 Case Report
Case Report
Bacillus cereus Bacteremia and Meningoencephalitis in a Twin-Preterm Neonate: A Case Report and Review of the literature
Eirini Koutsounaki*, Polyxeni Bitouni, Georgios Liosis, Iraklis Salvanos, Margarita Tzaki
Pediatrician-neonatologist, Helena Venizelou Hospital NICU, Athens, Greece
Eirini Koutsounaki, Pediatrician-neonatologist, Greece.
Received Date: September 19, 2018; Published Date: November 14, 2018
Abstract
Bacillus cereus is a Gram-positive spore-forming, motile and rod-shaped bacterium that produces tissue destructive toxins and it is commonly found in the environment. As a human pathogen, it is known for self-limited acute gastroenteritis after food poisoning. But it is also a rare cause of neonatal sepsis, highly aggressive and often fatal. Herein, we describe a case of Bacillus cereus bacteremia and meningoencephalitis in a 4-day-old female twin-preterm neonate. The neonate in only 12 hours after the initial clinical deterioration developed irreversible brain damage and fell to coma. The infant died on her sixth day of life due to cardiorespiratory deterioration and brain stem, central arrest. This case report aims to highlight the importance of clinical suspicion, early diagnosis and effective treatment.
Introduction
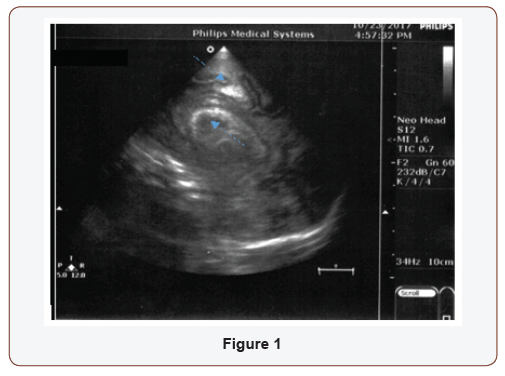
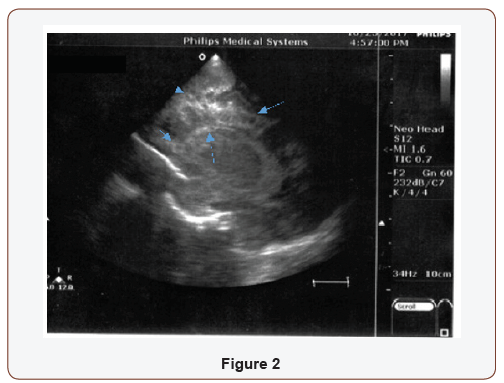
Female premature, IVF twin B, GA: 33+2-week, BW 1925 gr, born by cesarean section with normal Apgar scores. On postnatal day 4, previously asymptomatic N/G fed with IV fluid supplementation, the newborn presented with apnea, fever and tachycardia. The hematology study showed leucopenia with normal platelets and hemoglobin levels. The first sample of cerebral spinal fluid (CSF) was normal and, CRP initially, negative. The blood and CSF cultures were both positive for Bacillus cereus. Twelve hours after the initial signs and symptoms of sepsis, the neonate showed acute clinical deterioration and need of mechanical support: grunting, poor perfusion, tachycardia and opisthotonus. Elevated CRP and persistent leucopenia, as well as, lactic acidosis: pH 7.046, Lac 108 mg/dl were noted. To the broad spectrum –ampicillin and gentamycin-antibiotic treatment, ceftazidime and vancomycin was added at high doses. The ultrasound of the brain, only hours from the symptoms’ initiation, showed echogenicity of the choroid plexus and cysts in deep white matter around the ventricles (Figure 1 & 2).
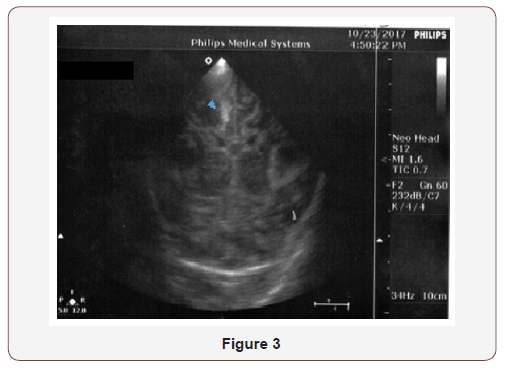
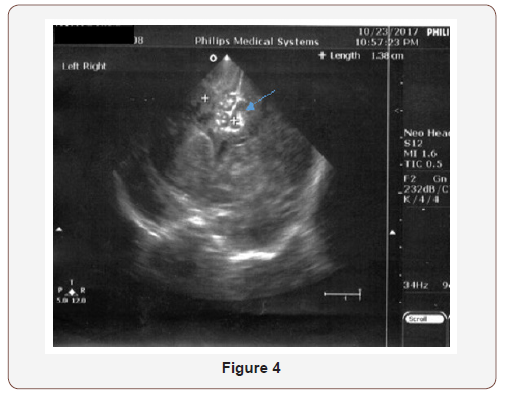
The clinical deterioration with cerebellar coma was accompanied by U/S progression to parenchymal subcortical and cortical echogenicity (Figure 3 & 4).
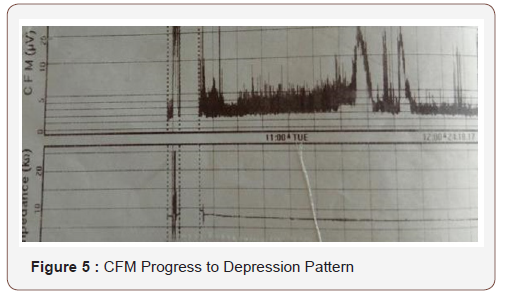
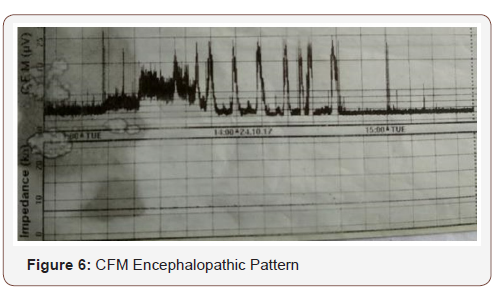
The neonate remained intubated, apneic, floppy and unresponsive with no signs of brainstem function. For a short period of time, focal rhythmic spasms of the jaw were noted probably reflexing primitive circuit enrolment, in the absence of upper neuron control. CFM followed suppression pattern (Figure 5 & 6). CSF repeated, direct indicative of meningitis, but sterile. At the end of the 6th day of life, the patient died. The other twin baby is healthy, growing and asymptomatic after a short course of antibiotics which was discontinued with negative infection control.
Discussion
The volatile progression of the clinical signs of the CNS infection by B. cereus is probably depicting the typical pathological findings described in the literature [7], as the bacilli seem to penetrate the vascular endothelium and also affect the luminal structure, causing the so named “liquefactive necrosis”, following a spatial distribution from central brain vessels to outer layers. A typical disease progression extends from the periventricular white matter, to the deep gray matter and finally the meninges. Probably the case in the vignette had the respiratory center being affected with initial apneic episodes but negative direct indexes for meningitis at the first stages (1st CSF: normal). Thus, evolved to irritability in a few hours, spasms and finally coma (2nd CSF: hemorrhagic/ infectious), after that no respiratory effort was detected on mechanical ventilation. Studies that correlate the pathophysiology with other strains e.g. B anthracis include suffocation at an end point stage of hemorrhagic meningoencephalitis [8].
The strains’ virulence is often attributed to the toxin production. Hemolytic and non-hemolytic gene products act either directly to the host peripheral organs affecting the end tissues either making pores onto cell membranes [9] or utilizing oxidative enzymes, such as enterotoxin, cytotoxin K and aerolysin O, which in the adult gut may cause diarrhea, or hemolysin BL strongly related to necrotic lesions [10].
Another proposed mechanism involves the toxins’ role against the host defense system, by increasing oxidative stress, which might have played a major role in our neonate. It has been described that macrophages insult may be the first target [11]. Earlyleukopenia was noted in our case, with normal initially platelets, impoverishing the naïve innate immunity system, allowing the bacilli spread, even more effectively, at the blood rich regions, such as the subarachnoid vasculature of the premature brain. Intrinsic individual features of the primary innate immunity seem to have determined the completely different clinical appearance of the IVF separate unaffected twin [12].
Hence the ubiquity of Bacilli spores in the environment is well known, the penetrance in the various nosocomial hosts can hardly been predicted [1,13]. Certainly, parts of the hospital equipment, previously thought as sterilized, can hardly been intact from latent microorganism forms. There are NICUs that after Bacillus’ contamination outbreak have altered the method of sterilization of the ventilator sensor [2], in the case described no respiratory support was being needed or so offered before the complete clinical deterioration. Though, “spore-form-bacteria” have been found to remain longitudinally over some surfaces. According to recent Space Stations Studies reference [3], when detected, quarantine remains for months or years. Nonetheless, biofilm formation in contact with inorganic material, may cause hardly any sign or mild symptoms as such in a healthy host. The potentially long latent period of the disease has not been extensively described in the literature. One of the most recent extensive surveys [13], including neonatal infected nosocomial population of various hospitals in France concludes that the same strain may remain longer than a two-year period in the same hospital, suggesting that in susceptible populations, having positive sample test for B. cereus should rather be considered as pathogenic rather than a contamination and imposes prompt treatment.
In contrast to acutely developed diarrheic syndrome, spores may last for long but, also the period of inoculation in the same host occasionally cannot be determined. In the case described, after the procedure of amniocentesis, the mother had mild bowel disturbances with diarrhea and was under broad spectrum antibiotic treatment. The twin B sac was diagnosed with 040.3 Polyhydramnios, third trimester, which was the indication for the cesarean section, prematurely. The twin sibling in our vignette was healthy and asymptomatic despite the first-days-similar conditions in the neonatal department. The two similar neonatal cases referred from the same period and hospital had shown different outcome [13]. Even, the total perinatal incubation period of time remains provocative. Impressively, a premature neonate, as described in literature, had shown latent carriage for weeks since a treated blood stream infection presided weeks before the lethal CNS infection [4].
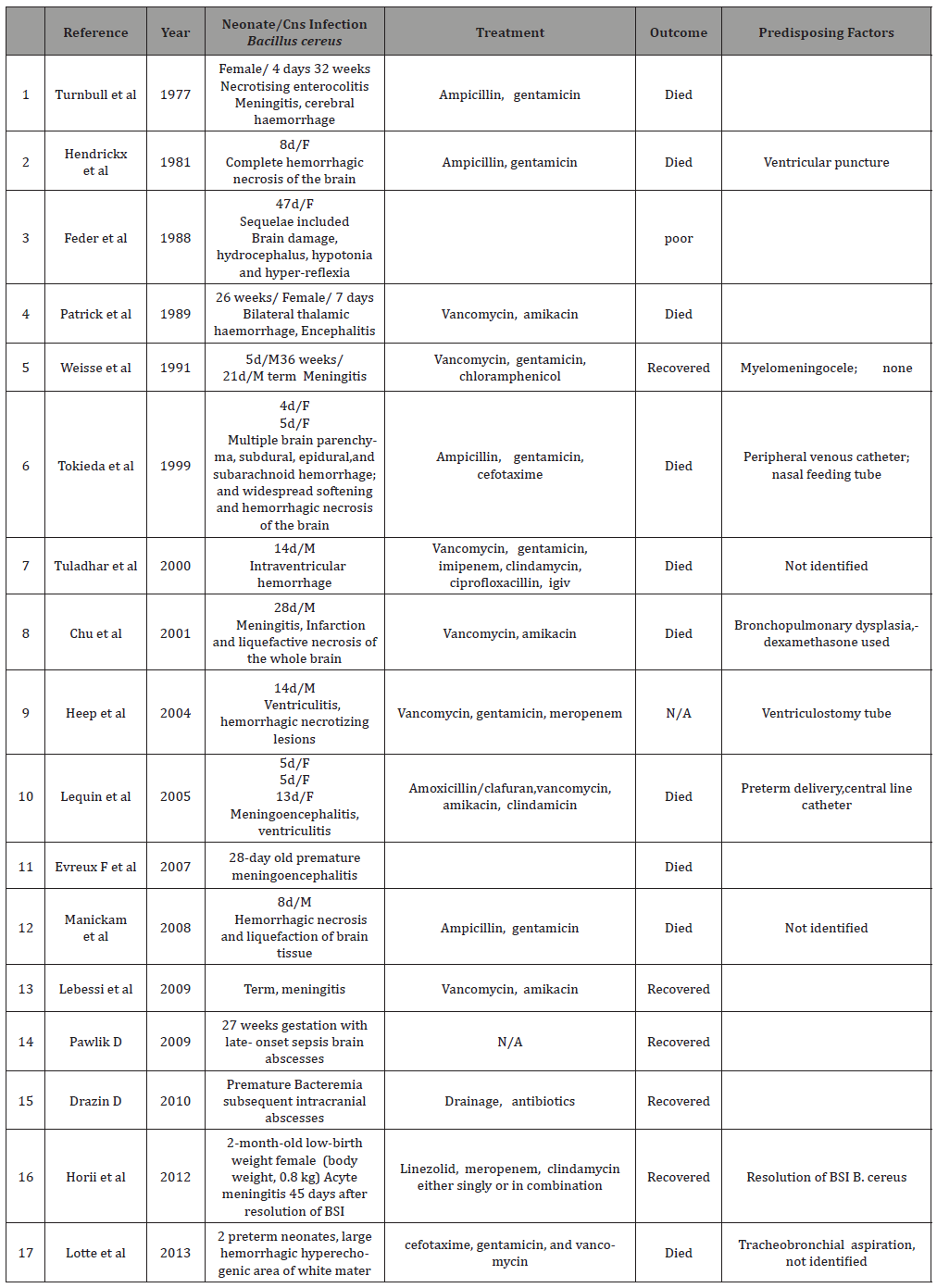
The difference between the twin siblings, as well as, the perinatal period individual events may impose variable vulnerability to the specific bacterial and response. Thus, infection from Bacillus cereus, an opportunistic pathogen may possibly be related to the host’s immune compromised response [5]. In most of the neonatal cases referred, were meningoencephalitis was involved, the outcome was lethal. Among the eight deaths, in total with patients of all ages, reported in the French Study, the four were premature neonates [13]. To our knowledge only a few neonatal cases, did rescue after appropriate treatment. They were term neonates and longitudinal follow up is not further described [6] (Table 1). In table 1, cases of neonates, both, premature and full term with CNS infection are depicted, though it is not extensive it exposes most of those which are referred as case reports.
Table 1:
In conclusion, B. cereus infection may be rare but there must be awareness concerning its toxicity and high mortality among neonates. It should always be suspected especially in preterm and extremely low birth weight infants with symptoms of sepsis.
Acknowledgement
None.
Conflict of Interest
No Conflict of Interest.
References
- Bottone EJ (2010) Bacillus cereus, a volatile human pathogen Clin Microbiol Rev 23(2):382-98.
- Turabelidze G, Gee JE, Hoffmaster AR, Farrin Manian, Cindy Butler, et al. (2013) Contaminated Ventilator Air Flow Sensor Linked to Bacillus cereus Colonization of Newborns. Emerging Infectious Diseases 19(5):781-783.
- Van Tongeren SP, Roest HIJ, Degener JE, Harmsen HJM (2014) Bacillus anthracis-Like Bacteria and Other B. cereus Group Members in a Microbial Community Within the International Space Station: A Challenge for Rapid and Easy Molecular Detection of Virulent B. anthracis. Schuch R, ed. PLoS ONE 9(6): e98871.
- Horii T, Tamai K, Notake S, Yanagisawa H. (2012) Bacillus cereus Bloodstream Infection in a Preterm Neonate Complicated by Late Meningitis. Case Reports in Infectious Diseases: 358789.
- Ramarao, Nalini Belotti, Laure Koebel, Christelle Escande, Benoit Guinebretière, et al. (2014) Two unrelated episodes of Bacillus cereus bacteremia in a neonatal intensive care unit. American Journal of Infection Control 42(6): 694-695.
- Brouland JP, Sala N, Tusgul S, Rebecchini C, Kovari E (2018) Bacillus cereus bacteremia with central nervous system involvement: A neuropathological study. Clin Neuropathol 37(1): 22-27.
- Maarten H Lequin, Jeroen R Vermeulen, Ruurd M van Elburg, Frederik Barkhof, René F Kornelisse, et al. (2005) Bacillus cereus Meningoencephalitis in Preterm Infants: Neuroimaging Characteristics. AJNR Am J Neuroradiol 26(8): 2137-2143.
- Zhu K, Didier A, Dietrich R, Heilkenbrinker U, Waltenberger E, et al. (2016) Formation of small transmembrane pores: An intermediate stage on the way to Bacillus cereus non-hemolytic enterotoxin (Nhe) full pores in the absence of NheA 469(3): 613-618.
- Beecher DJ, Wong AC (1994) Improved purification and characterization of hemolysin BL, a hemolytic dermonecrotic vascular permeability factor from Bacillus cereus. Infect Immun 62(3): 980-986.
- Oda M, Hashimoto M, Takahashi M, Ohmae Y, Seike S, et al. (2012) Role of sphingomyelinase in infectious diseases caused by Bacillus cereus. PLoS One.
- Hilliard NJ, Schelonka RL, Waites KB (2003) Bacillus cereus bacteremia in a preterm neonate. J Clin Microbiol 41(7):3441-3444.
- Stevens MP, Elam K, Bearman G (2012) Meningitis due to Bacillus cereus: A case report and review of the literature. Can J Infect Dis Med Microbiol 23(1): e16-19.
- Glasset B, Herbin S, Granier SA, Cavalie L, Lafeuille E, et al. (2018) Bacillus cereus, a serious cause of nosocomial infections: Epidemiologic and genetic survey 13(5): e0194346.
-
Koutsounaki E, Bitouni E, Liosis G, Salvanos I, Tzaki M. Bacillus cereus Bacteremia and Meningoencephalitis in a Preterm Neonate: A Case Report and bibliography review Glob J of Ped & Neonatol Car. 1(1): 2018. GJPNC.MS.ID.000501.
-
Vancomycin, Pediatric Patients, Antibiotic, Glycopeptide, Gram-Positive Organisms, Methicillin-Resistant Staphylococcus Aureus, Pharmacokinetics, Children, Clearance of Vancomycin, Empiric Pediatric, Pediatric Population, Potential Toxicity, Patient Age, Gender, Statistical Analysis, Unknown Origin, Leukocytosis, Sickle Cell Crisis and Empiric Indications
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






