressure, PAPP-A and uterine artery Doppler indices, is both feasible and effective in public healthcare settings. Various attempts continue for prevention. Aspirin is, most often suggested. Rolnik et al. [31] reported that although there was concern that aspirin might disrupt the vasoconstrictor/ vasodilator balance in high –risk women, there was no eclampsia in those women who were given aspirin. Hobmeyl et al. [32] reported the complex pathogenesis of preeclampsia, and the need to take a multi-pronged approach not only for identification of predicated risk but also subsequent prevention. Wide range of incidence of HDsP, their complications and sequela have been reported from Hospitals, Districts, States, Countries and Regions due to differences in occurrence, diagnosis, pregnancy care, and further complications and case fatality. There are differences in morbidity and mortality due to HDsP. The mortality rates are high in low-income countries due to various reasons including low quality maternal care due to various reasons, may be infrastructure, non-availability of services for investigations and management of severely ill women. At present it is unrealistic to assume that HDsP can be completely prevented. Better understanding of the etiology of HDsP is essential for preventing their occurrence and further complications. Some women enter pregnancy with pre-existing risk factors and pre-existing medical diseases, like diabetes, chronic hypertension, chronic kidney disease or autoimmune disease, or HDsP in previous pregnancy, in addition to other known risk factors such as obesity, primiparity, later age, family history of HDsP, or blood pressure higher than the normal range for the age.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Bhattacharya S, Ayansina D, Black C, Hall S, Afolabi E, et al. (2012) PP038. Are women with gestational hypertension or preeclampsia at an increased long term risk of kidney function impairment? Pregnancy Hypertension: An International Journal of Women's Cardiovascular Health 2(3): 262.
- Tuffnell D, Jankowcz D, Lindow S, Lyons G, Mason G, et al. (2005) Outcomes of severe pre-eclampsia/clampsia in Yorkshire 1999/2003. BJOG 112(7): 875-880.
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, et al. (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311(5768): 1770-1773.
- Zwart JJ, Richters A, Ory F, de Vries JI, Bloemenkamp KW, et al. (2008) Eclampsia in the Netherlands. Obstet Gynecol 112(4): 820-827.
- Keck Z, Wang W, Wang Y, Lau P, Carlsen TH, et al. (2013) Cooperativity in virus neutralization by human monoclonal antibodies to two adjacent regions located at the amino terminus of hepatitis C virus E2 glycoprotein. J Virol 87(1): 37-51.
- Montgomery AL, Morris SK, Bassani DG, Kumar R, Jotkar R, et al. (2012) Factors associated with physician agreement and coding choices of cause of death using verbal autopsies for 1130 maternal deaths in India. PLoS One 7: e33075.
- Eke AC, Eke UA, Okafor CI, et al. (2011) Prevalence, correlates and pattern of hepatitis B surface antigen in a low resource setting. Virol J 8: 12.
- Mehta B, Kumar V, Chawla S, Sachdeva S, Mahopatra D (2015) Hypertension in pregnancy: a community-based study Indian J Community Med 40(4): 273-278.
- Giordano JC, Parpinelli MA, Cecatti JG, Haddad SM, Costa ML, et al. (2014) The burden of eclampsia: results from a multicenter study on surveillance of severe maternal morbidity in Brazil. PLoS One 9(5): e97401.
- Ananth CV, Basso O (2010) Impact of pregnancy-induced hypertension on stillbirth and neonatal mortality. Epidemiology 21(1): 118-123.
- Clausen TD, Bergholt T (2014) Chronic hypertension during pregnancy. BMJ 348: g2655.
- Kuklina EV, Ayala C, Callaghan WM (2009) Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 113(6):1299-1306.
- Lawn Joy E (2009) 4 million neonatal deaths: An analysis of available cause-of-death data and systematic country estimates with a focus on “birth asphyxia”. PhD diss, UCL, UK.
- Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, et al. (2019) The Internatinal Federation of Gynecology and Obstetrics (FIGO) initative on pre-eclampsia: a pragmatic guide for first- trimester screening and prevention. Int J Gynaecol Obstet 145(Suppl 1): 1-33.
- Redman CW, Sargent IL, Staff AC (2014) IFPA Senior Award Lecture: Making sense of pre- eclampsia - two placental causes of preeclampsia? Placenta 35: S20-S25.
- Churchill D, Duley L, Thornton JG, Moussa M, Ali HS, et al. (2018) Interventionist versus expectant care for severe pre-eclampsia between 24 and 34 weeks' gestation. Cochrane Database Syst Rev 10(10): CD003106.
- Chhabra S, Singh A (2018) Perinatal Survival in Women with Low Resources with Early Onset, Late Onset Hypertensive Disorders. Ann Pregnancy Birth 1(1): 1-6.
- Broekhuijsen K, van Baaren GJ, van Pampus MG, Ganzevoort W, Sikkema JM, et al. (2015) Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet 385(9986): 2492-2501.
- Chappell LC, Bell JL, Smith A, Linsell L, Juszczak E, et al. (2019) Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial. Lancet 394(10201): 849-860.
- Mulder EG, Doha CG, Crutsen JRW, Kuijk SV, Thilaganathan B, et al. (2020) Effect of pregnancy prolongation in early‐onset preeclampsia on postpartum maternal cardiovascular, renal and metabolic function in primiparous women: an observational study. BJOG.
- Altman D, Carroli G, Duley L, Farrell B, Moodley J, et al. (2002) Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet 359(9321): 1877-1890.
- Mingyang Jiang, Huachu Deng, Shanggui Su (2019) Re: Global inequities in dietary calcium intake during pregnancy: a systematic review and meta‐analysis. BJOG: An International Journal of Obstetrics & Gynaecology 126(10): 1291-1291.
- Rudra P, Basak S, Patil D, Latoo MY (2011) Recent advances in management of pre-eclampsia . British journal of Medical Practitioners 4(3): a433.
- Young PC, Glasgow TS, Li X, Guest-Warnick G, Stoddard G (2007) Mortality of late-preterm (near-term newborns) in Utah. Pediatrics 119(3): e659-e665.
- Ridder A, Giorgione V, Khalil A, Thilaganathan B (2019) Preeclampsia: The Relationship between Uterine Artery Blood Flow and Tropphoblast Function Int J Mol Sci 20(13): 3263.
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, et al. (2014) Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2(6): e323-e333.
- Peguero A, Fernandez‐Blanco L, Mazarico E, Benitez L, Gonzalez A, et al. (2020) Added prognostic value of longitudinal changes of angiogenic factors in early‐onset severe pre‐eclampsia: a prospective cohort study, BJOG.
- Herraiz I (2020) New pathways to diagnose pre‐eclampsia, BJOG.
- Guy GP, Leslie K, Gomez DD, Forenc K, Buck E, et al. (2020) Implementation of routine first trimester combined screening for pre‐eclampsia: a clinical effectiveness study, BLOG.
- Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, et al. (2017) Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 377(7): 613-622.
- Hobmeyr R, Zörner B, Hrobarsch H (2013) Calibration of all pressure transducers in a hydrogen storage system. GM Global Technology Operations LLC, assignee. United States patent US 8,561,453.
-
Chhabra S, Jaiswal S, Suman A. Hypertensive Disorders During Pregnancy, Fatal Effects on Mother and Baby-Prevention a Challenge in Low Resource Settings. Glob J of Ped & Neonatol Car. 2(5): 2020. GJPNC.MS.ID.000550.
Hypertensive disorders, Pregnancy, Severe morbidity, Mortality, Perinatal mortality, Women, Eclampsia, Maternal deaths, Perinatal deaths, Preterm births
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






