 Research article
Research article
Complications of Central Venous Catheters in Neonates: A Comprehensive Level III Neonatal Intensive Care Unit Analysis
Sofia Branco1*, Ana Lídia Rouxinol-Dias2,3, Rita Magalhães Moita1,4, Henrique Soares1,4 and Susana Pissarra1,4
1Neonatal Intensive Care Unit, Pediatrics Department, Centro Hospitalar Universitário São João, Porto, Portugal
2Department of Anesthesiology, Centro Hospitalar Universitário de São João, Porto, Portugal
3Center for Research in Health Technologies and Information Systems (CINTESIS), Faculty of Medicine, University of Porto, Porto, Portugal
4Faculty of Medicine, University of Porto, Porto, Portugal
Sofia Branco, Neonatal Intensive Care Unit, Pediatrics Department, Centro Hospitalar Universitário São João, Porto, Portugal
Received Date: December 02, 2024; Published Date: December 13, 2024
Abstract
Background: To evaluate the use of central venous catheters (CVC), the incidence of related complications, to identify risk factors for these complications and their impact in a level III Neonatal Intensive Care Unit (NICU).
Methods: A retrospective cohort study at Centro Hospitalar Universitário de São João, Portugal, included neonates who underwent CVC placement from January 1, 2021, to December 31, 2022. Patient’s demographics, CVC’s characteristics, complications, and outcomes were collected. Statistical methods were used to assess CVC utilization rates, complication rates, associated risk factors and consequences.
Results: Out of 677 admissions, 288 neonates met the study criteria. A total of 463 CVCs were placed, resulting in a utilization ratio of 59.3%. CVCs’ complications occurred in 24.3% of neonates, corresponding to a complication rate of 22.9 per 1000 catheter days. Mechanical complications were the most common, followed by infectious and thrombotic events. Risk factors significantly associated with complications included inborn status and the cumulative number of CVCs. PICCs, in particular, had higher complication rates: infectious complications increased significantly after 14 days of placement, while mechanical complications were most prevalent within the first 4 days. CVC-related complications significantly impacted hospital length of stay and mortality.
Conclusions: Strategies to minimize CVC’s complications should focus on reducing the number of CVCs per patient, and improving insertion and maintenance techniques. PICCs, while beneficial, were associated with a higher risk of complications, suggesting a need for careful consideration of their use. Further research is required to refine guidelines for CVC management and enhance neonatal outcomes.
Keywords:Neonatal Intensive Care Unit; Neonate; Complications of central venous catheters
Abbreviations: CI: Confidence interval; CHUSJ: Centro Hospitalar Universitário de São João; CLABSI: Central line-associated bloodstream infections; CRBSI: Catheter-related bloodstream infections; CVCs: Central venous catheters; GA: Gestational age; HR: Hazard ratio; NICU: Neonatal Intensive Care Unit; OR: Odds ratio; PICC: Peripherally inserted central catheter; UVC: Umbilical venous catheter
Introduction
Central venous catheters (CVCs) are indispensable devices in daily clinical practice at Neonatal Intensive Care Units (NICUs), particularly in the management of preterm neonates and those with severe medical conditions [1-4].
Central venous access can be obtained through non-tunnelled and tunnelled catheters [5]. In the NICUs, the most common types of CVCs include umbilical venous catheters (UVCs) and peripherally inserted central catheters (PICCs), as non-tunnelled catheters, and Broviac, as a single-lumen tunnelled catheter [6]. UVCs offer a reliable central venous access during the first hours of life for urgent drug administration in critically ill neonates. They are generally well-tolerated for short-term use and should be removed within 5-7 days [7-10]. If longer-term access is needed, PICCs are usually placed. They are flexible catheters inserted into a peripheral vein and threaded to the superior/inferior vena cava [1,7]. Traditionally, PICCs are placed for short- to medium-term use, however its dwell time is not consensual [11,12]. When a peripheral access is unavailable or a longer dwell time for the CVC is required, surgical inserted catheters, such as Broviac, are considered [6].
Despite being lifesaving, the placement of a CVC is an invasive technique potentially associated with adverse outcomes that increase morbidity and mortality in NICUs patients, resulting in prolonged hospital length of stay and higher healthcare costs [7,13]. These adverse outcomes can be classified as mechanical, infectious and thrombotic CVC-related complications. Mechanical complications are wide and range from infiltration, occlusion, misplacement, breakage, external leaking and phlebitis to lifethreatening events such as pericardial effusion, cardiac tamponade and pleural effusion, which are rarer [7,14]. Among the infectious complications, central line-associated bloodstream infections (CLABSI) are one of the commonest causes of nosocomial infections in NICUs, with CVCs being a significant contributor to late-onset sepsis in neonates [15,16]. In terms of thrombotic events, it is wellestablished that CVCs account for over 90% of cases of neonatal venous thrombosis [17].
To decrease the risk of these complications, it is important to standardize practices related to the placement, maintenance and removal of CVCs in neonates, guided by knowledge of the main associated risk factors [14]. Therefore, the aim of this study was to assess the frequency and duration of CVC usage, the incidence of related complications, and to identify risk factors contributing to these complications and their impact among neonates in a level III NICU. The findings will aid clinicians in determining the optimal timing for CVC removal or replacement, thereby reducing the incidence of CVC-associated complications in neonates.
Materials and Methods
A retrospective cohort study was conducted in a level III NICU at Centro Hospitalar Universitário de São João (CHUSJ), Unidade Local de Saúde de São João in Porto, Portugal. Neonates admitted between January 1, 2021, and December 31, 2022, who had at least one CVC placement were included. Exclusion criteria consisted of neonates hospitalized for less than 2 days and CVCs in place for less than 24 hours.
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Ethics Committee of CHUSJ under protocol number 140/2023. Data were collected from patients’ medical records and maintained in an electronic database.
Data Collected
Patients’ demographic variables, including gender, gestational age (GA) at birth, birth weight, and birthplace (inborn or outborn), were recorded. GA, measured in completed weeks, was assessed using the time elapsed between the first day of the last menstrual period and the day of delivery, prenatal ultrasonography evaluation [18], or the New Ballard Score in cases where obstetrical indices were unavailable [19].
Regarding hospitalization, the following variables were collected: age and diagnosis at admission, length of hospital stay, quantity and type of CVCs inserted along with their duration of use, local where the CVC was placed (at the NICU or in an operating room during a surgical procedure), CVC-associated complications and final outcomes. Simultaneous multiple CVC were considered when more than one CVC were placed for more than 1 day. Maximum number of multiple CVCs, and cumulative period of simultaneous CVC were considered.
Outcomes
Utilizing this dataset, we computed the CVC utilization ratio by dividing the total number of CVC-days (according to CDC definition) by the total number of hospitalization days, expressed as a percentage [20,21]. Additionally, the CVC complication rate was determined by dividing the total number of CVC-related complications by the total number of CVC-days, expressed as the number of complications per 1000 days with CVC. The CVC-related complications were categorized into mechanical, infectious and thrombotic. Mechanical complications included occlusion, breakage, external leaking, infiltration, bleeding, phlebitis, exteriorization, pneumothorax, pericardial and pleural effusion, and cardiac tamponade. Regarding infectious complications, we distinguished CLABSIs from catheter-related bloodstream infections (CRBSIs). CLABSI was defined as a primary bloodstream infection occurring in patients with an indwelling central line either in place or within 48 hours preceding the onset of clinical signs of sepsis, without any other identifiable source of infection, with or without positive culture obtained through the CVC [6,15]. CRBSI was considered when patients displayed clinical signs of sepsis accompanied by a positive peripheral blood culture and either a positive catheter tip culture or a positive blood culture drawn from the CVC [6,15]. Laboratory parameters that were considered included peripheral, CVC drawn blood and tip cultures. CVC-related thromboembolism was defined as an intraluminal thrombus due to a CVC, confirmed through echocardiography or ultrasonography.
A death due to CVC-related complications was considered when the autopsy proved this association. As the design of the CHUSJ NICU was optimized during this period, transitioning from an openbay unit in 2021 to single-family rooms in 2022, we also tested whether this change had an impact on the rate of CVC-associated complications.
Data analysis
Descriptive and analytical analysis was performed regarding patient and CVC characteristics. Categorical variables were reported as absolute and/or relative frequencies and continuous variables were reported as median, interquartile range (percentile 25- percentile 75) and, when clinically relevant, minimum and maximum values. Univariate analysis comprised Mann–Whitney (MWT) for continuous independent variables and Pearson Chisquare/ Fisher’s exact test for categorical independent variables. A linear regression was computed for adjustment analysis of CVC related complications in length of NICU stay. For multivariate analysis for CVC complications two logistic regression models were computed (for NB related characteristics and CVC related characteristics) including all variables with p<0.1 in univariate analysis. Significance level of 5% was considered. Model’s accuracy was assessed using the area under the receiver operating characteristic (ROC-AUC) curve and tested calibration with the Hosmer–Lemeshow goodness-of-fit test. We expressed the results of the multivariate model as β coefficients and adjusted odds ratios (OR) with 95% confidence intervals (CI) and p-values.
Univariate Cox regression and log-rank test analysis were performed to determine the differences in the survival distribution to CVC complication for the different types of CVCs. We expressed the results as hazard ratios (HR) with 95% CI and p-values. Statistical analysis was performed using SPSS Statistics for Windows, Version: 28.0.1.0 (IBM Corp, Armonk, NY). A ROC curve analysis was performed to evaluate the optimal cutpoint for time to increase or decrease the risk of specific complications for PICCs, using R (version 4.3.0) [22]. Key packages used for this analysis included pROC [23]and cutpointr [24] for ROC curve creation and determining cutpoints.
Results
Admissions and eligibility
During the study period, 677 NICU admissions were recorded, with 288 NBs meeting the eligibility criteria. The distribution of eligible NBs showed a predominance of males (60.4%) and inborns (64.2%). The median gestational age at admission was 36 weeks (minimum 23 weeks and maximum 41), with a median hospital length of stay of 18 days (minimum of 48 hours and maximum 149 days). Eighty-two NBs (28.5%) had surgical intervention. Patients’ characteristics are presented in Table 1.
Table 1: Patients characterization.

Table 2: Mortality causes.

Use and complications associated with Central Venous Catheters (CVCs)
A total of 463 CVCs were placed, resulting in a CVC utilization ratio of 59.3% (4284 CVC days/7227 NICU days). Median number of CVCs was 1 per patient with maximum cumulative number of 8 per patient and maximum simultaneous of 3 CVCs placed simultaneously for two NBs. Median time with CVCs for a NB was 9 days (minimum 1 day and maximum 147 days), with a median length of each CVC stay of 7 days (minimum 1 day and maximum 83 days). PICCs were the most common type of CVC overall, while Broviac catheters were the most frequently inserted CVCs during surgical procedures (27 out of 49). The comparative analysis of CVC types is detailed in Table 2.
Table 3: Comparison of CVC types and outcomes.
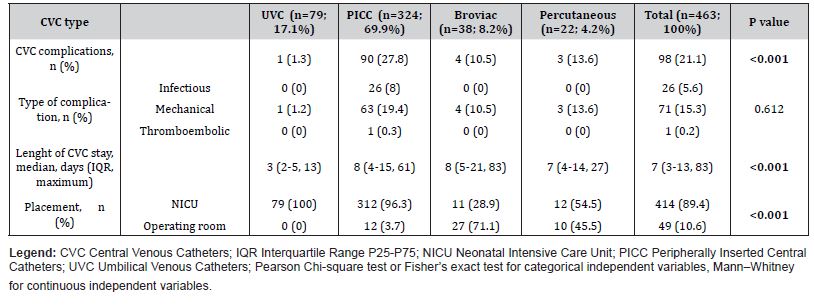
Seventy (24.3%) NBs experienced at least one CVC complication. The analysis of complications associated with CVC use revealed a rate of 22.9 complications per 1000 catheter days (98 complications/4284 CVC days*1000).
Mechanical complications were the most frequent, occurring in 72.4% of cases (71 out of 98). Infiltration was the most common mechanical complication, accounting for 42.2% of these cases (30 out of 71). Other mechanical complications included phlebitis (25.4%, n=18), breakage (11.3%, n=8), occlusion (8.5%, n=6), exteriorization (5.6%, n=4), and external leaking (4.2%, n=3). Notably, there was one case (1.4%) of a major mechanical complication involving pleural effusion, which corresponded to a PICC line with a non-central catheter tip position on radiographic imaging.
Table 4: Survival analysis of factors related with CVC complications – Cox univariate regression.

Overall, 55 cases of sepsis were recorded. Among these, there were 12 cases of CLABSI and 14 cases of CRBSI, corresponding to a CVC-related infection rate of 6.1 per 1000 catheter days. Staphylococcus epidermidis was the most commonly isolated organism (34.6%, n=9), followed by Klebsiella spp. and Staphylococcus haemolyticus (8.3%, n=2 each). Infectious complications were observed exclusively in NB with PICCs.
Additionally, one thromboembolic complication associated a PICC line was recorded during the study period.
Risk factors for developing complications
In univariate analysis, surgical intervention, inborn status, cumulative number of CVCs and having more than one CVCs simultaneously were significantly related with CVC complications (Table 4). Variables related to the newborn, such as gestational age, birth weight, and primary diagnosis, as well as the placement of CVC in the NICU versus the operating room, did not show a significant association with the occurrence of complications. Regarding CVC characteristics, the use of a PICC line was associated with a significantly higher odds of complications (OR 6.3, 95% CI: 3.0-13.4, X2 p<0.001), with significantly higher hazard ratio of complications compared to CVUs (HR 8.05, 95%CI: 1.11-58.44) and Broviac catheters (HR 3.79, 95%CI: 1.38-10.41) [figure 1].

Table 5: Univariate analysis of factors related with CVC complications and mortality.

Considering univariate logistic regression coefficient for CVC stay, PICCs’ infectious complications odds doubled every 8 days (p<0.001) (25). Mechanical complications, on the other hand, happened earlier, with their odds for PICCs decreasing by half after 7 days (p<0.001). ROC curve analysis for time cut-off for PICC’s infection was 14 days (AUC 0.809 – 95CI=0.741-0.877, sensitivity=80.8%, specificity=75.2%, risk of infection after 14 days =3.25) and for PICC’s mechanical complication was 4 days (AUC 0.731 – 95CI=0.655-0.807, sensitivity 64.5%, specificity 81.3%, risk ratio of mechanical complication after 4 days =0.21).
After performing a logistic regression that included surgical intervention, inborn status, presence of simultaneous CVCs and cumulative number of CVCs, only inborn status (p=0.05) and cumulative number of CVC (p<0.01) remained significantly associated with CVC complications.
The adjusted odds ratio (aOR) for inborn versus outborn NB was 2.1 (95% CI: 1.0-4.3). Furthermore, each additional CVC inserted increased the odds of complications fourfold (aOR 4.0, 95% CI: 2.5- 6.6, p<0.001). Performing a logistic regression with CVCs as the unit of analysis, PICCs kept with higher odds of complications compared to CVUs and Broviac catheters (p<0.001 and p=0.035, respectively), regardless of the CVC length of stay.
Impact of complications
CVCs complications were significantly associated with longer hospital stays, with a median (interquartile range) of 31 days (19- 63) compared to 14 days (7-26) for patients without complications (Mann–Whitney test p<0.001). Additionally, complications were linked to higher mortality (14.3% vs. 6.4%, Mann–Whitney test p=0.048). After adjusting for gestational age at admission, statistical analysis revealed that NB with CVC’s complications experienced significantly longer hospital stays (linear regression p<0.001), with an average increase of 22 days (95% CI: 17-28 days). Overall mortality was 8.3% (24 NB), with detailed causes of death described in supplementary table 1. There were two deaths specifically attributed to CVC-related complications: one case of CLABSI caused by Pseudomonas aeruginosa and one case of CRBSI due to Serratia marcescens, as confirmed by autopsy. CVC-related complications were associated with an increased risk of mortality (X2, p=0.043, OR 2.43, 95% CI: 1.03-5.75) and NBs submitted to surgical interventions also experienced higher mortality rates (X2, p=0.015, OR 2.78, 95% CI: 1.19-6.46). However, both effects disappeared in multivariate logistic regression (p=0.153 and p=0.057, respectively).
Discussion
Our study underscores the critical role of CVCs in managing neonates within a level III NICU, highlighting both their essential benefits and associated risks. The substantial utilization rate of CVCs, at 59.3%, demonstrates their indispensable role in providing stable intravenous access, particularly for preterm neonates and those with severe medical conditions, and reflects the clinical severity characteristics of our patient population (Table 1). Despite these benefits, CVCs are linked to a range of complications that can lead to increased morbidity, extended hospital stays and higher healthcare costs.
Complications and frequency
We found that 24.3% of neonates experienced at least one CVCrelated complication. Mechanical complications were the most common, followed by infectious and thrombotic complications.
Mechanical complications accounted for 72.4% of all complications, with infiltration being the most frequent. Infiltration, which occurs when fluids leak into the extra-vascular space, could be a result of improper placement or catheter dislodgement. Despite the routine confirmation of catheter tip position following the insertion of any CVC in our unit, migration of the catheter post-insertion remains a possibility and may contribute to this high rate. Addressing these issues through improved insertion techniques and regular monitoring could mitigate the incidence of such complications. Recent studies have explored new central line bundles incorporating cyanoacrylate glue at the catheter insertion site to reduce mechanical complications. Piersigilli et al. and D’Andrea et al. found that cyanoacrylate glue is safe and effective in preventing dislodgement of PICCs and UVCs, respectively [26,27].
Infectious complications, including CLABSI and CRBSI, were less frequent but still significant, with a CVC-related infection rate of 6.1 per 1000 CVC days. Reported CLABSI rates in neonates vary from 3.2 to 21.8 per 1000 central venous line days [28]. A recent study in our NICU reported a CLABSI rate of 12.4 per 1000 catheter days (1), demonstrating a sustained reduction in CVC-related infections. Nonetheless, continued efforts are essential to further decrease this rate. Notably, these infections were only observed in neonates with PICCs, indicating a potential area for targeted intervention.
Thromboembolic events, although rare, were observed in one term NB with a congenital malformation and a PICC in place for 24 days. Despite substantial research into the use of low molecular weight heparin (LMWH) for prophylaxis against central catheterrelated thrombosis, current evidence remains insufficient to recommend LMWH for thromboprophylaxis in neonates with central catheters [29]. This underscores the need for ongoing vigilance in monitoring for signs of thrombosis.
Risk factors for developing complications
The univariate analysis identified significant risk factors for CVC complications, including surgical intervention, inborn status, cumulative number of CVCs and simultaneous placement of more than one CVC. In multivariate analysis, only inborn status and the cumulative number of CVCs remained significant predictors of complications. Inborn neonates had twice the odds of experiencing complications compared to outborn neonates. This may suggest that inborn neonates, who often have more severe or complex conditions and are therefore referred to be born at our hospital, are at greater risk. Moreover, each additional CVC increased the odds of complications fourfold, highlighting the need for strategies to minimize CVC insertions per neonate. This could be achieved through the use of multi-lumen catheters or a more judicious assessment of the need for new CVCs.
Our study period included a transition from an open-bay unit to single-family rooms. Although this change did not significantly affect CVC-related complications, it is crucial to continue monitoring and evaluating the impact of such environmental changes on patient outcomes. Single-family rooms could potentially reduce nosocomial infections by decreasing exposure to cross-contamination [30], but further studies are needed to establish this relationship.
Notably, the use of PICCs was associated with a higher likelihood of complications compared to UVCs and Broviac catheters, even after adjusting for the length of CVC use. Several studies have yielded divergent results regarding the complication profiles of different CVC types, leaving the impact of prioritizing specific catheter types to prevent certain complications still unclear. For instance, one observational study reported that UVCs were associated with a two-fold increase in the risk of CLABSI compared to PICCs [31]. Conversely, two other observational studies found no significant difference in the incidence of CLABSI between UVCs and PICCs [32,33]. Furthermore, a multicenter study demonstrated that the incidence of CLABSI was 2.4 times higher for tunneled catheters compared to PICCs [34]. Regarding mechanical adverse events, two observational studies reported no difference in the occurrence of obstruction, extravasation, dislocation, and leakage between UVCs and PICCs [32,33]. On the other hand, a study conducted at our NICU found that PICCs were associated with higher rates of infiltration compared to UVCs and shortduration venous catheters (1). Unfortunately, this issue persists in our study, where infiltration was the most prevalent complication, particularly among PICCs. Addressing this issue requires targeted strategies, including improved insertion techniques, advanced catheter materials like cyanoacrylate glue to prevent dislodgment [27], and the establishment of a dedicated catheter care team to develop strict monitoring protocols.
Our study also revealed significant insights into the temporal dynamics of PICC-related complications.
The univariate logistic regression analysis indicated that the odds of infectious complications associated with PICCs double every 8 days (p<0.001). This finding underscores the escalating risk of infection with prolonged catheter dwell time, highlighting the need for vigilant infection control practices and timely interventions as the catheter remains in situ. Several studies have reported that extended PICC dwell time is a significant factor in the likelihood of developing infectious complications [35-37]. However, a defined optimal dwell time for PICCs to minimize this risk has not yet been established. In contrast, mechanical complications present a different temporal pattern. The odds of mechanical complications occur earlier, with a notable decrease in risk by half after 7 days (p<0.001). This suggests that while the risk of mechanical issues is initially higher, it diminishes significantly after the first week, potentially due to the stabilization of the catheter over time.
Further analysis using ROC curve methodology provided additional clarity on optimal time cut-offs for monitoring PICCrelated complications. For infectious complications, the ROC curve analysis identifies 14 days as the critical cut-off point. This indicates that the risk of infection significantly increases after 14 days, with a risk ratio of 3.25, supporting the necessity for enhanced surveillance and possible catheter replacement after this period. Conversely, the ROC curve analysis for mechanical complications identifies a 4-day cut-off as optimal, with the risk ratio decreasing to 0.21 beyond this period.
These findings emphasize the importance of tailoring monitoring and management strategies based on complication type and catheter duration. While early vigilance is essential for managing mechanical complications, longer-term management strategies should focus on preventing and addressing infectious complications as the CVC-dwell time extends. Further research is warranted to refine these cut-off points.
Impact of complications
CVCs’ complications were linked to longer hospital stays and higher mortality rates. Neonates with such complications had a median hospital stay of 31 days compared to 14 days for those without complications. This significant difference highlights the impact of CVC-related issues on hospital resources and patient outcomes. Moreover, CVC complications were associated with increased mortality; specifically, there were two recorded deaths attributed to PICC-related infections, representing 8.3% of the mortality rate. Although increased mortality was not significant in multivariate regression, these data suggest that CVC-related complications contribute to risk, but other factors may also influence mortality.
Limitations
This study has some limitations. First, its retrospective design limits the ability to control for confounding variables and introduces the risk of missing/incomplete data. Second, being a single-center study may restrict the generalizability of the findings to other settings. Third, the insertion of CVCs was performed by healthcare professionals with varying levels of expertise, which may have introduced outcome variability and bias. Finally, complications were reported based on clinical practice data, which raises the possibility that minor complications might not have been documented.
Conclusion
This study provides new insights into clinical practice and identifies key areas for targeted prevention of CVC-related complications in NICU patients. It underscores the need for a more judicious assessment of CVC use, particularly PICCs, which showed higher complication rates. While PICCs’ infectious complications increased significantly after 14 days, mechanical complications were most prevalent within the first 4 days of placement. Future research should focus on refining insertion and maintenance protocols and establishing precise dwell time guidelines.
Acknowledgement
We sincerely thank the physicians and nurses of the NICU at CHUSJ for their invaluable collaboration and dedication to patient care, which were essential to this study. We also thank the CHUSJ Research Ethics Committee for authorizing and supporting this research.
Author contributions
Sofia Branco – Data collection, data analysis, draft of the manuscript.
Ana Lídia Rouxinol-Dias – Data analysis, draft of the manuscript.
Rita Magalhães-Moita – Data collection, manuscript review.
Henrique Soares – Manuscript review.
Susana Pissarra – Conceptualization, manuscript review.
Conflict of Interest
The authors state no conflict of interest.
References
- Soares BN, Pissarra S, Rouxinol-Dias AL, Costa S, Guimarães H, et al. (2018) Complications of central lines in neonates admitted to a level III Neonatal Intensive Care Unit. The Journal of Maternal-Fetal & Neonatal Medicine 31(20): 2770-2776.
- Nielsen CL, Zachariassen G, Holm KG (2022) Central line-associated bloodstream infection in infants admitted to a level lllneonatal intensive care unit. Dan Med J 69(5): A05210463.
- Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM, et al. (2015) Complications of Central Venous Access Devices: A Systematic Review. Pediatrics 136(5): e1331-1344.
- Chiang VW, Baskin MN (2000) Uses and complications of central venous catheters inserted in a pediatric emergency department. Pediatr Emerg Care 16(4): 230-232.
- Kehagias E, Galanakis N, Tsetis D (2023) Central venous catheters: Which, when and how. Br J Radiol 96(1151): 20220894.
- Lee JH (2011) Catheter-related bloodstream infections in neonatal intensive care units. Korean J Pediatr 54(9): 363-367.
- Perme T (2023) Central Lines and Their Complications in Neonates: A Case Report and Literature Review. Children 11(1): 26.
- Ares G, Hunter CJ (2017) Central venous access in children: indications, devices, and risks. Curr Opin Pediatr 29(3): 340-346.
- Corso L, Buttera M, Candia F, Sforza F, Rossi K, et al. (2022) Infectious Risks Related to Umbilical Venous Catheter Dwell Time and Its Replacement in Newborns: A Narrative Review of Current Evidence. Life 13(1): 123.
- Keir A, Giesinger R, Dunn M (2014) How long should umbilical venous catheters remain in place in neonates who require long‐term (≥5–7 days) central venous access? J Paediatr Child Health 50(8): 649-6
- Gordon A, Greenhalgh M, McGuire W (2018) Early planned removal versus expectant management of peripherally inserted central catheters to prevent infection in newborn infants. Cochrane Database of Systematic Reviews 6(6): CD012141.
- Hugill K, van Rens M (2020) Inserting central lines via the peripheral circulation in neonates. British Journal of Nursing 29(19): S12-S18.
- Zhang Z, Brusasco C, Anile A, Corradi F, Mariyaselvam M, et al. (2018) Clinical practice guidelines for the management of central venous catheter for critically ill patients. Journal of Emergency and Critical Care Medicine 2: 53-53.
- Björkander M, Bentzer P, Schött U, Broman ME, Kander T, et al. (2019) Mechanical complications of central venous catheter insertions: A retrospective multicenter study of incidence and risks. Acta Anaesthesiol Scand 63(1): 61-68.
- Cho HJ, Cho HK (2019) Central line-associated bloodstream infections in neonates. Korean J Pediatr 62(3): 79-84.
- Al Bizri A, Hanna Wakim R, Obeid A, Daaboul T, Charafeddine L, et al. (2023) A Quality improvement initiative to reduce central line-associated bloodstream infections in a neonatal intensive care unit in a low-and-middle-income country. BMJ Open Qual 12(2): e002129.
- van Ommen CH, Bergman KA, Boerma M, Bouma HA, Donker AE, et al. (2022) NEOnatal Central-venous Line Observational study on Thrombosis (NEOCLOT): evaluation of a national guideline on management of neonatal catheter-related venous thrombosis. Journal of Thrombosis and Haemostasis 21(4): 963-974.
- Engle WA (2024) Age Terminology During the Perinatal Period. Pediatrics 114(5): 1362-1364.
- Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, et al. (1991) New Ballard Score, expanded to include extremely premature infants. J Pediatr 119(3): 417-423.
- Instructions for Completion of Denominators for Neonatal Intensive Care Unit (NICU) (CDC 57.116). National Healthcare Safety Network (NHSN). 2024. Centers for Disease Control and Prevention.
- General Key Terms. In: National Healthcare Safety Network (NHSN). Patient safety component manual. 2024. Centers for Disease Control and Prevention pp. 16-3.
- R Core Team (2023) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria.
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, et al. (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12: 77.
- Thiele C, Hirschfeld G (2021) cutpointr: Improved Estimation and Validation of Optimal Cutpoints in R. J Stat Softw 98(11).
- Zabor EC, Reddy CA, Tendulkar RD, Patil S (2022) Logistic Regression in Clinical Studies. International Journal of Radiation Oncology*Biology*Physics 112(2): 271-277.
- D’Andrea V, Prontera G, Pinna G, Cota F, Fattore S, et al. (2023) Securement of Umbilical Venous Catheter Using Cyanoacrylate Glue: A Randomized Controlled Trial. J Pediatr 260: 113517.
- Piersigilli F, Iacona G, Yazami S, Carkeek K, Hocq C, et al. (2023) Cyanoacrylate glue as part of a new bundle to decrease neonatal PICC-related complications. Eur J Pediatr 182(12): 5607-5613.
- Hightower HB, Young JA, Thomas J, Smith JJ, Hobby-Noland D, et al. (2022) Reduction of Central-line–Associated Bloodstream Infections in a Tertiary Neonatal Intensive Care Unit through Simulation Education. Pediatr Qual Saf 7(6): e610.
- Pelland-Marcotte MC, Amiri N, Avila ML, Brandão LR (2020) Low molecular weight heparin for prevention of central venous catheter-related thrombosis in children. Cochrane Database of Systematic Reviews 6(6): CD005982.
- O Callaghan N, Dee A, Philip RK (2019) Evidence-based design for neonatal units: a systematic review. Matern Health Neonatol Perinatol 5: 6.
- Sanderson E, Yeo KT, Wang AY, Callander I, Bajuk B, et al. (2017) Dwell time and risk of central-line-associated bloodstream infection in neonates. Journal of Hospital Infection 97(3): 267-274.
- Arnts IJJ, Bullens LM, Groenewoud JMM, Liem KD (2014) Comparison of Complication Rates Between Umbilical and Peripherally Inserted Central Venous Catheters in Newborns. Journal of Obstetric, Gynecologic & Neonatal Nursing 43(2): 205-15.
- Konstantinidi A, Sokou R, Panagiotounakou P, Lampridou M, Parastatidou S, et al. (2019) Umbilical Venous Catheters and Peripherally Inserted Central Catheters: Are They Equally Safe in VLBW Infants? A Non-Randomized Single Center Study. Medicina (B Aires) 55(8): 442.
- Greenberg RG, Cochran KM, Smith PB, Edson BS, Schulman J, et al. (2015) Effect of Catheter Dwell Time on Risk of Central Line–Associated Bloodstream Infection in Infants. Pediatrics 136(6): 1080-1086.
- Sanderson E, Yeo KT, Wang AY, Callander I, Bajuk B, et al. (2017) Dwell time and risk of central-line-associated bloodstream infection in neonates. Journal of Hospital Infection 97(3): 267-274.
- Sengupta A, Lehmann C, Diener-West M, Perl TM, Milstone AM, et al. (2010) Catheter Duration and Risk of CLA-BSI in Neonates with PICCs. Pediatrics 125(4): 648-653.
- Milstone AM, Reich NG, Advani S, Yuan G, Bryant K, et al. (2013) Catheter Dwell Time and CLABSIs in Neonates with PICCs: A Multicenter Cohort Study. Pediatrics 132(6): e1609-1615.
-
Sofia Branco*, Ana Lídia Rouxinol-Dias, Rita Magalhães Moita, Henrique Soares and Susana Pissarra. Complications of Central Venous Catheters in Neonates: A Comprehensive Level III Neonatal Intensive Care Unit Analysis. Glob J of Ped & Neonatol Car. 5(2): 2024. GJPNC.MS.ID.000609.
Neonatal Intensive Care Unit, Neonate, Complications of central venous catheters, Tunnelled catheters, Mechanical complications, CVC, Prioritizing, UVC
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






