 Research article
Research article
Anorectal Malformations in the Neonatal Intensive Care Unit – A Retrospective Analysis from 2008 to 2023
Sara Geraldes Paulino1*, Domingas Atouguia2, Mariana Borges Dias2, Henrique Soares3 and Susana Pissarra3
1Pediatric Department, Unidade Local de Saúde de São João, Porto, Portugal
2Pediatric Surgical Department, Centro Hospitalar Universitário de São João, Porto, Portugal
3Neonatal Intensive Care Unit, Serviço de Neonatologia, Unidade Local de Saúde de São João, Porto, Portugal
Sara Geraldes Paulino, Pediatric Department, Unidade Local de Saúde de São João, Porto, Portugal.
Received Date:December 03, 2024; Published Date:December 18, 2024
Abstract
Background: Anorectal malformations (ARM) occur in approximately 1:5.000 neonates and can be associated with other congenital
malformations. Treatment is surgical and is usually performed during the neonatal period. The aim of this study was to assess epidemiology, etiology, association with other malformations/syndromes, potential al risk factors, management and long-term prognosis.
Methods: This was a retrospective observational study in which clinical records of all neonates diagnosed with ARM admitted to the NICU of a
Level III Hospital between 2008 and 2023 were analyzed.
Results: Clinical records of 34 newborns (61.8% male) were analyzed. It was not possible to establish a causal relationship with maternal risk factors/pathologies. In 30 patients (88.2%), diagnosis was established during the first physical examination. Anal imperforation (AI) with perineal fistula was the most prevalent presentation (29.4%) - the most common in male neonates. In female newborns, the most frequent was AI with
recto-vestibular fistula (61,5%). Twenty-two patients (64.7%) had presented other malformations. A single-stage posterior sagittal anorectoplasty
(PSARP) was performed in 7 (70.0%) patients with AI with perineal fistula, 8 (88,9%) with vestibular fistula and 2 without fistula (28,6%). Colostomy followed by subsequent PSARP was performed in 14 patients (41.2%). The most common long-term complication after surgery was constipation
requiring medication (25,0%).
Discussion: Most ARM were associated with other malformations and their anatomy allowed correction in a single-stage surgery during
neonatal period in a significant percentage of cases. Further studies are needed to investigate the association with maternal risk factors and to
characterize long-term complications.
Keywords:Neonatology; Neonates; Anorectal malformations; Posterior sagittal anorectoplasty
Abbreviations:ARM: anorectal malformations; NICU: Neonatal Intensive Care Unit; PSARP: single-stage posterior sagittal anorectoplasty; SPSS:Statistical Package for Social Sciences; AI: anal imperforation; AGA: appropriate weight for gestational age
Introduction
Anorectal malformations (ARM) occur in approximately 1 in every 5000 newborns [1], resulting from abnormal development of the terminal portion of the digestive tract, which takes place between the 6th and 10th week of gestation [2,3]. These include anal imperforation, anal stenosis, and ectopic anus. The severity of ARM varies, depending on the level of disruption of the anorectal canal and of the associated caudal malformations (sacrum and spine). The diagnosis of ARM is typically made during the initial physical examination of the newborn, however further diagnostic tests are required for classification and to investigate other associated malformations. Over 50% of ARMs are associated with other malformations, notably esophageal, genitourinary, cardiovascular, and sacral. They may also be associated with syndromes such as VACTERL or Trisomy 21, among others [1,3]. The etiology is likely multifactorial and there can be an interplay between genetic elements and environmental influences. Supporting the existence of genetic factors, there are studies indicating that there is a higher risk of ARM in first-degree relatives of probands and in patients with chromosomal abnormalities, such as microdeletion of the chromosome 22 [4]. Evidence suggests that environmental factors are also involved in the development of ARM. Some maternal illnesses during the pregnancy might increase the risk of ARM (obesity, diabetes, asthma and asthma medication, epilepsy, vitamin A deficiency, folic acid deficiency and thyroid diseases). Meanwhile, although controversial, certain pertinent events have been considered as plausible risk factors for ARM, for instance maternal age, multiple pregnancy, fertility treatment/assisted reproduction, caffeine intake and exposure to tobacco smoke [3]. Treatment is surgical and, in most cases, performed during the neonatal period to restore normal anatomy of the digestive tract [5].
Long-term complications can emerge due to inadequate development of the anal sphincter and pelvic innervation, leading to fecal incontinence and constipation [6]. Currently, there are still limited studies on long-term complications, particularly regarding bowel, urinary tract and sexual function, as well as quality of life [7].
The aim of this study was to assess the epidemiology, association with other malformations/syndromes, potential risk factors (maternal age, multiple pregnancies, maternal illness, tobacco exposure, and assisted reproduction), management and long-term prognosis of children diagnosed with ARM, born and followed-up in Sa o Joa o Hospital and University Center.
Materials and Methods
A retrospective observational study of all neonates (inborns and outborns) diagnosed with ARM, admitted to the Neonatal Intensive Care Unit (NICU) of Centro Hospitalar Universita rio Sa o Joa, o between 2008 and 2023 (15 years) was conducted. Newborns whose clinical records lacked information about NICU admission and/or follow-up in outpatient consultation were excluded. Variables included socio-demographic data, maternal clinical data, newborn clinical data (prenatal, birth and postnatal), complementary diagnostic exams, surgical management and short and long-term complications. Statistical analysis was carried out using the Statistical Package for Social.
Sciences (SPSS) software version 29.0. In descriptive analysis, normally distributed continuous variables were represented by mean, while non-normally distributed variables were represented by median and upper and lower limits. Categorical variables were described by absolute and relative frequencies.
Results
A total of 34 neonates with ARM diagnosis were included, of whom 38.2% (n=13) were female and 61.8% (n=21) were male. The mean gestational age was 38 weeks, with a minimum of 36 weeks and a maximum of 41 weeks. We identified 31 neonates (91,2%) with weight appropriate for gestational age (AGA), 2 (5,9%) small for gestational age (SGA) and 1 (2,9%) large for gestational age (LGA). The median maternal age was 32 years, with a minimum age of 19 years and a maximum of 44 years. The descriptive analysis of maternal clinical data is summarized on Table 1 and Table 2.
Table 1:Descriptive analysis of maternal risk factors.
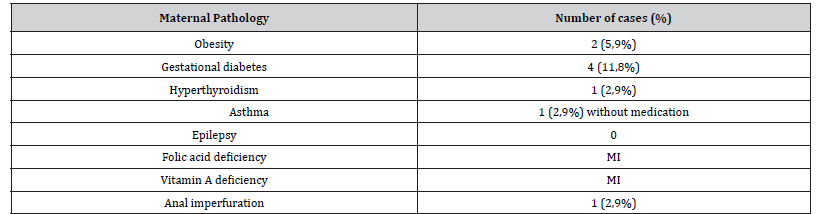
MI – Missing Information
Table 2:Descriptive analysis of controversial maternal risk factors.
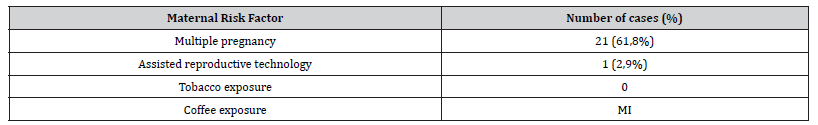
MI – Missing Information
Prenatal diagnosis is rare, however we found one case (n=1; 2,9%) where the diagnosis was established with prenatal ultrasound. In addition, 3 more cases had findings of intestinal obstruction on ultrasound which suggested possible anorectal malformation. Some of the remaining ultrasound findings were related to other malformations associated with ARM, such as cardiac and urogenital malformations and esophageal atresia. Most ARM diagnoses were made on the 1st day of life through physical examination (n=29; 85,3%). In 4 cases (11,7%), the diagnosis was suspected on the 2nd and 3rd days of life, through physical examination (n=1), due to the absence of meconium passage (n=1) or abdominal distension associated with vomiting (n=2). According to PEN A classification, the most prevalent presentation was anal imperforation (AI) with perineal fistula (29,4%), followed by AI with recto-vestibular fistula (26,5%) and AI without fistula (20,6%). The remaining types of ARM were less common.
The predominant presentation among male neonates was AI with perineal fistula (n=9; 42,9%), while among female newborns, it was AI with recto-vestibular fistula (n=9; 69,2%). Concomitant malformations, were present in 64,7% of the patients, with genitourinary malformations being the most frequent (58,8%), followed by cardiac (32,4%), vertebral (20,5%), central nervous system (11,8%) and finally digestive tract malformations (2,9%). Other malformations, such as agenesis of the left ear canal, preauricular appendages, branchial cyst, coloboma, cleft lip and palate, cervical hemangioma, 11 pairs of ribs, single umbilical artery, coccygeal dimple and bilateral hip dysplasia were present in 38,2% of the neonates. Seven neonates (20.6%) had a syndromic background – 3 cases of incomplete VACTERL association, 1 case of Trisomy 21, 1 microdeletion of chromosome 22, 1 tetrasomy 22 (Cat Eye Syndrome) and 1 Baraitser-Winter Syndrome (ACTB and ACTG1 gene mutation).
Twenty-seven neonates (79,4%) underwent surgical intervention during their hospitalization in the NICU. illustrates the surgical management according to the type of malformation. Only two patients did not undergo surgery - one with ectopic anus and another diagnosed with perineal fistula who had a polymalformative syndrome and deceased on the first day of life. Fourteen patients (41,2%) underwent protective colostomy, while 13 patients (38,2%) underwent single-stage PSARP. The median age at the time of surgery, whether for colostomy or PSARP, was 2 days of life, ranging from a minimum of 1 day to a maximum of 5 days of life. The median age for PSARP, following protective colostomy and after discharge from the NICU, was 10 months, with a minimum of 3 months and a maximum of 30 months of age. Five neonates (n= 4 AI with recto-vestibular fistula; n=1 ectopic anus with anterior fistula) underwent single-stage PSARP after discharge from the NICU, and after fistula dilation, with a median age of 8 months, ranging from a minimum of 6 months to a maximum of 14 months of age.
Short-term complications following colostomy surgery (n = 14) included local infection in 1 case (7.1%), which occurred in a patient with recto-prostatic fistula; and wound dehiscence that was observed in 2 cases (14.3%) – one in a patient with AI with recto-prostatic fistula (50.0%) and the other in a patient with an AI without fistula (50.0%). Wound dehiscence was also observed as a short-term complication after single-stage PSARP surgery (n = 18) in 3 cases, all of which occurred in patients presenting AI with recto-perineal fistula.
Short-term complications following PSARP surgery postcolostomy (n=14) included no cases of wound dehiscence (0%). Anal stenosis was observed in 2 cases (14.3%), both of which in patients with an AI with recto-prostatic fistula (100.0%). Additionally, 1 case of mislocated anus (7.1%) was reported, occurring in a patient with AI with recto-bulbar fistula (100.0%).
As for long-term complications (>1 year after surgery), the most common was constipation requiring medication (n=8; 25,0%). However, additional complications were observed in our study, all represented in Tables. Concerning patients experiencing soiling, 2 of them presented with sacral agenesis, and one of these neonates also had a tethered spinal cord. It is noteworthy that 3 adolescent patients continued to experience soiling.
Discussion
As previously reported in systematic reviews and observational studies [2,4,5], ARM was more prevalent in males (61,8%). In our sample, most patients had an appropriate weight for gestational age (AGA) which suggests that birth weight does not influence the occurrence of ARM. The small sample size and the lack of information in clinical records on variables, such as folic acid and vitamin A deficiency and coffee exposure precluded the establishment, in our study, of an association between ARM and maternal age, maternal pathology and complications or exposures during pregnancy. Further studies with larger study populations are needed to thoroughly investigate the association between anorectal malformations and maternal pathologies and risk factors.
Diagnosis was performed quite early in all cases, and in most of them, it was carried out through physical examination on the first day of life (85,3%). Mantho P et al in Douala5 have shown different results, since they found that up to 50% of the neonates had a late presentation, after the 2nd day of life. This fact demonstrates the thorough and systematic execution of the physical examination, including perineal examination, of our study population in the delivery room. According to Manasa Reddy et al [8], who compared the outcomes of early versus delayed presentation, an early diagnosis is associated with lower morbidity and mortality. In our study, mortality (n=2) was a result of factors not linked to ARM.
Although prenatal diagnosis is, as described by Herman R et al [4], noted for its low sensitivity and specificity, in our sample, we found a case where diagnosis was established prenatally and two more cases of suspected intestinal obstruction in prenatal ultrasound, that could suggest the presence of ARM.
Other malformations associated with ARM were identified on prenatal ultrasound of several patients in our case series. These included genitourinary malformations such as pyelocalyceal dilation, horseshoe or polycystic kidney, cardiac malformations, such as aortic coarctation, and esophageal atresia.
Enhancing ultrasound techniques to improve sensitivity in detecting subtle anomalies, along with establishing standardized protocols for assessing perineal anatomy – especially in high-risk populations – could improve diagnostic accuracy and ultimately lead to better clinical outcomes. The most prevalent in our study was AI with perineal fistula (29,4%). Findings regarding prevalence of different types of ARM described in published works are quite variable [5,9,10].
Additionally, we found that the most prevalent ARM in females was AI with recto-vestibular fistula while males presented mostly AI with perineal fistula. These findings are in line with what is reported in bibliography [4,10]. On the other hand, and contrary to what is reported in the literature [3], which states that the most commonly associated ARM in patients with trisomy 21 is AI without fistula, the sole newborn with trisomy 21 of our study presented with an AI with perineal fistula.
In what concerns associated malformations, our results revealed that 66% of patients exhibited additional anomalies, predominantly affecting the genitourinary system, surpassing the anticipated incidence (58.8%) [11]. This phenomenon may be attributed to the systematic exclusion of other malformations via ultrasound at our facility in case of MAR. Of the neonates who required surgical treatment, 43.8% (n=14) underwent PSARP surgery after colostomy, whereas 56.3% (n=18) underwent singlestage PSARP procedure. While anatomy allowed single-stage surgery during the neonatal period in a significant percentage of cases, timing of definitive PSARP surgery post-colostomy (from 3 to 30 months) underscores the prolonged challenges faced by certain infants, while early single-stage interventions offer notable benefits, including memory-free procedures, infection avoidance and ease of anal dilation in infants [5]. Among the 18 neonates who underwent single-stage PSARP surgery, 16.6% (n=3) presented wound dehiscence, while none of those who underwent PSARP post-colostomy developed this complication. Single-stage PSARP is indeed associated with a higher risk of wound infection and wound dehiscence compared to the multi-stage approach [6].
The existence and severity of sacral abnormalities, as well as the type of ARM, have demonstrated a significant correlation with long-term complications. Constipation stands out as a common long-term complication, emerging as the most prevalent in our study, with an incidence rate of 25%. In the present study, we verified that the most prevalent type of ARM in patients exhibiting soiling was AI without fistula and in patients with constipation was AI with perineal fistula, which is consistent with existing evidence [12,13]. Furthermore, we also observed that 2 out of the 4 patients who experienced soiling, presented sacral abnormalities (sacral agenesis and tethered spinal cord), which are more commonly related to this long-term complication [9,12].
Urinary incontinence and urinary infections are long-term complications described in literature [5,6,10], however none of the patients in our study experienced these complications. Sexual dysfunction can still arise even after successful anatomical repair, owing to associated issues such as underdeveloped sacrum, spinal cord anomalies or inadequate perineal nerve supply. It may present as erectile dysfunction, ejaculation problems, low sex drive and dyspareunia. The majority of studies addressing sexual dysfunction focus on patients older than 16 or 18 years old [5,6]. Our study does not address this topic, since it is a retrospective study and there is a lack of this type of information in our patients’ clinical records [14].
The most significant limitation of this study is the small sample size. Additionally, a notable bias arises from the retrospective nature of the study, leading to missing information for certain variables [15,16].
Conclusion
Most anorectal malformations were associated with other malformations. The physical examination at birth is vital for the prompt diagnosis and early intervention of the condition. Anatomy allowed for correction in a single-stage surgery during the neonatal period in a significant percentage of cases. Constipation remains a prevalent problem in patients even after surgery, potentially affecting their quality of life, particularly in those experiencing soiling. Further studies, ideally multicentric and involving larger study populations, are necessary to investigate the association of anorectal malformations (ARM) with maternal pathology and to better characterize long-term complications, including intestinal, urinary, and sexual dysfunction in adolescence and adulthood, with particular emphasis on quality of life.
Acknowledgement
Sara Geraldes Paulino – data collection, data analysis, draft of
the manuscript.
Domingas Atouguia – data collection, manuscript review.
Mariana Dias – manuscript review.
Henrique Soares – manuscript review.
Susana Pissarra – conceptualization, manuscript review.
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Conflict of Interest
The authors state no conflict of interest.
References
- Gomella T, Eyal F, Mohammed F (2020) Surgical diseases of newborn: alimentary tract obstruction. Lange pp. 1079-1080.
- Cretolle C (2013) Malformations ano-retales. Archives de Pe diatrie 1: S19-27.
- Wang C, Li L, Cheng W (2015) Anorectal malformation: the etiological factors. Pediatr Surg Int 31(9): 795-804.
- Herman R, Teitelbaum D (2012) Anoretal malformations. Clin Perinatol pp. 39
- Mantho (2022) Prevalence, Management and Outcome of Anorectal Malformations in Children Aged 14 Years or Less: A 7 Years Retrospective Study in Three Hospitals of Douala. Health Sci 23(4): 25-29
- Emre Divarci, Orkan Ergun (2020) General complications after surgery for anorectal malformations. Pediatric Surgery International 36(4): 431-445.
- Tomas Wester (2024) Long-term Outcomes of Individuals with Anorectal Malformations. US Clinical Trials Registry Clinical Trial NCT04901819.
- Manasa Reddy, Nilesh Tank, Monika Bawa, Ravi P Kanojia, Ram Samujh, et al. (2022) Anorectal Malformations: the earlier the diagnosis, the better the outcome. Indian Journal of Pediatrics 89(6): 537-540.
- Firdian Makrufardi, Dewi Novitasari Arifin, Dwiki Afandy, Dicky Yulianda, Andi Dwihantoro, et al. (2020) Anorectal malformation patients’ outcomes after definitive surgery using Krickenbeck classification: A cross-sectional study. Heliyon 6(2): e03435.
- Marin Pollakan (2017) Outcomes of Treatment of Anorectal Malformations: A 7-year Review at Queen Sirikit National Institute of Child Health. The THAI Journal of SURGERY 38: 14-21.
- Gomella T (2020) Alimentary tract obstruction, Gonmella´s Neonatology 8th edition, LANGE 2020 p. 1079.
- Divarci E, Ergun O (2020) General complications after surgery for anorectal malformations. Pediatr Surg Int 36(4): 431-445.
- Kyrklund K, Pakarinen MP, Rintala RJ (2017) Long-term bowel function, quality of life and sexual function in patients with anorectal malformations treated during the PSARP era. Semin Pediatr Surg 26(5): 336-342.
- Stoll C, Alembik Y, Dott B (2007) Associated malformations in patients with anorectal anomalies. Eur J Med Genet 50(4): 281-290.
- Marcelis C, de Blaauw I, Brunner H (2011) Chromosomal anomalies in the etiology of anorectal malformations: a review. Am J Med Genet A 155A(11): 2692-2704
- King S, Levitt M (2022) Advances in the Management of the Neonate Born with an Anorectal Malformation. Clin Perinatol 49(4): 965-979.
-
Sara Geraldes Paulino*, Domingas Atouguia, Mariana Borges Dias, Henrique Soares and Susana Pissarra. Anorectal Malformations in the Neonatal Intensive Care Unit – A Retrospective Analysis from 2008 to 2023. Glob J of Ped & Neonatol Car. 5(2): 2024. GJPNC.MS.ID.000610.
Neonatology, Neonates, Anorectal malformations, Posterior sagittal anorectoplasty, Genitourinary, Syndromes, Asthma medication, Diagnosis, Maternal clinical
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






