 Research article
Research article
Frequency and type of drug-related adverse effects in Pediatric Inflammatory Bowel Disease
Catarina Freitas1,2*, Carolina Castro1,2, Diana Alba1,3, Isabel Pinto Pais1, Maria do Ceu Espinheira1, Eunice Trindade1
1Department of Pediatrics, Centro Hospitalar Universitário de São João, Porto, Portugal
2Department of Pediatrics, Hospital Pedro Hispano, Matosinhos, Portugal
3Department of Pediatrics, Centro Hospitalar Tâmega e Sousa, Penafiel, Portugal
Catarina Freitas, Pediatrics Department of Hospital Pedro Hispano, Matosinhos, Portugal.
Received Date:January 19, 2024; Published Date:January 29, 2024
Abstract
Background: Inflammatory Bowel Disease (IBD) is a chronic inflammatory condition of the intestine, characterized by periods of remission and
relapse. The management of the disease implies long-term treatments in the majority of patients, with risk for adverse effects, ranging from mild
symptoms to severe complications that may necessitate adjustments or discontinuation of the therapy. Our objective was investigating the frequency
and types of these events in a pediatric cohort from a level 3 central hospital.
Methods: A retrospective observational analysis was performed of all paediatric patients with IBD followed in the Pediatric Gastroenterology
Unit from a level 3 central hospital.
Results: A total of 400 pediatric cases diagnosed with IBD were analyzed, comprising 246 with Crohn’s disease (CD), 137 with Ulcerative
Colitis (UC), and 17 with IBD-unclassified (IBD-U). In our cohort, 34 (8.5%) experienced at least one drug-related adverse effect (AE) necessitating
discontinuation of the medication. The most common AE was acute pancreatitis due to azathioprine. Infliximab was discontinued because
of anaphylactic reaction in 4 patients and psoriasis in 2 patients. For oral 5-ASA 3 patients had gastrointestinal intolerance, which required
discontinuation. 33 patients who underwent drug discontinuation, 18 were under combined therapy involving at least 2 concomitant drugs.
Conclusions: Discontinuation due to adverse drug events is common among paediatric patients with IBD. Despite all the efforts to minimize
such effects, the primary risk factor seems to be the utilization of combined therapy. Physicians should be aware of these to discuss safety profile and
search a continuous balance between efficacity and the occurrence of potential lateral damages.
Keywords:Pediatric; Inflammatory Bowel Disease; Crohn Disease; Ulcerative Colitis; Drug related adverse effect
Introduction
Inflammatory Bowel Disease (IBD) is a chronic inflammatory condition of the intestine with a course characterized by the occurrence of episodes of remission and relapse. One of the targets of treatment is to avoid disability of the organ related to the continuous inflammatory activity. As such, long-term treatments are required for disease control in most patients, not only to reduce the risk of bowel injury but also to guarantee adequate growth and normal development of children [1-3].
However, despite their efficacy in the control of inflammation, most of the used drugs have the potential to cause adverse effects, ranging from mild symptoms to life-threatening complications, requiring adjustment or discontinuation of therapy. In pediatric population, adverse effects have been related to different class of drugs, namely azathioprine with a need of discontinuation of 10% to 22%. Regarding tumor necrosis factor (TNF) – alpha antagonist, the percentage of adverse effects seem to be minor, namely risk for serious infections, when compared to patients treated with steroids [4-8].
This issue is particularly relevant in pediatric population considering the early age of use the drugs, predictably long time of exposure, frequent need of combination therapy and sequential use of biologic therapy.
A great variety of new biological agents are available now days, most of them used off-label in pediatric patients, while phase 2 and 3 trials are ongoing. Real-world studies in pediatric patients suggest positive outcomes but strict vigilance concerning safety issues should be maintained [9-13].
Given the reduced number of studies with specific pediatric evaluation of adverse effects associated to treatment discontinuation, it was our purpose investigate frequency and type of this events in a pediatric cohort from a level 3 central hospital.
Materials and Methods
We performed a retrospective review, that included paediatric patients with IBD (up to 18 years old). Data about demographic variables (gender, age) and IBD treatment, including the drug and drug-related adverse effects necessitating treatment discontinuation, were collected. For these cases we characterized the type of adverse effect, IBD type and age at diagnosis, the type of therapy (combined or single).
We investigated also what was the frequency of adverse events related to the use of multiple (at least two) drug treatments in IBD patients.
Results
Table 1:Number and proportion of patients with AE necessitating drug discontinuation among the total number of patients exposed to that drug, by diagnosis.
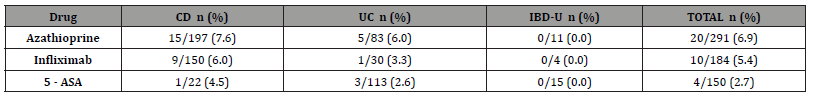
CD = Crohn disease; UC= ulcerative colitis; IBD-U= inflammatory bowel disease; 5-ASA (5-aminosalicylic acid)
A total of 400 pediatric cases with a diagnosis of IBD were analysed, of which 246 (61.5%) with Crohn disease (CD), 137 (34.3%) with ulcerative colitis (UC) and 17 (4.3%) with IBDunclassified (IBD-U). In our cohort, 34 (8.5%) had at least one drugrelated adverse effect (AE) that required drug discontinuation. Azathioprine caused AE requiring drug cessation in 6.9% (20/291) of patients, followed by TNF-alpha antagonist infliximab (5.4%; 10/184) and oral 5-ASA (2.3%; 4/150) as represented in Table 1.
Demographic characteristics of patients with AE compared to patients without AE are presented in Table 2. There was no differences between these two groups of patients with regards to gender, diagnosis and age at diagnosis.
Table 2:Characteristics of Inflammatory bowel disease patients grouped by drug-related adverse effects (AE) versus no drug AE.
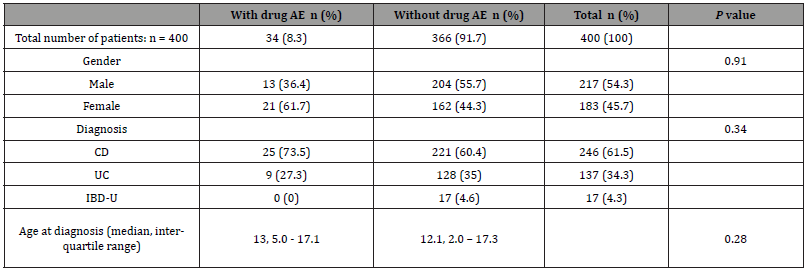
AE = adverse events; CD = Crohn disease; UC= ulcerative colitis; IBD-U= inflammatory bowel disease
Regarding the group with AE that required drug cessation, the most common was acute pancreatitis due to azathioprine (documented in 8 cases), followed by digestive intolerance including vomiting and abdominal pain (in 7 cases). The most severe case occurred with a female patient who discontinued treatment with azathioprine due to the diagnosis of Hodgkin´s lymphoma (unrelated to EBV infection). The mean duration of treatment until discontinuation was 17.3 [2-56] weeks. None of these patients had genetic variations in the thiopurine methyltransferase gene (TPMT).
Infliximab was discontinued because of anaphylactic reaction in 4 of 10 (40%) patients and psoriasis onset in 2 of 10 (20%) patients. For oral 5-ASA 3 patients had gastrointestinal intolerance, which required discontinuation.
There was a total of 4 cases of opportunistic infection, 2 pulmonary tuberculosis under treatment with infliximab and 2 cases of symptomatic primary EBV infection in children under azathioprine.
Among the 33 patients experiencing drug cessation, 18 (54.4%) were under combined therapy (at least 2 concomitant drugs). Azathioprine was part of the concomitant drug in 23 (69.7%) cases.
Additionally, we had 33 patients treated with different classes of biologicals, without evidence of adverse events: Adalimumab 20; Vedolizumab 6; Ustekinumab 6 and Tofacitinib 1 patient. Among these patients, all were previously exposed to 1 or 2 different biological agents, namely Infliximab.
Discussion/Conclusion
In our study, we present data of a pediatric cohort with significant number of patients, evaluating the frequency and type of IBD drug-related side effects that required treatment discontinuation. Treatment of paediatric IBD patients should be looked like a continuous balance between the need of guaranty efficacity in different domains (control of the inflammation, growth, mental health, etc) and safety in order to minimize the occurrence of potential lateral damages.
In our cohort, the total prevalence of drug cessation because of AE was 8.5%. Azathioprine was the drug most frequently associated with adverse effects, followed by infliximab, and 5-ASA. Twenty of 315 (6.3%) patients had to stop azathioprine mainly because pancreatitis or gastrointestinal intolerance, which are the most associated side effects according to the literature. However, this prevalence is lower when compared with other paediatric studies, where 10.3% - 22% paediatric IBD patients had to discontinue azathioprine due to AE [4-8]. As expected, evaluation of thiopurine methyltransferase (TPMT) do not predict all cases of leukopenia and azathioprine-specific hypersensitivity reactions. In our group, and according the ECCO/ESPGHAN guidelines, the determination of TPMT genotype or phenotype is systematically performed to identify patients at greater risk of profound myelosuppression and that way enables us to the adjust the maximal dose to be used with security in those that are herterozygous mutants or even contraindicate its use in those that are homozygous mutants for TPMT [1, 2].
Two patients under azathioprine presented symptomatic primary EBV infection and azathioprine was promptly discontinued. We know that a significant proportion of new pediatric Portuguese IBD patients are EBV-naïve; for that reason, and as previously described, we preform systematic screening of EBV status, which enables the identification of patients at risk of primoinfection; the occurrence of symptoms suggestive of acute EBV infection in seronegative patients should lead to rapid confirmation of the diagnosis because timely diagnosis may allow the adjustment of therapeutic strategy sparing patients from potentially severe iatrogenesis [14].
In our cohort a female patient had the diagnosis of Hodgkin lymphoma; in this case there was no evidence of EBV infection before or after the diagnosis of the lymphoproliferative disease. The possibility of occurrence of lymphoproliferative disorders in IBD patients is a serious concern. The risk seems to be similar or slightly higher than that in the general population and is related to the patient’s age and disease duration. The literature suggests that thiopurines are associated with a 4- to 5-fold increase in risk of lymphoproliferative disorders, it increases gradually for successive years of therapy, and that discontinuing thiopurines reduces the risk of lymphoma. However, risk-versus-benefit models suggest that benefits outweigh the risks in the case of thiopurine use [15- 22].
Most of these cases of lymphoproliferative disorders in IBD patients on thiopurines are associated with EBV and of the non- Hodgkin type, what was not the case of our patient [18, 23-25]. She had the diagnosis of Crohn disease and uveitis 6 years before the diagnosis of lymphoma, was exposed to adalimumab in the first 2 years when she was submitted to ileal resection for ileal stenosis; after surgery azathioprine was started and maintained for 4 years. Clinical surveillance should be ensured, always keeping in mind the possibility of occurrence of malignancy related or not with IBD treatment.
The TNF-alpha antagonist infliximab was the second more frequent drug causing AE requiring treatment discontinuation (5.4%; 10/184), which is a low risk, as described in the literature. Anaphylactic reactions occurred in 4 patients and were promptly recognized and treated; these patients were subsequently treated with adalimumab with good response. The patients with psoriasis were switched to ustekinumab with control of the disease.
Two of the 184 cases on infliximab treatment had pulmonary tuberculosis. This risk has been documented in studies carried out in adults but has not been demonstrated in paediatric patients.[5] Annual re-screening could be considered especially for patients with a higher tuberculosis risk; the risk is dependent on the local disease burden of tuberculosis, and the benefit/risk should always be considered individually [26,27]. It should be noted that Portugal, and the North of Portugal in particular, still have a considerable number of new cases of tuberculosis per year [28]. For that reason, once a year we repeat the QuantiFERON and proactive inquire the patients in each visit about respiratory complaints. One of our patients was symptomatic and the other presented the annual QuantiFERON test positive. Both were treated with success and restarted the previous treatment with infliximab, after concluding the treatment of tuberculosis disease.
According to the literature, 5-ASA are well tolerated, which was also seen in our sample. Only 4 of 150 cases had to discontinue treatment, most due to gastrointestinal intolerance. No pancreatitis or nephritis was reported in our cohort.
When evaluating associated factors, there was no difference between the AE group and the other, with regards to gender, IBD subtype and age at diagnosis. However, we found a strong association with AE necessitating drug cessation and combined therapy (at least 2 concomitant drugs). Azathioprine was the commonest concomitant drug in our cohort. Current guidelines recommend the use of combined therapy between azathioprine and infliximab for maintenance of remission in Crohn’s and ulcerative colitis, ideally for 6 to 12 months; but in clinical practice we all have the experience that some patients need to maintain combined treatment for longer periods of time because of the aggressivity of the disease in order to obtain a better control and prevent bowel damage [1,2]. In an Swiss IBD paediatric cohort study, it was identified that the use of at least 3 IBD drugs increased the risk of AE [4]. A combination of different new class of biological therapy has been reported in small series of patients but we do not have personal experience in this field [29].
We found an association of concomitant use of 2 or more drugs and an increased risk of AE. However, we cannot determine whether an increased risk of AE is due to an accidental drug interaction or a cumulative effect of each drug when using 2 or more drugs at the same time.
In our study drug levels were not captured. Therefore, we were not able to filter, for example, for dose-dependent AE.
In conclusion, adverse drug events leading to discontinuation are frequent in pediatric IBD patients despite all the efforts to minimize these effects. The strongest risk factor appears to be the use of combined therapy. Physicians should be aware of these aspects related to “old” drugs and be alert to possible adverse effects of “new” drugs, in order to be able to discuss safety profile and treatment strategies with patients.
Funding
This study did not receive any funding.
Acknowledgement
None.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Turner D, Ruemmele FM, Orlanski-Meyer E, Anne M Griffiths, Javier Martin de Carpi, et al. (2018) Management of paediatric ulcerative colitis, part 1: ambulatory care-an evidence-based guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 67:257-291.
- Rheenen PF, Aloi M, Assa A, Jiri Bronsky, Johanna C Escher, et al. (2021) The Medical Management of Paediatric Crohn’s Disease: an ECCO-ESPGHAN Guideline Update. Journal of Crohn's and Colitis pp. 171-194.
- Turner D, Ricciuto A, Lewis A, Ferdinando D'Amico, Jasbir Dhaliwal, et al. (2021) STRIDE-II: An Uptade on the Selecting Therapeutic Targets in Inflamatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study od IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 160(5): 1570-1583.
- Godat S, Fournier N, Safroneeva E, Pascal Juillerat, Andreas Nydegger, et al. (2018) Frequency and type of drug-related side effects necessitating treatment discontinuation in the Swiss Inflammatory Bowel Disease Cohort. Eur J Gastroenterol Hepatol 30(6): 612-620.
- Salzmann M, Graffenried T, Righini-Grunder F, Christian Braegger, Johannes Spalinger, et al. (2020) Drug-Related Adverse Events Necessitating Treatment Discontinuation in Pediatric Inflammatory Bowel Disease Patients. J Pediatr Gastroenterol Nutr 75(6): 731-736.
- Wintzell V, Svanström H, Olén O, Mads Melbye, Jonas F Ludvigsson, et al. (2019) Association between use of azathioprine and risk of acute pancreatitis in children with inflammatory bowel disease: a Swedish–Danish nationwide cohort study. Lancet Child Adolesc Health 3(3): 158-165.
- Chun JY, Kang B, Lee YM, Soo Youn Lee, Mi Jin Kim, et al. (2013) Adverse Events Associated with Azathioprine Treatment in Korean Pediatric Inflammatory Bowel Disease Patients. Pediatr Gastroenterol Hepatol Nutr 16(3): 171-7.
- Dulai PS, Thompson KD, Blunt HB, Marla C Dubinsky, Corey A Siegel, et al. (2014) Risks of serious infection or lymphoma with anti-tumor necrosis factor therapy for pediatric inflammatory bowel disease: a systematic review. Clin Gastroenterol Hepatol 12(9): 1443-1451.
- Dayan JR, Dolinger M, Benkov K, David Dunkin, Jacqueline Jossen, et al. (2019) Real world Experience with Ustekinumab in Children and Young Adults at a Tertiary Care Pediatric Inflammatory Bowel Disease Center. J Pediatr Gastroenterol Nutr 69(1): 61-67.
- Bishop C, Simon H, Suskind D, Dale Lee, Ghassan Wahbeh, et al. (2016) Ustekinumab in pediatric Crohn disease patients. J Pediatr Gastroenterol Nutr 63(3): 348-51.
- Singh N, Rabizadeh S, Jossen J, Nanci Pittman, Morgan Check, et al. (2016) Multi-Center Experience of Vedolizumab Effectiveness in Pediatric Inflammatory Bowel Disease. Inflamm Bowel Dis 22(9): 2121-6.
- Moore H, Dubes L, Fusillo S, Robert Baldassano, Ronen Stein, et al. (2021) Tofacitinib Therapy in Children and Young Adults with Pediatric-onset Medically Refractory Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr 73(3): e57-e62.
- Hyams JS, Turner D, Cohen SA, Erzsébet Szakos, Kinga Kowalska-Duplaga, et al. (2022) Pharmacokinetics, Safety and Efficacy of Intravenous Vedolizumab in Paediatric Patients with Ulcerative Colitis or Crohn’s Disease: Results from the Phase 2 HUBBLE Study. J Crohns Colitis 16(8): 1243-1254.
- Espinheira MC, Pais IP, Afonso I, Jorge Ferreira, Eunice Trindade, et al. (2020) Epstein-Barr Virus Infection and Thiopurine Therapy in a Pediatric Population with Inflammatory Bowel Disease. GE Port J Gastroenterol 27: 318-323.
- Vos ACW, Bakkal N, Minnee RC, M K Casparie, D J de Jong, et al. (2011) Risk of malignant lymphoma in patients with inflammatory bowel diseases: a Dutch nationwide study. Inflamm Bowel Dis 17(9):1837-45.
- Subramaniam K, D Rozario J, Pavli P (2013) Lymphoma and other lymphoproliferative disorders in inflammatory bowel disease: a review. J Gastroenterol Hepatol 28(1): 24-30.
- Sokol H, Beaugerie L (2009) Inflammatory bowel disease and lymphopcroliferative disorders: the dust is starting to settle. Gut 58(10): 1427-36.
- Beaugerie L, Brousse N, Bouvier AM, Jean Frédéric Colombel, Marc Lémann, et al. (2009) Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 374(9701): 1617-25.
- Barzilai M, Polliack A, Avivi I, Yair Herishanu, Ron Ram et al. (2018) Hodgkin lymphoma of the gastrointestinal tract in patients with inflammatory bowel disease: portrait of a rare clinical entity. Leuk Res 71:1-5.
- Khan N, Abbas AM, Lichtenstein GR, Edward V Loftus Jr, Lydia A Bazzano, et al. (2013) Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology 145(5): 1007-1015.e3.
- Kotlyar DS, Lewis JD, Beaugerie L, Ann Tierney, Colleen M Brensinger, et al. (2015) Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol 13(5): 847-58.e4.
- Lewis JD, Schwartz JS, Lichtenstein GR (2000) Azathioprine for maintenance of remission in Crohn’s disease: benefits outweigh the risk of lymphoma. Gastroenterology 118(6): 1018-24.
- Dayharsh GA, Loftus EVJ, Sandborn WJ, William J Tremaine, Alan R Zinsmeister, et al. (2002) Epstein-Barr virus–positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology 122(1): 72-7.
- Francisco R, Castaño-García A, Martínez-González S, Isabel Pérez-Martínez, Ana J González-Huerta, et al. (2018) Impact of Epstein-Barr virus serological status on clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther 48(7): 723-730.
- Afif W, Sandborn WJ, Faubion WA, Meher Rahman, Scott W Harmsen, et al. (2013) Risk factors for lymphoma in patients with inflammatory bowel disease: a case-control study. Inflamm Bowel Dis 19(7): 1384-9.
- Kucharzika T, Ellulb P, Greuterc T, J F Rahier, B Verstockt, et al. (2021) ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J Crohns Colitis 15(6): 879-913.
- Kedia S, Mouli VP, Kamat N, Jeeva Sankar, Ashwin Ananthakrishnan, et al. (2020) Risk of Tuberculosis in Patients with Inflammatory Bowel Disease on Infliximab or Adalimumab Is Dependent on the Local Disease Burden of Tuberculosis: A Systematic Review and Meta-Analysis. Am J Gastroenterol 115(3): 340-349.
- Vigilância Laboratorial da Tuberculose em Portugal Relatório 2020-2022. DSG 2022.
- Dolinger MT, Spencer EA, Lai J, David Dunkin, Marla C Dubinsky et al. (2021) Dual Biologic and Small Molecule Therapy for the Treatment of Refractory Pediatric Inflammatory Bowel Disease. Inflamm Bowel Dis 27(8): 1210-1214.
-
Catarina Freitas*, Carolina Castro, Diana Alba, Isabel Pinto Pais, Maria do Ceu Espinheira, Eunice Trindade. Frequency and type of drug-related adverse effects in Pediatric Inflammatory Bowel Disease. Glob J of Ped & Neonatol Car. 4(3): 2024. GJPNC. MS.ID.000589.
Pediatrics, Inflammatory Bowel disease, Crohn Disease, Ulcerative Colitis, Durg related adverse effect, life-threatening, Biological agents, Tuberculosis disease, Lymphoproliferative disease
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






