 Review Article
Review Article
Selection of Antifungals in Bone Cements for the Treatment of Fungal Prosthetic Joint Infections - A Systematic Review
Wen Po Jonathan Tan1*, Amelia Tan Gek Min2, Renjy Nelson3, David Campbell4,5 and Peter Jonathan Smitham2,5
1Department of Orthopaedic Surgery, National University Health System, Singapore
2Adelaide Medical School, Australia
3Department of Infectious Diseases, Central Adelaide Local Health Network, Adelaide, Australia
4Wakefield Orthopaedic Clinic, Adelaide, South Australia, Australia
5Department of Orthopaedics and Trauma, Royal Adelaide Hospital, South Australia, Australia
Corresponding AuthorWen Po Jonathan Tan, Department of Orthopaedic Surgery, National University Health System, Singapore
Received Date:November 30, 2023; Published Date:March 26, 2024
Abstract
Background:Although antibiotic-impregnated bone cement has been widely used for the treatment of bacteria prosthetic joint infections, the use of antifungal-impregnated bone cement (AF-BC) in the treatment of Fungal Prosthetic Joint Infections (F-PJIs) remains unclear. This systematic review aims to summarise the use of AF-BC for the treatment of F-PJIs.
Methods:A literature search was performed using Ovid Medline, Embase, CINHAL and Cochrane via the Ovid platform from inception until August 2023. Screening was performed by two independent reviewers with a third for discrepancies.
Results:Out of 191 articles identified, 25 articles met the inclusion criteria describing 102 joints in which AF-BC was employed. All studies were case reports or case series, and no randomized controlled trials. Majority of the cases were caused by Candida species (95%). Amphotericin B was the preferred antifungal (86%) with a mean dose of 0.37g ± 0.25g per 40g bag of cement but ranged from 0.1-1.2g. Of the 81 cases that achieved infection free survival, the mean time for AF-BC was 25 weeks (range 3-60).
Conclusion:Our systematic review showed that a 2-stage reimplantation approach using AF-BCs combined with systemic antifungal therapy was successful in treating majority of F-PJIs. However, due to the small sample size, specific recommendations regarding the use of antifungal treatment in bone cements cannot be made. The combination of 0.3g of amphotericin B and 1.8g of vancomycin per 40g of bone cement demonstrated successful infection-free survival at the 12-month follow-up in most reported cases.
Introduction
Fungal prosthetic joint infections (F-PJIs) are rare, accounting for less than 1% of all PJIs [1]. With few reported cases and the lack of a specific treatment protocol, F-PJIs represent a therapeutic challenge [2]. F-PJIs pose greater challenges compared to bacteria PJIs for several reasons: 1) they are less common, leading to potential misdiagnosis and delayed treatment; 2) F-PJIs tend to be chronic and insidious, making eradication more difficult; 3) fungal organisms have complex cell walls and unique biochemical pathways, making them more resistant to antifungal agents than bacteria are to antibiotics. Some fungal species may also form biofilms, which are dense and protective communities that are difficult for antifungal agents to penetrate; and 4) Surgical management of F-PJIs is more complex, requiring extensive debridement and leading to increased morbidity and longer hospital stays.
In the absence of therapeutic guidelines for the management of F-PJI, most studies adopt the two-stage revision arthroplasty as the treatment of choice [2,3]. The largest F-PJI clinical study to date by Herndon et al reported a success rate of less than 50% with most cases associated with bacterial co-infection [4]. Systemic antifungals have limited effectiveness at the implantation site, leading to considerations of local antifungal treatments as effective adjuncts [5]. Antifungal-impregnated bone cements (AFBCs) are preferred for local drug delivery. However, AF-BCs have some limitations including antifungal resistance development, hypersensitivity reactions, decreased mechanical strength of bone cements, increased surgical time and increased cost. Due to limited clinical data, the efficacy of AF-BCs remains controversial. This systematic review aims to consolidate available evidence on AFBCs use in managing F-PJIs and try to identify optimal management regimes.
Material and Methods
Study Design
A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [6].
Literature Search
The authors conducted a literature search to identify studies on the use of AF-BCs for the treatment of F-PJIs. Various databases including Ovid Medline, Embase, CINHAL and Cochrane via the Ovid platform were searched without date restrictions but limited to English language publications. Specific keywords and Medical Subject Headings (MeSH) were used in the search strategy that is supplied in Appendix 1. The initial search was performed on 22 July 2021 and an update was conducted on 14 August 2023. Additionally, related references and cited articles were manually searched to find any additional relevant studies for inclusion.
Inclusion and Exclusion Criteria
The inclusion criteria for the study were PJIs caused by fungal pathogens; and patients undergoing surgical revision with the use of AF-BCs irrespective of the pathogen type or surgical treatment strategy. Studies were excluded if they did not report outcomes for AF-BC use in F-PJIs, if necessary, data could not be extrapolated or calculated from published results, non-English texts, and studies falling into categories like reviews, animal studies, in-vitro studies, or mechanical studies.
Selection Process
After the literature search, duplicates were removed, and the remaining citations were screened for eligibility. Covidence, a web-based systematic review tool was employed to assist with citation importing and screening, full text review, study selection, data extraction and data exporting. WPJT and ATGM independently screened all titles and abstracts to identify eligible studies based on the predefined criteria. The studies were then reviewed in full text by both reviewers for final inclusion. Disagreements were resolved by consensus between the two reviewers and the senior author (PJS) was available to resolve any disagreements if consensus could not be met.
Data Items
The following information were collected: author, year of publication, demographic data (age, gender), site of infection, causative fungal pathogen, presence of bacteria/ fungal coinfection, cement type, details of antifungals with or without antibiotics impregnated in bone cements, surgical approach, type and length of systemic antifungals, complications and length of follow-up (Tables 2 &3).
Table 1:Antifungal impregnated into bone cements.

Δ two antifungal agents (amphotericin B and voriconazole) were used in one case.
Table 2:Overview of treated cases, demographic data and causative organism(s).
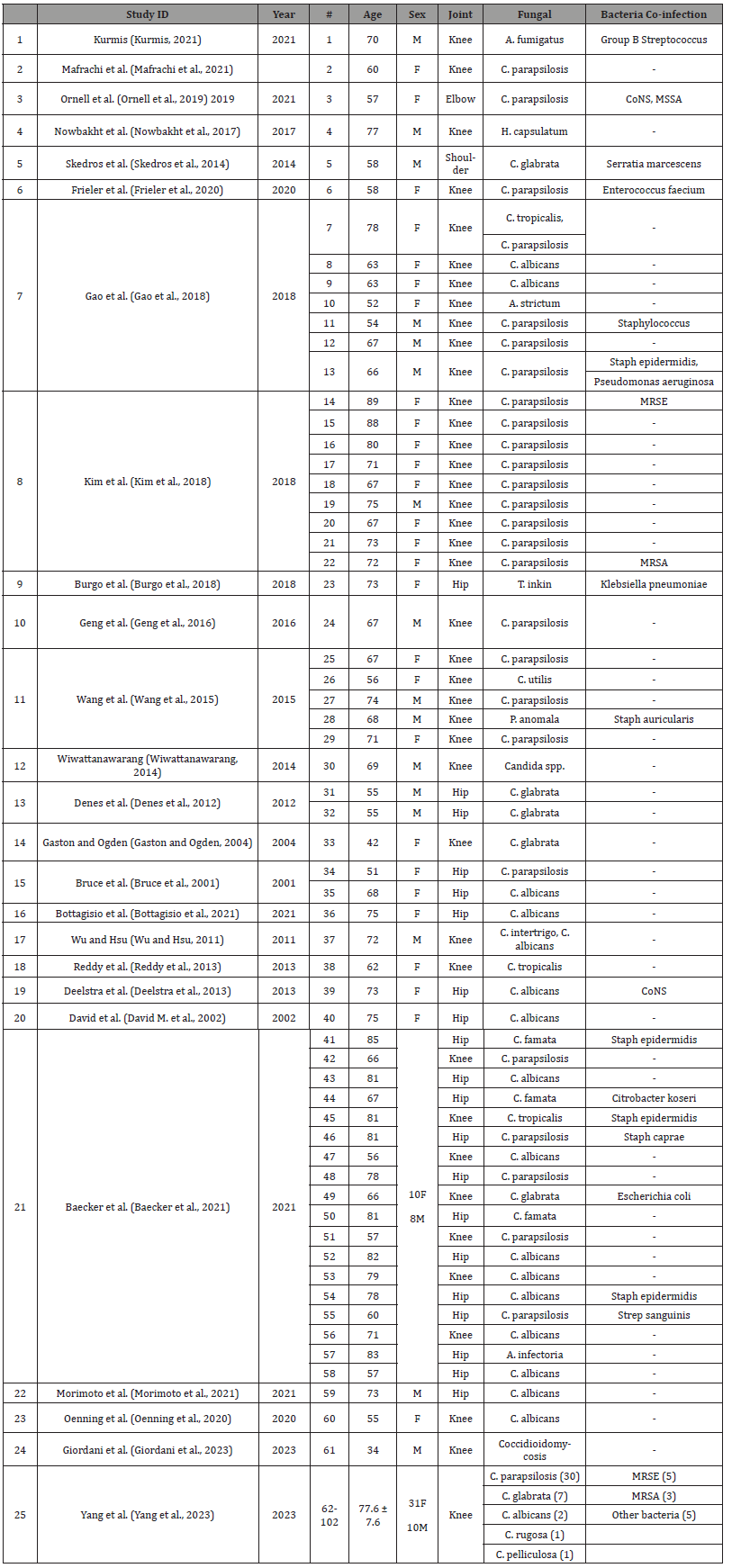
CoNS: coagulase negative staphylococcus; MSSA: methicillin sensitive staphylococcus aureu; MRSA: methicillin resistant staphylococcus aureus; MRSE: Methicillin resistant staphylococcuys epidermidis
Table 3:Surgical and Antifungal Treatment, Follow-up, and Infection Outcome of Reported Cases..
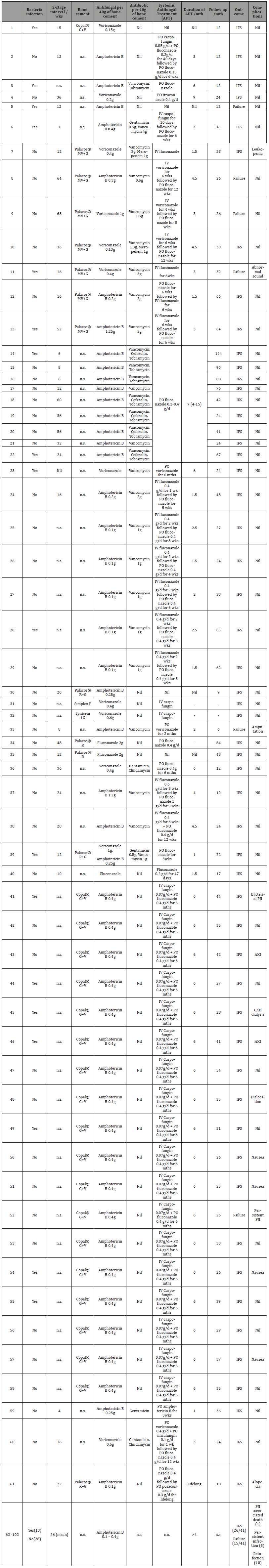
Palacos MV+G: medium viscosity bone cement with gentamicin Palacos R: high viscosity bone cementPalacos R+G: high viscosity bone cement with gentamicin Copal G+V: high viscosity bone cement with gentamicin and vancomycin Simplex P: dual viscosity bone cement with tobramycin Synicem 1G: high viscosity bone cement with gentamicin n.s.: not specified
Effect Measures
The primary outcome assessed in this systematic review was remission of F-PJI defined as: (i) absence of clinical signs of infection attributed to the original microorganism (relapse of infection) or a different strain (re-infection) after a minimum follow-up period of 12 months post-surgery; and (ii) no need for continuing antifungal therapy for suppressive treatment (iii) no death related to prosthetic joint infection.
Result
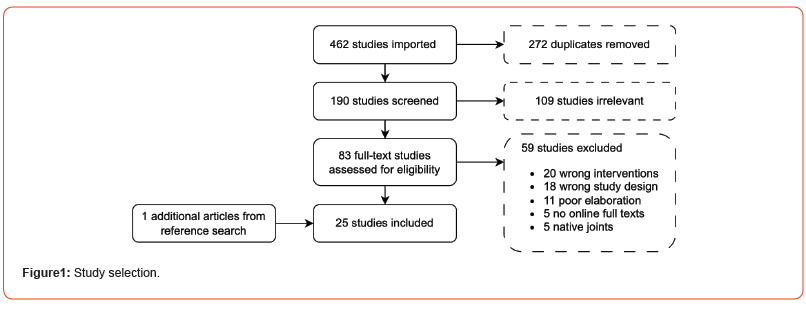
After searching the electronic databases, we identified 462 studies, of which 272 were duplicates and 109 studies were deemed irrelevant by title and abstract alone. Reference lists were screened, and an additional article was included. Full text evaluation led to the exclusion of 59 studies (Figure 1). Among the 25 included studies, 20 were case reports, 4 were retrospective studies and 1 was a prospective study. A total of 102 cases were included.
General Characteristics
Of the 102 included cases, there were 34 men (33%). The mean age of patients was 71.9 years (SD 9.7, range 34-89). The joints involved were the knee in 80 (78%), hip in 20 (20%), elbow in 1 (1%) and shoulder in 1 (1%). The length of follow-up was variable with a mean of 38 months (SD 24.7, range 6 to 144).
Causative pathogen (s)
The most frequently isolated fungal pathogens were Candida parapsilosis in 56 cases (55%), followed by Candida albicans in 18 (17%), Candida glabrata in 12 (12%), Candida famata in 3 (3%), Candida tropicalis in 3 (3%), while there was one case of Candida pelliculosa, Candida utilis, Candida intertrigo, Candida rugosa, Candida species, Acremonium strictum, Alternaria infectoria, Aspergillus fumigatus, Coccidioidomycosis, Histoplasma capsulatum, Trichosporon inkin and Pichia anomala each (1%). Fungal-fungal co-infection was present in 2 cases (2%). Bacteriafungal co-infection was present in 31 cases (30%) and the most common bacterial pathogen being staphylococcus spp. in 20 cases (65%) followed by streptococcus spp. in two (6.5%) while serratia, enterococcus, pseudomonas, klebsiella, citrobacter and escherichia in one case each (5.5%).
Bone Cements and Cement Loading
A wide variety of proprietary bone cements were used. Of the 33 cases that specified the type of bone cements used: Copal® G+V in 19 cases (58%), Palacos® MV+G in 7 (21%), Palacos® R+G in three (9%), Palacos® R in two (6%), Simplex P and Synicem 1G in one case each (3%). The remaining 69 studies did not specify the type of bone cement used (Table 3). A single antifungal agent was impregnated in bone cement in 101 cases (99%). Amphotericin B was the preferred antifungal in 88 cases (87.1%) followed by voriconazole in 12 (11.9%) and fluconazole in 3 (3.0%). Two antifungal agents were impregnated in 1 case (1%) that used a combination of voriconazole and amphotericin B. 16 cases failed to report the dose of antifungals impregnated per 40g of bone cement. The data on antifungals impregnated are summarised in Table 1.
For the 43 cases that had no bacterial co-infection, 23 (54.8%) specified that they had additional antibiotics impregnated into the bone cements. 81 cases obtained Infection-free Survival (IFS) for more than 12 months with an average follow-up of 38 months (range: 12-144). For cases that obtained IFS, AF-BCs were placed in-situ for an average of 25 weeks (range 3-60).
Discussion
Antibiotic loaded bone cements are commonly used in 2-stage resection arthroplasty for the treatment of bacteria PJIs [7]. The rarity and complexity of F-PJIs have resulted in a paucity of strong clinical evidence guiding treatment of F-PJIs. Despite the widely accepted use of AF-BCs to treat F-PJIs, there are some concerns. These include empirical treatment with limited clinical relevance to antifungal’s ability to penetrate pathogen-specific biofilms; nonstandardized; and unknown release kinetics [8]. A survey of 33 Australian arthroplasty surgeons in 2023 revealed that liposomal amphotericin, fluconazole and voriconazole were the common antifungals used in AF-BCs [9]. Amphotericin-B is an ideal agent to mix with bone cement due to its heat stability, broad antimicrobial spectrum and availability in powdered form. It has successfully used in vitro and in vivo to eradicate F-PJIs even with extensive bone loss [10] Although previous research suggests a minimum dose of 0.5g per 40g PMMA to prevent Candida’s biofilm formation, our review shows successful treatment of candida PJI with lower doses [11].
Voriconazole can retain its antifungal activity for at least two weeks when impregnated into bone cements and its elution rate is higher than amphotericin-B [12-14]. However, the addition of voriconazole can decrease the compressive strength of bone cements to a level unsuitable for fixation. Miller, et al. [14] were the first to investigate compressive strength in antifungal-loaded Simplex B bone cements and concluded that although the initial compressive strength for a 0.3 g/bag voriconazole formulation was above the acceptable strength for fixation, the strength decreased rapidly by the first day in elution to a strength lower than what is recommended for fixation [14,15]. However, argues that the reduced compressive strength does not depend on the elution of antifungals but on the excessive powder quantity by admixing of voriconazole in PMMA [15]. The dose recommendations for voriconazole range from 0.2g to 0.6g per 40g of PMMA cement [16].
The elution rate of antifungal bone cements is dose dependent. When antifungals are added, it increases the cement matrix porosity, which has an influence on antifungal delivery at cost of mechanical deterioration. Palacos® R cement exhibits a higher elution rate compared Simplex® P cement [14,17]. However, most studies in this review do not specify the type of bone cement used [17].
Pathogen characteristics
Fungal pathogens form biofilms on implant and tissues, showing varying tolerance to antifungal agents, with Candida albicans forming larger and more complex biofilms than other fungi pathogens [18-20]. Each fungi have its unique virulence potential, antifungal susceptibility and epidemiology [21] and limited data exists on the antifungal concentrations needed to achieve minimum biofilm eradication concentration (MBEC) for each pathogen [22]. In our review, most F-PJI cases with bacterial super-infections had antibiotic added to the bone cement spacer. When compared to pure AF-BC, the success rates were slightly lower but the reason for this is unclear.
The ideal interval between implant removal and reimplantation for F-PJIs is unknown as fungal pathogens are notoriously indolent. In our review, the mean interval was 25 weeks (range 4-68 weeks). Differentiating successful eradication from persistent PJI using serologic tests is challenging [2]. Although Wang, et al [23] propose that reimplantation should only be performed in the absence of clinical signs and symptoms with CRP and ESR within the normal range [23], our group challenges this notion on the basis that fungal infections are commonly associated with normal inflammatory markers [24].
Elution Rate
The elution rate of AF-BC is influenced by the addition of poragen and the formulation of the antifungal. Kweon et al. reported that adding 10g of cephazolin to AF-BC improved the elution rate of amphotericin B by seven times. Other antibiotics like vancomycin, tobramycin, meropenem, gentamicin and clindamycin were identified in our study as additions for treating F-PJIs without bacterial co-infection [25].
The formulation of amphotericin influences its elution rate when used in bone cements. A comparison between liposomal and deoxycholate amphotericin B revealed that the liposomal formulation had better elution rates. Another study explored an alternative non-liposomal formulation, N-methyl-D-glucamine/ palmitate amphotericin B, which exhibited a higher elution rate than deoxycholate amphotericin B [26]. However, our review lacked clear descriptions on the form of amphotericin B used in the identified studies to comment on these differences [11].
Limitations and Strengths
Our study faced limitations due to the lack of high-quality evidence regarding the benefits of AF-BCs. Our study identified case reports, retrospective studies and prospective studies with a notable absence of long-term prospective studies evaluating the effectiveness of AF-BCs in treating F-PJIs. While many reported cases had long-term follow-ups, there was inadequate documentation of the progress of the follow-ups often with only the end-outcome being reported. Our study also showed high success rates in the treatment of fungal prosthetic joint infection, but registry data paints a bleaker picture. This raises concerns about publication bias, skewing our perception of success rates in published literature [4].
Additionally, there was a lack of documentation on the treatment protocol such as the type of bone cement used and the form and dose of antifungals, thereby limiting our ability to draw conclusions on the most effective combination for antifungal impregnated bone cements. Another issue noted include the lack of sufficient studies to analyse the effectiveness of AF-BC equally across four different joints. We acknowledge that the principle of fixing joint prosthesis, the implant-cement-bone interface and the antifungal-cement filling agent system are similar irrespective of the joints. The review noted most of the case reports involving a Total Knee Arthroplasty (TKA), with the next majority being a total hip arthroplasty and only a case reported each for total elbow and shoulder arthroplasty. Therefore, the findings of the review are a more accurate representation of the efficacy of AF-BC in patients who previously had a TKA.
The lack of consistency or specific treatment guidelines of AF-BC continue to pose a problem in the decision-making prior to reimplantation. Several criteria to consider include the type, dosage and form of anti-fungal to be added into the bone cement. Our review showed that the concentration of anti-fungal additive ranged between 0.1g to 1g per 40g of bone cement, either the powdered or liposomal form could be considered. However, the lack of randomised controlled trials makes the selection of antifungals in bone cements a difficult endeavour even for experienced orthopaedic surgeons. Despite the limitations, our review managed to have an in-depth analysis and breakdown of the case studies. We captured specific AF-BC concentrations and duration and dosage of adjunctive systemic therapy for each case, to identify a potential pattern in the treatment methods. To the best of our knowledge, this systematic review is the most comprehensive summary of reported use of antifungals in bone cements. A recent publication in July 2022 by Anagnostakos, et al. [27] also reviewed the efficacy of AF-BC in F-PJI but concluded that further evaluation of this subject matter is still required [27-51].
Conclusion
In conclusion, our systematic review showed that a 2-stage reimplantation approach using AF-BCs alongside systemic antifungal therapy was successful in treating majority of F-PJIs. Based on the information gathered, the most effective anti-fungal identified was the liposomal Amphotericin B (0.37±0.25g, range 0.1-1.2g per 40g of bone cement) with subsequent use of systemic antifungal therapy for at least 6 months.
Authors Contributions
All authors contributed to the study design, provided thorough feedback, and gave final approval of the version to be published. W.P.J.T and A.T.G.M analysed the data and designed the figures and tables. All authors can take responsibility for the integrity and accuracy of the data analysis.
Competing Interests
We declare that there is no potential conflict of interest and that no funding was provided in this research.
Ethical Statement
Not applicable.
Acknowledgement
We are thankful to Ms Natalie Dempster, South Australia Health reference librarian, for her contribution through assistance of the search strategy.
References
- Riaz T, Tande AJ, Steed LL, Demos HA, Salgado CD, et al. (2020) Risk Factors for Fungal Prosthetic Joint Infection. J Bone Jt Infect 5(2): 76-81.
- Azzam K, Parvizi J, Jungkind D, Hanssen A, Fehring T, et al. (2009) Microbiological, clinical, and surgical features of fungal prosthetic joint infections: a multi-institutional experience. J Bone Joint Surg Am 6: 142-149.
- Kuiper JW, Van Den Bekerom MP, Van Der Stappen J, Nolte PA, Colen S, et al. (2013) 2-stage revision recommended for treatment of fungal hip and knee prosthetic joint infections: An analysis of 164 patients, 156 from the literature and 8 own cases. Acta orthopaedical 84(6): 517-523.
- Herndon CL, Rowe TM, Metcalf RW, Odum SM, Fehring TK, et al. (2023) Treatment Outcomes of Fungal Periprosthetic Joint Infection. The Journal of Arthroplasty 38: 2436-2440.
- Wang J, Zhu C, Cheng T, Peng X, Zhang W, et al. (2013) A systematic review and meta-analysis of antibiotic-impregnated bone cement use in primary total hip or knee arthroplasty. PloS one 8(12): e82745.
- Moher D, Liberati A, Tetzlaff J, Altman D G (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535.
- Anagnostakos K (2017) Therapeutic Use of Antibiotic-loaded Bone Cement in the Treatment of Hip and Knee Joint Infections. J Bone Joint Infect 2(1): 29-37.
- Schwarz EM, McLaren AC, Sculco TP, Brause B, Bostrom M, et al. (2021) Adjuvant antibiotic-loaded bone cement: Concerns with current use and research to make it work. J Orthop Res 39: 227-239.
- Hillock NT, Campbell DG, Nelson R, Teoh A, Tan J, et al. (2023) Antimicrobial-loaded bone cement use is highly variable in joint replacement surgery: a survey of Australian arthroplasty surgeons. ANZ J Surg.
- Zhu ES, Thompson GR, Kreulen C, Giza E (2013) Amphotericin B-impregnated bone cement to treat refractory coccidioidal osteomyelitis. Antimicrob Agents Chemother 57(12): 6341-6343.
- Czuban M, Wulsten D, Wang L, Di Luca M, Trampuz A (2019) Release of different amphotericin B formulations from PMMA bone cements and their activity against Candida biofilm. Colloids Surf B Biointerfaces 1:183:110406.
- Sealy PI, Nguyen C, Tucci M, Benghuzzi H, Cleary JD, et al. (2009) Delivery of antifungal agents using bioactive and nonbioactive bone cements. Ann Pharmacother 43(10: 1606-1615.
- Grimsrud C, Raven R, Fothergill AW, Kim HT (2011) The In Vitro Elution Characteristics of Antifungal-loaded PMMA Bone Cement and Calcium Sulfate Bone Substitute. Orthopedics 34: e378-e381.
- Miller RB, McLaren AC, Pauken C, Clarke HD, McLemore R, et al. (2013) Voriconazole Is Delivered from Antifungal-Loaded Bone Cement. Clinical Orthopaedics and Related Research® 471(1): 195-200.
- Krampitz B, Steiner J, Trampuz A, Kühn KD (2023) Voriconazole Admixed with PMMA-Impact on Mechanical Properties and Efficacy. Antibiotics 12(5): 848.
- Fusini F, Aprato A, Massè A, Bistolfi A, Girardo M, et al. (2020) Candida periprosthetic infection of the hip: a systematic review of surgical treatments and clinical outcomes. Int Orthop 44(1): 15-22.
- Silverberg D, Kodali P, Dipersio J, Acus R, Askew M, et al. (2002) In Vitro Analysis of Antifungal Impregnated Polymethylmethacrylate Bone Cement. Clinical Orthopaedics and Related Research® 403: 228-231.
- Ramage G, Rajendran R, Sherry L, Williams C (2012) Fungal biofilm resistance. Int J Microbiol 2012: 528521.
- Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, et al. (2001) Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol 183: 5385-5394.
- Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA, et al. (2002) Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 46(6): 1773-1780.
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, et al. (2016) Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 62(4): e1-50.
- Feng-Chih Kuo, McLaren A (2018) Which antifungal agents are heat-stable and what dose of these agents should be used in cement spacers for fungal periprosthetic joint infection (PJI)?. International Consensus Meeting.
- Wang QJ, Shen H, Zhang XL, Jiang Y, Wang Q, Chen Y, Shao JJ, et al. (2015a) Staged reimplantation for the treatment of fungal peri-prosthetic joint infection following primary total knee arthroplasty. Orthopaedics & Traumatology: Surgery & Research 101(2): 151-156.
- Wang QJ, Shen H, Zhang XL, Jiang Y, Wang Q, et al. (2015b) Staged reimplantation for the treatment of fungal peri-prosthetic joint infection following primary total knee arthroplasty. Orthopaedics & traumatology, surgery & research: OTSR 101(2): 151-156.
- Kweon C, McLaren AC, Leon C, McLemore R (2011) Amphotericin B Delivery from Bone Cement Increases with Porosity but Strength Decreases. Clinical Orthopaedics and Related Research® 469(11): 3002-3007.
- Cunningham B, McLaren AC, Pauken C, McLemore R (2012) Liposomal Formulation Increases Local Delivery of Amphotericin from Bone Cement: A Pilot Study. Clin Orthop Relat Res 470: 2671-2676.
- Anagnostakos K, Becker SL, Sahan I (2022) Antifungal-Loaded Acrylic Bone Cement in the Treatment of Periprosthetic Hip and Knee Joint Infections: A Review. Antibiotics 11(7): 879.
- Baecker H, Frieler S, Geßmann J, Pauly S, Schildhauer TA, et al. (2021) Three-stage revision arthroplasty for the treatment of fungal periprosthetic joint infection: outcome analysis of a novel treatment algorithm: a prospective study. Bone Jt Open 2(8): 671-678.
- Bottagisio M, Bidossi A, Logoluso N, Pellegrini A, De Vecchi E (2021) A spacer infection by Candida albicans secondary to a Staphylococcus capitis prosthetic joint infection: a case report. BMC Infectious Diseases 21(1): 416.
- Bruce A, Kerry R, Norman P, Stockley I (2001) Fluconazole-impregnated beads in the management of fungal infection of prosthetic joints. J Bone Joint Surg Br 83: 183-184.
- Burgo FJ, Mengelle DE, Abraham A, Kremer G, Autorino CM (2018) Periprosthetic fungal infection of a hip caused by Trichosporon inkin. Arthroplasty today 4: 24-26.
- David MP, Douglas RO, Michael RK, Arlen DH (2002) Delayed Reimplantation Arthroplasty for Candidal Prosthetic Joint Infection: A Report of 4 Cases and Review of the Literature. Clin Infect Dis 34(7): 930-938.
- Deelstra JJ, Neut D, Jutte PC (2013) Successful treatment of Candida albicans–infected total hip prosthesis with staged procedure using an antifungal-loaded cement spacer. The Journal of arthroplasty, 28(2): 374.e5-e8.
- Denes E, Fiorenza F, Saint-Marcoux F, Megherbi M, Dupon M, et al. (2012) Voriconazole stability in cement spacers. Med Mal Infect 42: 567-568.
- Frieler S, Yilmaz E, Goodmanson R, Hanusrichter Y, Schildhauer TA, et al. (2020) Conversion From Knee Arthrodesis Back to Arthroplasty: A Particular Challenge in Combination With Fungal Periprosthetic Joint Infection. Arthroplast Today 6(4): 1038-1044.
- Gao Z, Li X, Du Y, Peng Y, Wu W, et al. (2018) Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study, Medical science monitor. Med Sci Monit 24: 5549.
- Gaston G, Ogden J (2004) Candida glabrata periprosthetic infection: a case report and literature review. J Arthroplasty 19: 927-930.
- Geng L, Xu M, Yu L, Li J, Zhou Y, et al. (2016) Risk factors and the clinical and surgical features of fungal prosthetic joint infections: A retrospective analysis of eight cases. Exp Ther Med 12(2): 991-999.
- Giordani FA, Kiernan B, Giordani M, Darrow M, Thorpe S, et al. (2023) Coccidioidomycosis in Joint Replacement: A Review of the Literature With Case Presentations. Arthroplast Today 21: 101123.
- Kim JK, Lee DY, Kang DW, Ro DH, Lee MC, et al. Efficacy of antifungal-impregnated cement spacer against chronic fungal periprosthetic joint infections after total knee arthroplasty. The Knee 25: 631-637.
- Kurmis AP (2021) Eradicating Fungal Periprosthetic TKA “Super-infection”: Review of the Contemporary Literature and Consideration of Antibiotic-Impregnated Dissolving Calcium Sulfate Beads as a Novel PJI Treatment Adjunct. Arthroplasty today 8: 163-170.
- Mafrachi B W, Al Debei AH, Al Muhtaseb F M, Al-Ajlouni JM, Hammad Y S, et al. (2021) Fungal Prosthetic Joint Infection Following Total Knee Arthroplasty: A Case Report. Journal of Orthopaedic Case Reports 11(2): 95.-98.
- Morimoto Y, Yo H, Ohashi H (2021) Two-stage revision using antifungal-loaded cement beads for the treatment of Candida infection following revision total hip arthroplasty: A case report. Journal of Orthopaedic Science 26(3): 505-509.
- Nowbakht C, Garrity K, Webber N, Eraso J, Ostrosky-Zeichner L, et al. (2017) Prosthetic joint infection due to Histoplasma capsulatum complicating a total knee arthroplasty, Open Forum Infectious Diseases 4(3): ofx118.
- Oenning S, Moellenbeck B, Gosheger G, Schmidt-Bräkling T, Schwarze J, et al. (2020) Fungal periprosthetic knee joint infection in a patient with metamizole-induced agranulocytosis. Arthroplasty today 6(4): 726-730.
- Ornell SS, Dang KH, Bois AJ, Dutta AK (2019) Fungal infection following total elbow arthroplasty. Case Reports in Orthopedics 2019: 7927914.
- Reddy KJ, Shah JD, Kale RV, Reddy TJ (2013) Fungal prosthetic joint infection after total knee arthroplasty. Indian Journal of Orthopaedics 47(5): 526-529.
- Skedros JG, Keenan KE, Updike WS, Oliver MR (2014) Failed reverse total shoulder arthroplasty caused by recurrent Candida glabrata infection with prior Serratia marcescens coinfection. Case Reports in Infectious Diseases 2014:
- Wiwattanawarang N (2014) Fungal periprosthetic joint infection after total knee arthroplasty. J Med Assoc Thai 97: 1358-1363.
- Wu MH, Hsu KY (2011) Candidal arthritis in revision knee arthroplasty successfully treated with sequential parenteral-oral fluconazole and amphotericin B-loaded cement spacer, Knee Surgery. Sports Traumatology Arthroscopy 19(2): 273-276.
- Yang HY, Shin HH, Kim JW, Seon JK (2023) The fate of fungal periprosthetic joint infection after total knee arthroplasty. Int Orthop 47(11): 2727-2735.
-
Wen Po Jonathan Tan*, Amelia Tan Gek Min, Renjy Nelson, David Campbell and Peter Jonathan Smitham. Selection of Antifungals in Bone Cements for the Treatment of Fungal Prosthetic Joint Infections - A Systematic Review. Glob J Ortho Res. 4(4): 2024. GJOR.MS.ID.000595.
-
Osteogenesis imperfecta, non-telescoping rods, Fracture risk reduction, Recurrent fractures, Osteogenesis Imperfecta, Elastic Stable Intramedullary Nail
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






