 Research Article
Research Article
Altitude at Birth on Critical Congenital Heart Disease Screening Through Pulse Oximetry in Newborns in Colombia - A Validation by Experts
Gloria Amparo Troncoso Moreno1, Alejandra Fonseca2, Maria Teresa Domínguez Torres2*, Néstor Fernando Sandoval Reyes3, Alejandra TabordaRestrepo4, Hernán Camilo ArangurenBello2, Sandra Vanessa Romero Ducuara3, Cindy Lorena Chamorro4, Rodolfo José Dennis Verano2 and Darío Londoño Trujillo4
1Neonatal Intensive Care Unit, Cardioinfantil Foundation - Institute of Cardiology, Bogotá, Colombia
2Research Department, Cardioinfantil Foundation - Institute of Cardiology, Bogotá, Colombia
3Institute of Congenital Heart Diseases, Cardioinfantil Foundation - Institute of Cardiology, Bogotá, Colombia
4Axis of Public Health, Santa Fe Foundation of Bogotá, Colombia
Maria Teresa Domínguez, Research Department, Cardioinfantil Foundation - Institute of Cardiology, Bogotá, Colombia.
Received Date: June 05, 2020; Published Date: June 18, 2020
Abstract
Critical Congenital Heart Diseases (CCHDs) are heart anomalies that if untreated within the first year of life, can lead to death. Current strategies for an early diagnosis involve fetal echocardiography and physical examination. However, these practices fail to diagnose more than 50% of the cases. Since most CCHDs are hypoxic, the use of pulse oximetry to measure arterial oxygen saturation (SaO2) is considered as a complementary method for their detection. Studies have reported that the combination of echocardiography, physical examination and pulse-oximetry increases CCHDs diagnosis in newborns (NBs). Pulse oximetry screening algorithms are affected by altitude, and in Colombia, with a population concentrated at 1,000-2,700 meters above sea level, specific cut-off points for oxygen saturation are necessary.A construction and validation of an algorithm that would detect CCHDs in NBs in a range of 0-2,700 meters was done, based on 1) scientific literature available on pulse-oximetry in NBs, 2) SaO2 values reported at different altitudes, 3) Neonatology and Pediatric Cardiovascular Surgery experts’ opinion, and 4) assessment by the Colombian Society of Neonatology, Cundinamarca.A SaO2<90% cut-off point was defined, measured by pulse oximetry during the first 24 hours of life, or a difference >3% in measurements for saturation in hand and foot, with referral for chest X-ray and transthoracic echocardiogram in case of <90% readings. Combined with the physical examination, this strategy will allow early detection of CCHDs in NBs, and it may be used as basis for the design of public policies in the National screening panel.
Keywords: Critical congenital heart diseases; Screening; Pulse oximetry; Newborns; Altitude
Abbreviations: (CCHDs): Critical Congenital Heart Diseases; (NB): Newborns; (SaO)2: Arterial Oxygen Saturation; (HLHS): Hypoplastic Left Heart Syndrome; (PA): Pulmonary Atresia; (TOF): Tetralogy of Fallot; (TAPVR): Total Anomalous Pulmonary Venous Return; (TGV): Transposition of Great Vessels; (AT): Tricuspid Atresia and (TAr): Truncus Arteriosus; (MAMSL): meters above sea level.
Introduction
Congenital Heart Diseases (CHDs) are anatomic anomalies of the heart and its blood vessels originating at the prenatal period, from day 18 of the second week of intrauterine life, due to genetic and environmental factors [1,2]. This type of anomaly has a prevalence of approximately 8-9/1,000 live births and it is one of the main causes of pediatric mortality in high, medium and low in come countries. It is responsible for 5.7% neonatal deaths in the United States [1,3,4,5]. In Latin America it is estimated that every year 54,000 children are born with Congenital Heart Diseases, and only 17,000 of them undergo any treatment [6,7]. Within this type of anomaly, we find Critical Congenital Heart Diseases (CCHDs), which require surgery or catheter intervention in the first year of life [3,8,9]. If diagnosed late they may provoke circulatory collapse, shock, acidosis, intracranial hemorrhage, hypoxic-Ischemic encephalopathy, heart failure and even death [5,8,10]. CCHDs may be ductal-dependent and cyanotic, correlated with some degree of hypoxia. Among them we find Hypoplastic Left Heart Syndrome (HLHS), Pulmonary Atresia (PA), Tetralogy of Fallot (TOF), Total Anomalous Pulmonary Venous Return (TAPVR), Transposition of Great Vessels (TGV), Tricuspid Atresia (AT) and Truncus Arteriosus (TAr) [10-12].
Due to the severity of CCHDs, the Local Health Authorities and the Department of Health and Human Services (HHS) in the United States, and the American Academy of Pediatrics (AAP) have recommended that CCHD screening be added to the uniform screening panel in that country [8]. Many NBs with CCHDs, in particular with anomalies like HLHS, TGV and TAPVR do not present the characteristic symptoms for these types of heart diseases (i.e. heart murmur or cyanosis), and do not receive timely diagnosis [10,13]. So far, the main strategy used in the United States focuses on performing fetal echocardiography and the general postnatal physical examination, involving pulse assessment and cyanosis detection within the first 24 to 48 hours after birth [14], the conventional physical clinical examination being the most widely used method around the world [8,15].
The routine physical examination of the newborn has a low rate in the detection of CCHD, as it fails to detect from 30% to 50% of NBs with CCHD, leading to an early discharge from hospital, before diagnosis [9,10,13,15,16]. It has also been described that approximately 1/15,000 -1/26,000 cases of CCHD with fatal outcomes may be prevented if they are detected early [5]; for this reason, CCHD screening by means of a more sensitive and specific tool is essential in the early detection of CCHD [9].
Since CCHD may be associated with a certain degree of hypoxemia [10], a noninvasive test that measures Arterial Oxygen Saturation (SaO2), even in the absence of other physical symptoms, such as pulse oximetry, is considered a complementary strategy in the early detection of CCHDs [15,17,14]. To this end, a meta-analysis conducted by Thangaratinam et al. [10], which included 13 studies on a total population of NBs, revealed that the use of pulse oximetry as a screening method had a specificity and sensitivity of 99.9% (I.C. 99.7-99.9) and 76.5% (I.C. 67,7-83,5) respectively [10]; the latter being higher than the figure resulting from the use of the conventional postnatal physical examination as a screening method [10]. Thus, pulse oximetry is considered a good complementary strategy to the general physical examination in the detection of CCHD in NBs.
At present, SaO2 levels are measured through pulse oximetry in the NBs’ limbs. The saturation limit corresponds to 90% and figures lower than this may be markers for heart anomalies [10]. Hence, SaO2 may be measured at the hand or foot. However, a combination of 1) an SaO2<90% from the hand or foot and 2) a difference larger than 3% between the SaO2 measured at the hand and the one measured at the foot may allow for the detection of little common conditions in which the only saturation reading taken from the limbs may provide normal values; as in the case of interrupted aortic arches, in which saturation at the hand may be 99% and 95% at the foot [13,14]. Both values are considered as normal individually; however, if we consider the difference between saturation measured in the upper limbs and the one measured in the lower limbs, there is a difference larger than 3%, a possible indicator for heart anomaly.
At present, SaO2 levels are measured through pulse oximetry in the NBs’ limbs. The saturation limit corresponds to 90% and figures lower than this may be markers for heart anomalies [10]. Hence, SaO2 may be measured at the hand or foot. However, a combination of 1) an SaO2<90% from the hand or foot and 2) a difference larger than 3% between the SaO2 measured at the hand and the one measured at the foot may allow for the detection of little common conditions in which the only saturation reading taken from the limbs may provide normal values; as in the case of interrupted aortic arches, in which saturation at the hand may be 99% and 95% at the foot [13,14]. Both values are considered as normal individually; however, if we consider the difference between saturation measured in the upper limbs and the one measured in the lower limbs, there is a difference larger than 3%, a possible indicator for heart anomaly.
The use of pulse oximetry in combination with the general physical examination may drastically improve the detection of CCHD, avoiding late diagnosis of these alterations and allowing for timely treatment. It has been described that, in spite of the differences in study protocols and the cut-off values in the studies performed, the use of pulse oximetry is a useful strategy in the detection of CCHD [9], and its false positive rate has been reported to be only 0.10-0.17 cases [9,18]. For this reason, the AAP has recently published recommendation strategies for the implementation of pulse oximetry as a screening method in NBs [14].
According to Valmari et.al. [19], 1.56 cases of CCHD in 1,000 live births were not detected through the conventional physical examination. However, when pulse oximetry and physical examination are combined, this figure is noticeably reduced to 0.25 cases in 1,000 [13,19]. However, it is important to notice that pulse oximetry has a sensitivity of 76.5% in the detection of CCHDs, so its use in isolation, without a complementary technique like the physical examination is not recommended [10,20].
Current practices for CCHD screening by means of pulse oximetry involve measuring SaO2 through this technique. Saturation figures higher than the cut-off point are considered negative and a physical examination is performed. In turn, saturation levels lower than the cut-off point should be referred to consultation with cardiology, and those showing SaO2 levels similar to the cut-off point are submitted to a second pulse oximetry measurement with a time interval of 6 to 12 hours [13].
The altitude at which SaO2 levels are measured may have an influence on the SaO2 cut-off point, since, as altitude increases, atmospheric pressure decreases. Hence, at sea level, atmospheric pressure is 760 mm Hg; at an altitude of 1,000m it corresponds to 674 mm Hg, and at 4,000m it is 462 mm Hg. However, since the oxygen concentration remains almost the same (21%), its partial pressure drops and the presence of oxygen in the alveoles is reduced. This results in a lower amount of oxygen being transferred from the alveoles to the blood stream [21,26]. As mentioned earlier, pulse oximetry allows for a more efficient detection of CCHD [22]; however, due to the physiological characteristics of newborns at higher altitudes above sea level, the design of a screening algorithm considering these altitudes is necessary. Colombia has an altitude range varying from 0 to 6,000 meters above sea level (MAMSL), and the more densely populated geographical areas are located between 1,000 and 2,700 MAMSL. Therefore, the design of SaO2 cut-off points that consider these altitude ranges is necessary.
Currently, in spite of the AAP recommendations to include CCHD screening within the national uniform screening panel in the United States [8], Colombia does not count on a screening model for these abnormalities in NBs in the design of public policies for the early detection of these heart anomalies. For this reason, the purpose of this study was to design a flow diagram with the main decisions to be taken for the early detection of CCHD in NBs at altitudes from 0-2,700 MAMSL.
Materials and Methods
The study was carried out by means of a four-phase flow diagram: the first phase involved the review of the literature available on the use of pulse oximetry for measuring SaO2 at different altitudes above sea level, and the referencing of some studies on the levels of saturation conducted on different age groups at altitudes above 1,600 MAMSL [23,24]. The second phase corresponded to the determination of the median values of SaO2 found among newborns at altitudes above 1,600 MAMSL. A systematic review of the literature was conducted to determine these values. Based on the first version of the flow diagram proposed during the first phase and the validation of members of the Sociedad Colombiana de Neonatología, capitulo Cundinamarca (Neonatology Colombian Society, Cundinamarca) a new flow diagram was designed. The third phase included the presentation of the flow diagram by these specialists and the review of the study conducted by Wright [25], in which a cut-off SaO2 of 85% is used at 1,600 MAMSL; the use of different cut-off points was adopted, depending on the altitude above sea level of the cities involved. In the fourth and last phase, the flow diagram was reviewed and assessed by different medical specialists from the Colombian Society of Neonatology, from the Cundinamarca regional office.
Results and Discussion
The influence of atmospheric pressure on optimal arterial oxygen saturation affects both preductal and post ductal saturation in healthy NBs, as these are lower than the readings reported above sea level. Because of the differences in the amounts of oxygen in the blood, the AAP has declared that the algorithms designed for these altitudes may be different [8], and research has been carried out on the SaO2 cut-off points used in the screening of CCHDs at altitudes different from sea level [21,26].
In line with this idea, in the United States, the study conducted by Ravert et al. revealed that, at an altitude higher or equal to 6,800 ft. (2,073 MAMSL), healthy NBs showed an SaO2 between 91 and 96% [21]. In La Paz, Bolivia, a study conducted at an altitude of 3,665 MAMSL, including 122 NBs within the first 24 hours after birth, revealed an SaO2 between 88.7 and 88.2% [24]; on the other hand, in El Alto, Bolivia, at an altitude of 4,018 MAMSL, 168 newborns with ages ranging from 1 day to 60 months showed an average SaO2 of 87.3% [27]. In Israel, at 780 MAMSL, 119 NBs had an SaO2 of 97.86% during the first 24-72 hours of life [26]. In Bogota, Colombia, at 2,640 MAMSL, SaO2 levels were measured in 189 infants between 5 days and 24 months of age and an average SaO2 of 92.6% was obtained for the first month of life [13]. Conversely, as described by Paralikar and Paralikar [28], heights between 1,500 and 3,500 mts are considered high altitudes, and within this range, there is a light disability in the transportation of arterial oxygen, which corresponds to an SaO2 of at least 90%. Due to the fact that in the studies previously mentioned the SaO2 oscillates between 91 and 97%, at altitudes between sea level and 2,640 mts [21,23,26], in Colombia, it is possible to use a unique cut-off point for the design of the algorithm to screen CCHD in NBs.
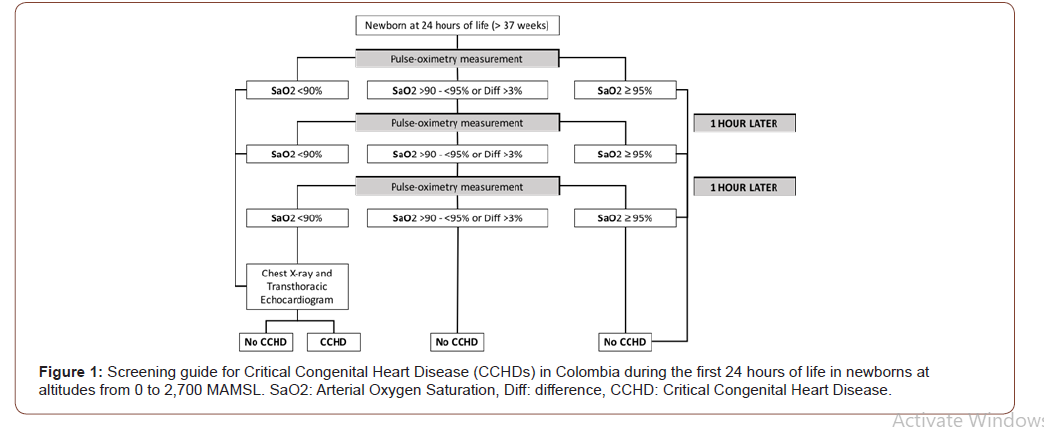
In this way, and based on the scientific literature available, the experience observed in Colombian hospitals, and after a consensus with members of the Colombian Association of Neonatology, Cundinamarca Regional Office, an algorithm was designed that considered the different altitudes found in Colombia (Figure 1). Within the first 24 hours of life, SaO2 will be measured by preductal (in the hand) or post ductal (in the foot) pulse oximetry, with a cut-off point of SaO2< 90%, or a combination of pre-and post-ductal reading; with an SaO2 limit of 90% and/or a difference larger than 3% between the SaO2 levels taken at the upper and lower limbs. Newborns with SaO2<90% will undergo chest X-rays and transthoracic echocardiogram, and those with SaO2 readings between 90 and 95% will undergo sequential SaO2 measurements with one-hour intervals in between measurements.
Due to the severity of CCHDs and the fatal consequences they may entail in the absence of timely treatment, a specific screening method is necessary. Most CCHDs are hypoxic; hence the use of pulse oximetry to measure SaO2 is useful in the optimal screening of CCHDs [10,13]. Detection of CCHDs through pulse oximetry depends directly on SaO2 cut-off points and the altitude at which the detection is carried out. However, till now, studies determining the average SaO2values in NBs at an altitude different from sea level are scarce. Those studies available focus on measuring SaO2 at a specific altitude - e.g. Ravert’s, at 2,073 m [21], Salas’ at 3,665 m [24], Gamponia’s at 4,018 m [27], Samuel’s at 780 m [26]and Lozano’s at 2,640 m [23]. In spite of this, a study where an algorithm is designed to study different SaO2 levels at different heights has not yet been conducted.
Altitude levels between 1,500 and 3,000 MAMSL are considered high altitudes, and healthy NBs report SaO2 levels of at least 90% [28]. In Colombia, a country with a high population density, mostly located between 0 and 2,700 MAMSL, it is possible to design an algorithm with the same cut-off points, to be used all over the country. The algorithm was developed on the bases of the existing literature on the use of pulse oximetry in NBs, followed by its validation and revision by specialists in pediatric cardiovascular surgery and neonatology at Fundacion Cardioinfantil – Institute of Cardiology, and different health specialists from the Colombian Society of Neonatology, Cundinamarca Regional Office. The strategy designed takes as the cut-off point an SaO2<90%, measured by pulse oximetry during the first 24 hours of life, or a difference larger than 3% in the saturation level readings for hand and foot, followed by chest X-ray and transthoracic echocardiogram in case of obtaining an SaO2<90%. The combination of this algorithm and the conventional physical examination will allow for the detection of CCHDs in a more sensitive way in the Colombian territory with a higher population density
Our study shows some limitations, as the little amount of literature available on SaO2 levels in NBs at altitudes different from sea level may bias the SaO2 cut-off points chosen to determine the presence of CCHD. Likewise, this algorithm was designed for altitudes between 0 and 2,700 MAMSL; hence, in remote regions with higher altitudes and low population densities its use is not recommended, and NBs should be referred to healthcare centers located at up to 2,700 m of altitude to perform the CCHD screening.
Conclusion
present, the most widely used strategy for the screening of CCHDs is the conventional physical examination; however, this exam fails to detect between 30 and 50% of newborns suffering from CCHD. For this reason, the use of a more sensitive and specific strategy like pulse oximetry is necessary. However, the algorithms designed according to this methodology are directly related with the altitude level at which they are going to be used. The algorithm presented in this study has been designed for altitudes between 0 and 2,700 MAMSL (heights corresponding to Colombia); its use in combination with the conventional physical examination will allow for higher levels of reliability in SaO2 measurements and a larger sensitivity in the detection of CCHD.
Funding Source
This study was supported by the Fondo Nacional de Financiamiento para la Ciencia, la Tecnología y la Innovación, Francisco José de Caldas – COLCIENCIAS, Programa para la Innovación en Cardiopatías Congénitas Humanas Infrecuentes para Colombia (PINOCCHIO)-Contract 662-2015 and by the Consortium of Pediatricians at Fundación Cardioinfantil - Instituto de Cardiología.
Acknowledgement
We wish to thank all the team involved in the project “Cost-effectiveness economic assessment and Budget impact of neonatal screening with oximetry in the detection of Critical Congenital Heart Diseases” from the Program for Innovation in Uncommon Congenital Heart Diseases in Humans in Colombia - PINOCCHIO, from Fundación Cardioinfantil- Instituto de Cardiología, Eje de Salud Pública from Fundacion Santa Fe de Bogota and the Colombian Association of Neonatology, Cundinamarca Regional Office.
Conflict of Interest
No conflict of interest.
References
- Benavides-Lara A, Faerron Ángel JE, Umaña Solís L, Romero Zúñiga JJ (2011) Epidemiology and registration of congenital heart diseases in Costa Rica. Rev Panam Salud Publica 30(1): 31-38.
- García A, Moreno K, Ronderos M, Sandoval N, Caicedo M, et al. (2016) Differences by Altitude in the Frequency of Congenital Heart Defects in Colombia. Pediatr Cardiol 37(8): 1507-1515.
- Han LM, Klewer SE, Blank KM, Seckeler MD, Barber BJ (2013) Feasibility of Pulse Oximetry Screening for Critical Congenital Heart Disease at 2643-Foot Elevation. Pediatr Cardiol 34(8): 1803-1807.
- Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, et al. (2005) Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis. Health Technol Assess (Winchester, England) 9(44): 1-152.
- Schultz AH, Localio AR, Clark BJ, Ravishankar C, Videon N, et al. (2008) Epidemiologic Features of the Presentation of Critical Congenital Heart Disease: Implications for Screening. Pediatrics 121(4): 751-757.
- Sandoval Néstor (2015) Congenital heart diseases in Colombia and around the world. Revista Colombiana de Cardiología 22(1): 1-2.
- Sandoval Nestor, Kreutzer C, Jatene M, Sessa T Di, Novick W, et al. (2010) Pediatric Cardiovascular Surgery in South America: current status and regional differences. World J Pediatr Congenit Heart Surg 1(3): 321-327.
- Mahle W, Martin G, Beekman 3rd, Morrow R, Rosenthal G, et al. (2012) Endorsement of Health and Human Services Recommendation for Pulse Oximetry Screening for Critical Congenital Heart Disease. Pediatrics 129(1): 190-192.
- Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, et al. (2010) Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine - results from a prospective multicenter study. Eur J Pediatr 169(8): 975-981.
- Thangaratinam S, Brown K, Zamora J, Khan K, Ewer A (2012) Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet 379(9835): 2459-2464.
- Liberman RF, Getz KD, Lin AE, Higgins CA, Sekhavat S, et al. (2014) Delayed Diagnosis of Critical Congenital Heart Defects: Trends and Associated Factors. Pediatrics 134(2): e373-81.
- Talner CN (1998) Report of the New England Regional Infant Cardiac Program, by Donald C. Fyler, MD, Pediatrics 1980; 65(suppl):375-461. Pediatrics, 102(1 Pt 2): 258-259.
- Hoffman JIE (2011) It Is Time for Routine Neonatal Screening by Pulse Oximetry. Neonatology 99(1): 1-9.
- Peterson C, Ailes E, Riehle-Colarusso T, Oster ME, Olney RS, et al. (2014) Late Detection of Critical Congenital Heart Disease Among US Infants. JAMA Pediatr 168(4): 361.
- Ewer AK, Middleton LJ, Furmston AT, Bhoyar A, Daniels JP, et al. (2011) Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study. Lancet 378(9793): 785-794.
- Bakr AF, Habib HS (2005) Combining Pulse Oximetry and Clinical Examination in Screening for Congenital Heart Disease. Pediatr Cardiol 26(6): 832-835.
- Meberg A, Andreassen A, Brunvand L, Markestad T, Moster D, et al. (2009) Pulse oximetry screening as a complementary strategy to detect critical congenital heart defects. Acta Paediatr 98(4): 682-686.
- De-Wahl Granelli, A, Wennergren M, Sandberg K, Mellander M, Bejlum C, et al. (2009) Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ 338: a3037.
- Valmari P (2007) Should pulse oximetry be used to screen for congenital heart disease? Archives of Disease in Childhood. Arch Dis Child Fetal Neonatal Ed 92(3): F219-224.
- Ewer AK (2013) Review of pulse oximetry screening for critical congenital heart defects in newborn infants. Curr Opini Cardiol 28(2): 92-96.
- Ravert P, Detwiler TL, Dickinson JK (2011) Mean Oxygen Saturation in Well Neonates at Altitudes Between 4498 and 8150 Feet. Adv Neonatal Care 11(6): 412-417.
- Ewer AK, Martin GR (2016) Newborn Pulse Oximetry Screening: Which Algorithm Is Best? Pediatrics 138(5): e20161206.
- Lozano JM, Duque OR, Buitrago T, Behaine S (1992) Pulse oximetry reference values at high altitude. Arch Dis Child 67(3): 299-301.
- Salas AA (2008) Pulse oximetry values in healthy term newborns at high altitude. Ann Trop Paediatr 28(4) 275-278.
- Wright J, Kohn M, Niermeyer S, Rausch CM (2014) Feasibility of Critical Congenital Heart Disease Newborn Screening at Moderate Altitude. Pediatrics 133(3): e561-e569.
- Samuel TY, Bromiker R, Mimouni FB, Picard E, Lahav S, et al. (2013) Newborn oxygen saturation at mild altitude versus sea level: implications for neonatal screening for critical congenital heart disease. Acta Paediatr 102(4): 379-384.
- Gamponia MJ, Babaali H, Yugar F, Gilman RH (1998) Reference values for pulse oximetry at high altitude. Arch Dis Child 78(5): 461-465.
- Paralikar SJ, Paralikar JH (2010) High-altitude medicine. Indian J Occup Environ Med 14(1): 6-12.
-
Maria Teresa Domínguez Torres, Néstor Fernando Sandoval Reyes etc..., all. Altitude at Birth on Critical Congenital Heart Disease Screening Through Pulse Oximetry in Newborns in Colombia - A Validation by Experts. Glob J of Ped & Neonatol Car. 2(3): 2020. GJPNC. MS.ID.000540.
Critical congenital heart diseases, Screening, Pulse oximetry, Newborns, Altitude, Birth, Neonatology, Colombia, SaO2, Physical examination, Cundinamarca
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






