 Review Article
Review Article
Diagnosis and Management of Persistent Pulmonary Hypertension of the Newborn
Omar Abu Sa’da*
Division of Neonatology Tawam Hospital and Department of Pediatrics, College of Medicine and Health Sciences (CMHS), the United Arab Emirates University, UAE
Division of Neonatology Tawam Hospital and Department of Pediatrics, College of Medicine and Health Sciences (CMHS), the United Arab Emirates University, UAE
Received Date:January 30, 2024; Published Date:February 22, 2024
Abstract
Persistent pulmonary hypertension of the newborn (PPHN) is characterized by sustained elevation of pulmonary vascular resistance (PVR), caused by a failure in the circulatory adaptation that normally occurs directly after delivery. This leads to right-to-left shunting of blood through foramen ovale and ductus arteriosus and prevents the increase in pulmonary blood flow (PBF), essential for extrauterine oxygenation and survival. Therefore, PPHN usually presents shortly after birth, causing severe respiratory distress and hypoxemia. This review aims to discuss the pathophysiology, causes, management and outcome of PPHN. The management of PPHN includes a discussion of the use of oxygen, ventilation, medical management of PPHN, and the utilization of extracorporeal membrane oxygenation (ECMO).
Keywords:Persistent Pulmonary Hypertension; Pulmonary Vascular Resistance
Definition of Terms/Abbreviations:BP: Blood Pressure; BPD: Bronchopulmonary Dysplasia (BPD); CDH: Congenital Diaphragmatic Hernia; cGMP: Cyclic
Guanosine Monophosphate; ECMO: Extracorporeal Membrane Oxygenation; ET-1: Endothelin-1 Receptors; HIE: Hypoxic ischemic
encephalopathy; IVH: intraventricular hemorrhage; LV: Left Ventricle
MAP: Mean Airway Pressure; MAS: Meconium Aspiration Syndrome; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; OI:
Oxygen Index; OSI: Oxygen Saturation Index; PBF: Pulmonary Blood Flow; PDA: Patent Ductus Arteriosus; PDE: Phosphodiesterase;
PEEP: Positive End Expiratory Pressure; PFO: Patent Foramen Ovale; PG: Prostaglandin; PGI: prodtaglandin Inhibitor; PIP: Peak
Inspiratory Pressure; PPHN: Persistent Pulmonary Hypertension of the Newborn; PPM: Parts Per Million; PVR: Pulmonary Vascular
Resistance; RDS: Respiratory Distress Syndrome; SSRIs: Selective Serotonin Reuptake Inhibitors; SVR: Systemic Vascular Resistance;
TTN: Transient Tachypnea of the Newborn; UAC: Umbilical Arterial Catheter; UVC: Umbilical Venous Catheter; V1: Vasopressin
Receptors 1; V/Q: ventilation/perfusion
Introduction
The incidence of PPHN is around 2 per 1,000 live births [1, 2], being highest in term and late preterm infants. Despite advances in neonatal cardiorespiratory care, PPHN is still one of the main causes of neonatal morbidity and mortality, with a mortality rate ranging between 4–33% [1, 2]. Inhaled nitric oxide has been proven to treat PPHN successfully with improved oxygenation in 60-70% of patients and to significantly reduce the need for extracorporeal membrane oxygenation (ECMO). About 14%-46% of the survivors develop long-term impairments such as hearing deficits, chronic lung disease, cerebral palsy and other neurodevelopmental disabilities [3].
Discussion
Pathophysiology:
The whole mark of PPHN is failure in the circulatory adaptation which is the drop in pulmonary vascular resistance that normally occurs directly after delivery. This leads to right-to-left shunting of blood through foramen ovale and ductus arteriosus and prevents the increase in pulmonary blood flow (PBF), essential for extrauterine oxygenation and survival. Therefore, PPHN usually presents shortly after birth, causing severe respiratory distress and hypoxemia [1]. The essential mechanisms of PPHN pathogenesis are increased pulmonary arteriolar vasoconstriction, vascular structural remodeling, and the underdevelopment of pulmonary vascular bed. However, many different perinatal disorders might be involved in causing these phenomena and are recognized as PPHN etiologies, as described in table 1 [1, 2].
Table 1:Causes of PPHN.

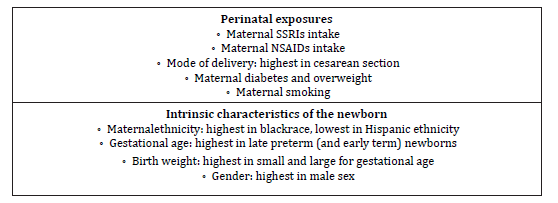
Several perinatal risk factors such as maternal exposures are linked to the pathogenesis of PPHN. The specific mechanism linking these factors and PPHN remains unclear for most of them. However, these factors predict higher risk and should be considered when evaluating the causes of PPHN (Table 2), [1 2].
Table 2:Perinatal risk factors of persistent pulmonary hypertension of the newborn (PPHN). SSRIs, selective serotonin reuptake inhibitors; NSAIDs, non-steroidal anti-inflammatory drugs.
Assessment of PPHN [2-5]:
When Persistent pulmonary hypertension of the newborn is
suspected the following assessment steps should be considered:
- Clinical: Respiratory distress with cyanosis and hypoxaemia
which is refractory to oxygen therapy, in the absence of
congenital heart disease.
- Chest X Ray: May reveal features associated with secondary
PPHN (such as a CDH or pneumonia), but hypoxemia
disproportionate to the severity of parenchymal disease on CXR
should suggest PPHN.
- Pre and postductal oxygen saturations: Postductal maybe
5-10% lower than preductal, consistent with right to left
shunting (PPHN is not excluded if ≤ 5% difference).
- Blood gas (arterial): The blood gas (arterial) is likely to show
severe hypoxemia with PaO2 < 50mmHg.
- Oxygen Index (OI) = Mean Airway Pressure X FiO2 X 100 / PaO2
- In a situation when arterial line couldn’t be inserted, then
calculation of Oxygen Saturation Index (OSI) can be alternative
to OI.
OSI= Mean Airway Pressure (MAP) X FiO2 X100 / Preductal Oxygen Saturation. OI = OSI X 2
Severity of hypoxic respiratory failure based on OI:
• Mild ≤15
• Moderate 15 to ≤25
• Severe 25 to ≤40
• Very severe >40
- Echocardiogram: It is essential in ruling out congenital cyanotic heart disease, supporting a diagnosis of PPHN, and in estimation of pulmonary pressures. Evaluation of cardiac function and haemodynamic assessment will help in the management of fluid therapy and choosing appropriate medications such as inotropes, lusitropes or vasopressors (See Appendix 3 below).
Management of PPHN:
Early recognition of PPHN and correction of factors that prevent decrease in PVR are important to the successful management.
General Support of PPHN [2, 3]:
- General management principles for PPHN include maintenance
of normal temperature, electrolytes (particularly calcium and
magnesium), glucose, hemoglobin, and intravascular volume and
correction of acidosis, commence antibiotics if early onset neonatal
sepsis is suspected.
- A hemoglobin concentration between 14 and 16 gm/dL is
reasonable to preserve adequate tissue oxygenation. On the
contrary, polycythemia should also be corrected with partial
exchange transfusions.
- Oxygen concentration should be adjusted to maintain a
targeted PaO2 goal of between 50 and 80 mmHg and preductal
oxygen saturation 90-95 percent.
- Optimize nutrition and fluid balance.
- Minimal handling, nurse in quiet environment.
- Consider insertion of umbilical arterial line (UAC) and umbilical
venous line (UVC) if FiO2 > 40% and O2 saturation<90% or PaO2<5
0mmHg.
- Start early invasive blood pressure monitoring.
- Consider urgent Echocardiography: To rule out congenital
heart disease, to assess pulmonary artery pressures and to
assess right to left shunting across the patent foramen ovale
(PFO) and the patent ductus arteriosus (PDA).
- Until stable, calculate OI regularly Q2-6 hours using the
formula (OI= FiO2 X MAP X100 / PaO2)
- Regular blood gases Q2-6 hours until stable
All of these modalities for general support are meant to optimize lung and cardiac function as well as to support sufficient oxygen delivery.
Specific Supportive Measures
Oxygenation & Ventilation [2-5]:
The two most potent natural pulmonary vasodilators are oxygen and good lung inflation.
(1) Oxygenation and Ventilation:
Aim for preductal O2 saturations 90- 95% with minimal
difference between pre-and post-ductal saturations. Maintain PaO2
in the range of 50-80 mmHg and PaCO2 between 40 and 50 mmHg.
Hypoxia increases pulmonary vascular resistance (PVR).
Hyperoxia does not further decrease PVR, instead results in free radical injury. Optimal lung expansion is essential for adequate oxygenation as well as the effective delivery of iNO. Keeping babies in high level of FiO2 >45-50% and on noninvasive support can potentiate hypoxia and increase the risk of development and exacerbation of PPHN. Consider intubation and ventilation, if oxygen requirement is > 45-50%. Early ventilation can lead to optimisation of oxygenation, PaCO2, Ph and prevention of severe PPHN. Aim for normocapnia, avoid hypocapnia. Gentle ventilation strategies with optimal positive end expiratory pressure (PEEP), relatively low peak inspiratory pressure (PIP) and some permissive hypercapnia are now being recommended to ensure adequate lung expansion (8-9ribs) without causing lung injury. Optimal lung recruitment with the use of PEEP/MAP decreases PVR. High frequency oscillatory ventilation (HFOV) in combination with iNO can improve oxygenation in newborns who has severe PPHN complicated by diffuse parenchymal lung disease, such as respiratory distress syndrome (RDS) and meconium spiration syndrome (MAS).
Sedation [2, 3]:
Infants with PPHN may breathe out of synchrony with the ventilator and become agitated. Agitation may further increase right-to-left shunting, as well as catecholamine release, resulting in increased PVR. A patient-triggered ventilator mode may improve synchrony. If asynchrony presents, application of sedatives is a common practice in management of PPHN, either by benzodiazepine or narcotic agents. Paralysis may be considered after discussing with Consultant.
Surfactant therapy [3]:
Surfactant deficiency or inactivation is commonly attributed to PPHN, and administration of surfactant may be useful in the management of PPHN when significant parenchymal lung disease exists. Previous evidence revealed beneficial effects in infants with parenchymal lung diseases such as MAS and sepsis, for which surfactant can improve oxygenation and decrease the need for EC MO.
Pulmonary Vasodilators [1-6, 8-10]:
Inhaled Nitric Oxide [1, 2, 4, 5, 7, 8]:
Inhaled NO (iNO) improves oxygenation and reduces the need
for ECMO therapy in patients with diverse causes of PPHN. Several
studies have suggested an effective dosage ranging from 5 to 20
ppm and increasing the dose beyond 20 ppm in non-responders
does not significantly improve outcomes.
- iNO is the preferred first choice pulmonary vasodilator
therapy. It acts via cGMP pathway causing pulmonary vasodilation
and improved V/Q matching.
Criteria to commence iNO include:
- Infant > 34 weeks gestation with hypoxic respiratory failure.
- Diagnosis of Persistent Pulmonary Hypertension (PPHN):
1. Difference in pre & post-ductal saturations ≥ 5%
2. Oxygen index (OI) >15
3. Evidence of moderate to severe PPHN on Echocardiography
and the absence of congenital heart disease for which iNO is
contraindicated.
- In preterm infants<34 weeks gestational age with documented
acute PPHN and severe hypoxic respiratory failure, iNO may be
considered after reviewing the potential risks and benefits.
Monitoring During iNO Therapy
- Monitor blood methemoglobin levels at baseline, 2- and 8-h
following initiation, and every 12-24 hours thereafter.
Aim to keep methemoglobin levels below <2.5%.
Methemoglobinemia rarely occurs with iNO dose <20 ppm.
(Methemoglobin < 2.5% normal; reduce
iNO if ≥4%; give methylene blue if methaemoglobin > 7%).
- Nitrogen Dioxide level should be kept below 0.5ppm.
Weaning Nitric Oxide
- Wean Oxygen first maintaining saturations >95%.
- iNO weaning should be done under direct observation.
- iNO weaning may be initiated as early as 4-6hrs after starting
treatment.
- Haemodynamic stability & oxygenation should be monitored
for 30-60mins after each weaning step.
- Increase iNO to previous level if oxygen saturation drops
below 90% or PaO2 <50mmHg & wait at least 4-6 hours before
attempting weaning again.
Weaning steps:
- First, wean FiO2 slowly until < 40%
- If stable, start weaning iNO in steps of 5ppm every 6 hours
until at level of 5 ppm.
- Once at 5ppm, if stable, wean by 1ppm every 4-6 hours until
complete stop.
- Once iNO has been stopped, other adjunct medications’
weaning may then commence (If the patient is on these
medications. See below).
Sildenafil (Oral /IV) [1-6, 8]:
Acts by inhibition of the cGMP degrading phosphodiesterase (PDE5).
Consider adding Sildenafil in severe PPHN if no proper response to iNO and persistent hypoxemia, or in chronic pulmonary hypertension due to congenital diaphragmatic hernia (CDH) or bronchopulmonary dysplasia (BPD), where iNO may not be effective. Sildenafil may reduce the rebound pulmonary hypertension noted during iNO weaning. Recommended dosing regimen for oral Sildenafil is 0.5–2 mg/kg/dose every 6 hours. Sildenafil can be weaned once iNO has been successfully weaned and stopped. Sildenafil can be reduced by 0.5mg/kg/dose every 12- 24 hours.
Prostaglandins (PGI2 analogues) [2-5, 8]:
Prostacyclin (PGI2) stimulates adenylate cyclase to increase intracellular cAMP level, which produces vasodilation through a decrease in intracellular calcium concentration. Intravenous PGI2 may be used as a rescue therapy for infants with severe PPHN. However, systemic hypotension may raise some concern. The inhaled PGI2 (e.g., iloprost or treprostinil) is possessed of vasodilator effects limited to pulmonary circulation, and its efficacy has been reported to be similar to that of iNO. Inhaled iloprost may be a therapeutic option in newborns with PPHN when the patient has severe PPHN not responding to iNO and ECMO is not available.
The dose of inhaled iloprost is 1–2.5 microgram/Kg every 2–4 hours by nebulization.
Milrinone [1-4, 8-10]:
Acts as a selective PDE-3 inhibitor in cardiac myocytes as well as in the vascular smooth muscle.
Milrinone may be the pulmonary vasodilator of choice in the presence of PPHN with left ventricular dysfunction and stable blood pressure. The dose of milrinone is 0.1 – 0.5 microgram/Kg/ min via a central line. Milrinone can cause hypotension and should be used with caution in renal impairmet and thrombocytopenia.
Bosentan (Endothelin Receptor nonselective agonist) [3, 4, 8, 10, 11]:
Endothelin-1 (ET-1), which is synthesized by vascular endothelial cells, acts on two kinds of endothelin receptor (ET-A and ET-B) to cause vasoconstriction. Higher plasma level of ET-1 was also found in newborns with PPHN. Bosentan is a non-selective ET-1 antagonist acting on both ET-A and ET-B receptors. The dose of bosentan is 1-2 mg/kg/dose twice daily orally via nasogastric tube. Baseline liver function test should be considered before starting bosentan as it can affect the liver enzymes’ levels.
Inotropes and Vasopressors [1-3, 5, 8, 9, 12-14]:
The mean arterial pressures should be kept above 40 mmHg in term infants or higher if right ventricle pressure calculated to be greater than this. Systemic hypotension due to low systemic vascular resistance or left ventricular dysfunction is common in infants with PPHN. Right ventricular dysfunction and failure occurs due to increased afterload in infants with severe PPHN. Optimizing cardiac output with adequate volume expansion and inotropic support is important for achieving optimal gas exchange and systemic oxygen delivery.
In the presence of systemic hypotension without cardiac dysfunction, 1-2 boluses of 10 ml/kg of normal saline should be considered, followed by dopamine. Some centers prefer the use of norepinephrine or vasopressin. If high doses of vasopressors are needed, cortisol level and starting stress dose of hydrocortisone should be considered. If the cortisol levels are low relative to the infant’s stress level and there is no evidence of infection (viral, bacterial or fungal), the stress dose of hydrocortisone should be continued. When systemic hypotension is associated with cardiac dysfunction, epinephrine or a combination of dopamine and vasopressin and milrinone are the agents of choice. While in the presence of stable systemic blood pressure and cardiac dysfunction, milrinone is the agent of choice.
Dopamine:
Dopamine is a central neurotransmitter and is also a precursor to norepinephrine. It directly stimulates D1 and D2 receptors and directly (or through metabolism to norepinephrine) stimulates α1, β1 and β2 receptors. Therefore, dopamine can result in vasoconstriction, vasodilation, inotropy, and/or chronotropy depending on the dose.
Dopamine can be started at 5mcg/Kg/min, can be increased to
20mcg/Kg/min.
• Dose range of 4-10 microgram/Kg/min – inotropic and
chronotropic effect
• Dose range of 11-20 microgram/Kg/min – vasopressor,
increased systemic and pulmonary vascular resistance.
Dobutamine:
Dobutamine is predominantly a β1 agonist resulting in significant inotropic effect. Thus, it may be useful for neonates with decreased cardiac function, when milrinone is contraindicated. However, the potential chronotropic effect must be considered. The dose range dobutamine is 5-20 microgram /Kg/min, which can cause inotrope, reduced SVR and increases cardiac output.
Norepinephrine:
Norepinephrine is more selective than dopamine with regards to receptor stimulation, acting primarily on α1 receptors resulting in vasoconstriction and minimal inotropic effect on β1 receptors. The vasoconstriction mechanism could affect both systemic and pulmonary arterial pressure. Norepinephrine may decrease the basal pulmonary vascular tone through stimulation of α2 receptors and nitric oxide release. Norepinephrine can be useful to increase SVR, reduce PVR and increase BP, however if SVR is too high, then the ability of myocardium to pump against the resistance may become compromised. The dose of norepinephrine is 0.05-0.5 microgram / Kg/min and should be administered via Central line.
Epinephrine:
Epinephrine is less selective than norepinephrine and its stimulation on α and β receptors vary by dose. At lower doses, epinephrine has predominant β effect causing chronotropy and inotropy. Thus, for infants with depressed myocardial function, epinephrine may be useful. In pediatric trials, epinephrine has been shown to be superior to dopamine with faster resolution of shock and lower mortality. In neonatal trials, epinephrine and dopamine were comparable. However, epinephrine was associated with more metabolic disturbances such as hyperglycemia and lactic acidosis. Epinephrine has dose dependent effects and is useful in neonates with vasodilatory shock with or without myocardial dysfunction.
A dose range of 0.03-0.1 microgram /Kg/min causes mainly inotropic effect, while the dose range of 0.1-1.0 microgram mcg/ Kg/min causes vasopressor and increased SVR.
Vasopressin:
There are three subtypes of vasopressin receptors, V1-3. V1 receptors are located in the vasculature beds and commonly known for their potent vasoconstrictive properties on systemic vasculature with minimal increase/decrease in PVR leading to a decrease in the pulmonary/systemic arterial pressure ratio. The mechanism of vasodilation in the pulmonary vasculature is thought to be from stimulation of oxytocin endothelial receptors and subsequent NO pathway activation. Case series publications have suggested that vasopressin use was associated with improvement in systemic BP, reduction in OI, steady reduction in iNO use and enabled weaning of other inotropes. Assumingly, when considering its mechanism of action, vasopressin could be beneficial for a patient with low SVR and good LV function and potentially harmful for a newborn with poor LV dysfunction by increasing SVR (or LV afterload).
The effect vasopressin on sodium and water balance has to be considered prior to initiation. Vasopressin can alter sodium and water balance via two mechanisms – V1 receptors result in peripheral vasoconstriction and thus improve renal blood flow, and V2 receptors have antidiuretic effects resulting in reabsorption of free water. This last mechanism has the potential to cause hyponatremia. The recommended dose used of vasopressin in PPHN: 0.0001-0.001 Unit/Kg/min.
Hydrocortisone [2, 3, 5, 12, 14]:
The mechanisms of cardiovascular effects of hydrocortisone administration are not completely understood, but both genomic and nongenomic steroidal effects seem to play a role. Hydrocortisone administration to preterm and term infants with vasopressor-resistant hypotension is associated with an improvement in BP, stoke volume (and a trend to increase in cardiac output) with a decrease in heart rate and need for vasoactive medications. Genomic upregulation of cardiovascular adrenergic and angiotensin receptors and inhibition of inducible nitric oxide synthase and prostaglandins are potential mechanisms. In addition, non-genomic effects such as better capillary integrity, inhibition of catecholamine metabolism and increase in intracellular calcium may be mainly responsible for the rapid onset of the hydrocortisoneinduced BP improvement. High dose hydrocortisone inhibits phosphodiesterase 5 enzyme (PDE 5) and enhances oxygenation response in PPHN. It may be helpful to start hydrocortisone when second line inotropes are commenced as it may take 2-3 hours to have an effect. The dose of hydrocortisone is 4 mg/kg, followed by 1 mg/kg every 6 hours. Obtaining cortisol level before starting hydrocortisone should be considered.
Escalation to ECMO [2, 5, 15]:
Infants who fail to respond to medical management with persistent OI > 40 and metabolic acidosis require treatment with ECMO.
Criteria for referral:
1. Failure to respond to maximal conventional treatment
2. Reversible disease
3. Weight > 2Kg
4. Gestational age more than 34 weeks gestation
5. Oxygenation Index >40
6. No Ccontraindication to systemic anticoagulation (such as
IVH)
7. No lethal congenital abnormalities
8. No irreversible organ dysfunction
9. No Severe HIE
APPENDICES:
Appendix 1: Flow Chart for Management of PPHN

Appendix 2: Flow Chart for Inotropes use in PPHN

For Inotropes doses see section 5
Appendix 3: Echocardiographic Findings in PPHN [2, 3, 4, 12]:
1- Tricuspid regurgitation 2- Atrial shunting:
Some degree of right-to-left atrial shunting through the patent foramen ovale is common.
Bowing of the interatrial septum to the left is commonly seen. Right-to-left atrial shunting reflects right atrial filling (diastolic) pressure
If a VSD is present, bidirectional shunting may be noted.
Ductus Artiosus Flow:
The direction and velocity of ductal blood flow can gives useful
information on PAP.
• Pure right-to-left flow indicates Pulmonary arterial pressure is
higher than the aortic pressure throughout the cardiac cycle.
• Bidirectional flow occurs when the aortic and pulmonary
arterial pressures are approximately equal. Flow is left-toright
during diastole and right-to –left, in systole.
Bidirectional flow is common in healthy babies in the first 12 hours but changes to pure left-to-right when aortic pressures become higher than pulmonary pressures.
4- Cardiac function:
• There may be enlargement of Right atrium, Right ventricle and
main pulmonary artery.
• There may be flattening (RV: LV pressure >0.5) and or even
bowing (RV: LV pressure ≥1.0) of interventricular septum to
the left as RV pressure rises.
• Quantitative assessment of cardiac function may assist with
decisions and assessments of the roles of inotropes and
inhaled nitric oxide.
Funding
This research received no external funding.
Acknowledgment
None.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Sofia Martinho, Rui Adão, Adelino F Leite-Moreira, Carmen Brás-Silva (2020) Persistent Pulmonary Hypertension of the Newborn: Pathophysiological Mechanisms and Novel Therapeutic Approaches. Front. Pediatr 8: 342.
- Mei-Yin Lai, Shih-Ming Chu, Satyan Lakshminrusimha, Hung-Chih Lin (2018) Beyond the inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Pediatrics and Neonatology 59(1): 15-23.
- Jonas Pedersen, Elise R Hedegaard, Ulf Simonsen, Marcus Krüger, Manfred Infanger, et al. (2018) Current and Future Treatments for Persistent Pulmonary Hypertension in the Newborn. Basic & Clinical Pharmacology & Toxicology 123: 392-406.
- Bobby Mathew and Satyan Lakshminrusimha (2017) Persistent Pulmonary Hypertension in the Newborn. Children 4: 63.
- Uthaya Kumaran and Arvind Shenoi (2021) Management of Pulmonary Hypertension in Term Infants—A Review. Journal of Neonatology 35(1): 29-37.
- Kelly LE, Ohlsson A, Shah PS (2017) Sildenafil for pulmonary hypertension in neonates. Cochrane Database of Systematic Reviews 8(8): CD005494.
- Anita M Ware, Sergio G Golombek (2015) Weaning of inhaled nitric oxide: is there a best strategy? Journal of Pediatric and Neonatal Individualized Medicine 4(1): 9e040124.
- Satyan Lakshminrusimha, Bobby Mathew, Corinne L Leach (2016) Pharmacologic Strategies in Neonatal Pulmonary Hypertension other than Nitric Oxide. Semin Perinatol 40(3): 160-173.
- Amish Jain, Regan E Giesinger, Shyamala Dakshinamurti, Yasser ElSayed, Robert P Jankov, et al. (2022) Care of the critically ill neonate with hypoxemic respiratory failure and acute pulmonary hypertension: framework for practice based on consensus opinion of neonatal hemodynamics working group. Journal of Perinatology 42(1):3-13.
- Amna Qasim (2020) Milrinone Use in Persistent Pulmonary Hypertension of the Newborn. NeoReviews 21: 3.
- Gunlawadee Maneenil, Anucha Thatrimontrichai, Waricha Janjindamai, Supaporn Dissaneevate (2018) Effect of bosentan therapy in persistent pulmonary hypertension of the newborn. Pediatrics and Neonatology 59: 58-64.
- Heather M Siefkes, Satyan Lakshminrusimha (2021) Management of Systemic Hypotension in Term Infants with Persistent Pulmonary Hypertension of the Newborn (PPHN) – An Illustrated Review. Arch Dis Child Fetal Neonatal Ed 106(4): 446-455.
- Patrick J McNamara, Regan E Giesinger, Satyan Lakshminrusimha (2022) Dopamine and Neonatal Pulmonary Hypertension—Pressing Need for a Better Pressor? The Journal of Pediatrics 246: 242-250.
- Mitali Sahni, MD Sunil Jain (2016) Hypotension in Neonates. NeoReviews 17: 10.
- Cornelia Heleen Van Ommen (2018) Neonatal ECMO. Frontier in Medicine Volume 5: 289.
- Pei-Ni Jone, D Dunbar Ivy (2014) Echocardiography in pediatric pulmonary hypertension. Frontiers in Pediatrics 2: 124.
-
Omar Abu Sa’da*. Diagnosis and Management of Persistent Pulmonary Hypertension of the Newborn. Glob J of Ped & Neonatol Car. 4(4): 2024. GJPNC.MS.ID.000591.
Persistent Pulmonary Hypertension, Pulmonary Vascular Resistance, Oxygenation, Pathogenesis, Electrolytes, Hemoglobin, Methaemoglobin, Pathogenesis, Inotropy
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






