 Review Article
Review Article
Percutaneous Ultrasound-Guided Techniques for Carpal Tunnel Release: A Descriptive Review
Javier de la Fuente1*, Jose F Aramendi2, Davila Fernando3, Balius Ramon4 and Marc Blasi5
1Orthopedic Department, Pakea-Mutualia Clinic, Spain
2Primary Care Department, Pakea-Mutualia Clinic, Spain
3Orthopedic Department, Pakea-Mutualia Clinic, Spain
4Diagonal Clinic,Esplugues de Llobregat, Spain
5Plastic Surgery Department, Hospital Germans Triasi Pujol University, Spain
Corresponding AuthorJavier de la Fuente, Orthopedic Department, Pakea-Mutualia Clinic, Paseo de Arriola 26, 20018 San Sebastian, Spain.
Received Date: August 27, 2021; Published Date: October 13, 2021
Abstract
Carpal tunnel syndrome (CTS) is a prevalent compressive peripheral neuropathy that often requires surgical treatment due to symptom severity or medical treatment failure. The recent popularity of ultrasound-guided (US) percutaneous surgery to treat these neuropathies has made this minimally invasive technique a good option over traditional open techniques. This review sought to identify, classify, and describe the minimally invasive percutaneous US-guided techniques reported in the literature for carpal tunnel release (CTR). Thirty reports were identified describing 22 different techniques. No systematic reviews addressing this topic were found. Of the 30 studies reviewed, only five (describing four techniques) were randomized controlled trials (RCT), three were non-randomized controlled trials, and 22 were uncontrolled studies. The technical characteristics most frequently described by the different authors were: 13 MHz US-probe, incisions 2 to 15 mm, section direction retrograde, incision orientation longitudinal, entry point carpal, with a single incision technique. As further characteristics, cutting instruments varied widely, few studies reported on the use of tourniquet and nearly all of them used local anesthesia. Our descriptive review shows that minimally invasive percutaneous US-guided surgery for CTR offers multiple technical possibilities. As only four of these techniques have been the focus of RTCs, more work is needed to assess the efficacy of this approach in improving the pain and hand functionality problems experienced by patients with CTS.
Keywords: Minimally invasive surgery; interventional ultrasound; percutaneous technique; carpal tunnel syndrome
Abbreviations: BCTQ: Boston carpal tunnel syndrome questionnaire; CT: Carpal tunnel; CTR: Carpal tunnel release; CTS: Carpal tunnel syndrome; DLA: Daily life activities; FR: Flexor retinaculum; MCID: Minimal clinical important difference; MeSH: Medical Subject Headings; MHz: Megahertz; MSN: Hanzhang miniscalpel-needle; NCV: Nerve conduction velocity; NoC: Studies without a control or comparison group; NoRCT: Non-randomized controlled studies; n.r: Not reported; PUSG: Percutaneous ultrasound-guided; Quick DASH: Quick–disabilities of the arm, shoulder, and hand questionnaire; RCT: Randomized controlled trial; SAPA: Superficial arterial palmar arch; SPA: superficial palmar aponeurosis; SR: Systematic reviews; TCL: Transverse carpal ligament; US: Ultrasound; WALANT: Wide awake local anesthesia no tourniquet
Introduction
Carpal tunnel syndrome (CTS) is the most common of all compressive peripheral neuropathies affecting some 88 men and 193 women per 100,000 [1]. While it is frequently idiopathic, it may be the consequence of increased carpal tunnel (CT) pressure, ischemic changes within the nerve, or compression of adjacent structures [2,3]. Clinically, CTS causes tingling, pricking, numbness, pain, swelling, or stiffness in the first three fingers of the hand. These symptoms will often awake the patient during the night and lead to hand weakness and atrophy of the thenar muscles [2,4]. The diagnosis is mainly clinical and confirmed by tests such as nerve conduction velocity (NCV), monofilament, and 2-point discrimination [3,5]. In milder cases, treatment for CTS can be conservative, but there is strong evidence to support the use of surgical treatment to improve symptoms [4]. The prognosis for surgically treated CTS is good, with a success rate of 86% [6].
Although open release of the CT is the most common surgical procedure, it has been associated with complications like postsurgical pain, dysesthesia and loss of grip strength [7]. To minimize these complications, new surgical techniques have been developed in the past 30 years for sectioning the flexor retinaculum (FR) and releasing the median nerve in the CT. These techniques can be open, mini-open, or percutaneous. The latter can be guided by ultrasound (US) or by endoscopic visualization [8]. The objectives of these newer procedures have been to reduce the length of the surgical incision, generating an ever-smaller scar associated with less postoperative pain and a sooner return to work. However, these approaches also have some shortcomings related to the compromised visualization of the median nerve and its terminal branches, the vascular structures around the wrist, and any anatomic variants that might be present, thereby increasing the risk of complications during CT release (CTR) surgery [9,10]. Several CTR procedures conducted under direct US-guidance have been described since, in 1997, Nakamichi and Tachibana described the first US-guided CTR technique [11]. These procedures have been classified according to the surgical access route (anterograde or retrograde), approach (palmar or wrist), and cutting instrument [8]. In this article, we review and update all percutaneous USguided (PUSG) techniques described in the scientific literature that we were able to identify.
Materials and Methods
Eligibility criteria
The studies identified were those in which only a PUSG technique of CTR was examined. There were no filters on study design, comparison intervention, follow-up duration, or publication date or language (English, Spanish, German, or French). Participants were required to have primary CTS. Cadaveric studies were also included. Exclusion criteria were patient studies testing percutaneous techniques with endoscopic visualization, and participants of those studies with secondary CTS or undergoing repeat CTR surgery. Besides original articles, abstracts of presentations at conferences and articles only describing the surgical technique were also included.
Search methods
The MEDLINE database was searched through Pubmed in December 2020 using the terms: (systematic [sb]) AND””Carpal Tunnel Syndrome / surgery”” [Mesh]. However, as no systematic review that met the inclusion criteria was found, we extended the search to: (Ultrasonography [Mesh]) AND “Carpal Tunnel Syndrome/ surgery” [Mesh]. We also revised the reference lists of the most relevant studies and also included one RCT by the present authors [12].
Studies were classified according to evidence level into systematic reviews (SR), randomized controlled clinical trials (RCT), non-randomized controlled studies (NoRCT), and studies without a control or comparison group (NoC). Due to the descriptive nature of this work, we did not assess the quality of the studies. To avoid further bias, we only consider the results obtained only in RCTs.
To describe the techniques used, we compiled data regarding: 1) the resolution of the US probe; 2) sonographer experience; 3) incision sizes; 4) anterograde or retrograde sections; 5) longitudinal or transverse incisions; 6) proximal or distal entry point; 7) number of incisions; 8) cutting instruments used; 9) use of tourniquet; and 10) anesthesia.
Result
Result of the search
Thirty studies meeting the inclusion/exclusion criteria were identified in our search. The results of the search are shown in (Figure 1).
Types of study
No SR comparing PUSG techniques of CTR with any other intervention were found. Only 5of the 30 studies reviewed here describing 4 different techniques were RCT [11–15]; a further 3 studies were NoRCT [10,16,17]; and 22 were NoC [18–39].
We considered the risk of bias of the study by Guo et al. [24] as high and classified it as NoC without taking into account its results. In this study, conducted in an initial series of 116 patients, 159 wrists were operated on. In the first 23 wrists (not specified how many patients), triamcinolone mixed with 0.5% lidocaine was used for hydro dissection. For the remaining 136 wrists, the authors no longer used steroids. After surgically treating 48 wrists, one step of their intervention was modified. The authors compared their results in 159 wrists (116 patients) with those of other authors [40] who reported on a group of 75 patients (96 wrists) who underwent endoscopic CTR, and on another group of 72 patients (95 wrists) who underwent open CTR surgery.
Furthermore, Buncke et al. and Markison [18,34] described in the same year the use of the same technique in a series of 3 patients, 2 men and 1 woman. Although we have considered them as different studies, we cannot be sure that this was really the case.
Publication years
The earliest study reviewed was the first to describe a PUSG technique [11]. A further 26 of the 30 studies included were published between 2013 and 2020.
Participants
Of the 30 studies identified, in 18 participants were patients with CTS. A further 9 were cadaver studies and the remaining 3 articles were descriptions of the surgical technique. In the 5 RCTs included, 378 patients were treated, mostly women (range 54% to 100%) of a mean age of 55.1 years (range 47 to 63 years). One NoC study [28] included patients with some disability (4 of the 10 patients).
Interventions
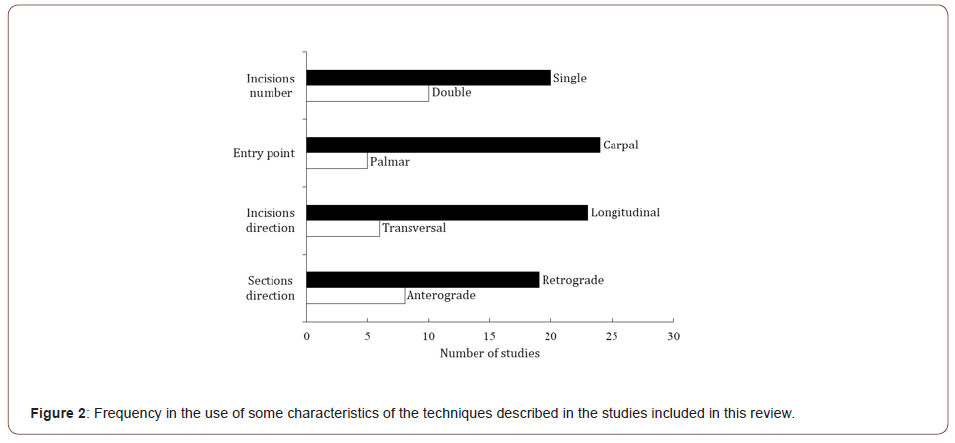
The 30 studies reviewed described 22 different surgery techniques. The technical characteristics described by the different researchers varied widely and are described in Tables 1, 2, and 3, for RCT, NoRCT, and NoC, respectively. The most used US-probe was 13 MHz (6 of the 25 studies reported on this), although frequencies ranged from 10 to 18 MHz. Only six reports considered sonographer experience, and this varied from 5 to 20 years. Incision size varied between 2 and 15 mm. With regard to section direction, 19 groups preferred retrograde, 8 anterograde, and two groups sectioned in the direction dorsal to volar. Incision orientation was preferably longitudinal (23 out of 29 studies). The most common entry point was carpal or proximal (24 out of 29 studies). Most surgeons opted for a single incision technique, 23 out of 30. Cutting instruments varied widely. In five reports, the US-guided technique was combined with the endoscopic one, and different types of endoscopes were used for the FR section [29,30,33,34,39]. Few studies reported on the use of a tourniquet while most used local anesthesia. Eight research groups reported they performed hydro dissection, and those of one study [20] reported they used the WALANT (wide awake local anesthesia no tourniquet) technique. Three studies by two groups [15–17] undertook steroid injection in the same surgical act as FR section.
Table 1: Details of the RCTs included in this review and their outcomes.
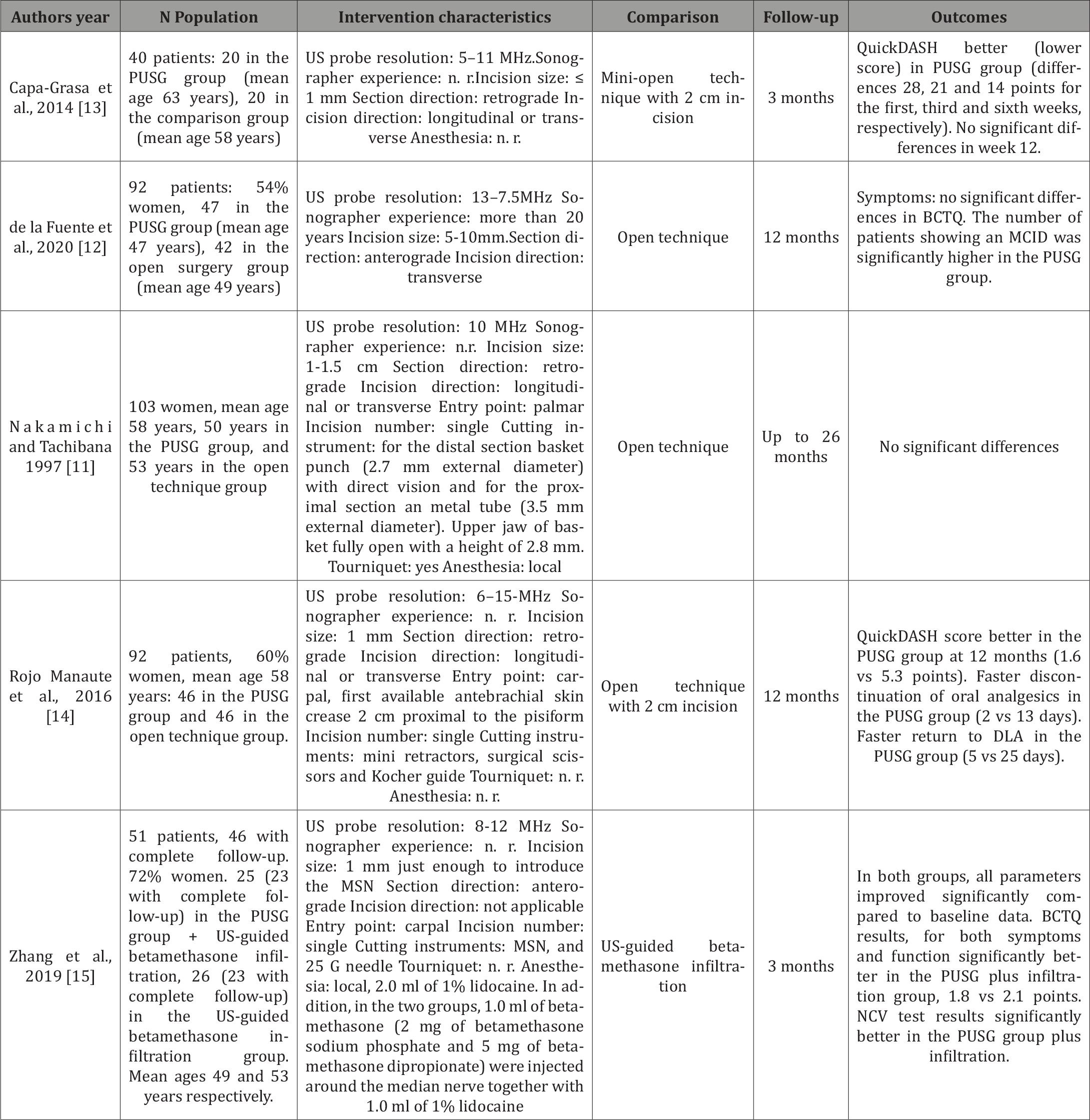
Table 2: Details of the non-randomized controlled studies included in this review.
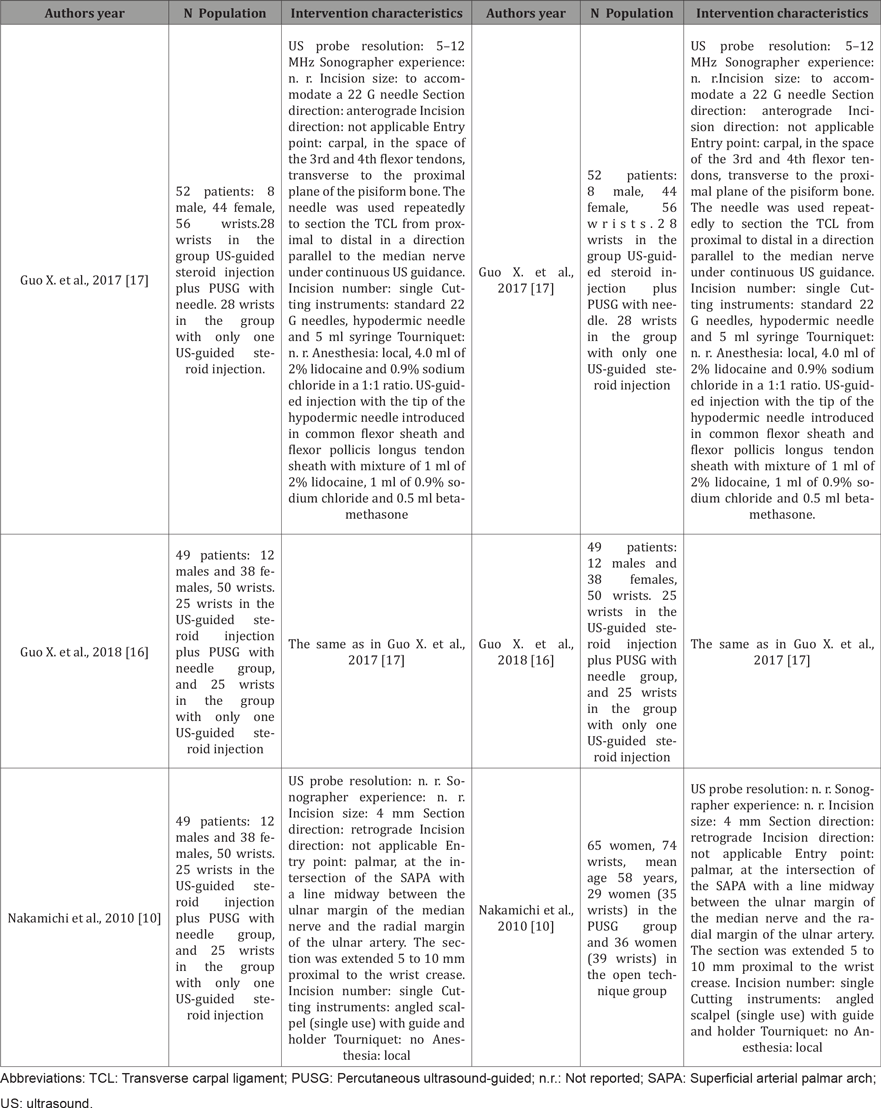
Table 3: Details of the uncontrolled studies included in this review.
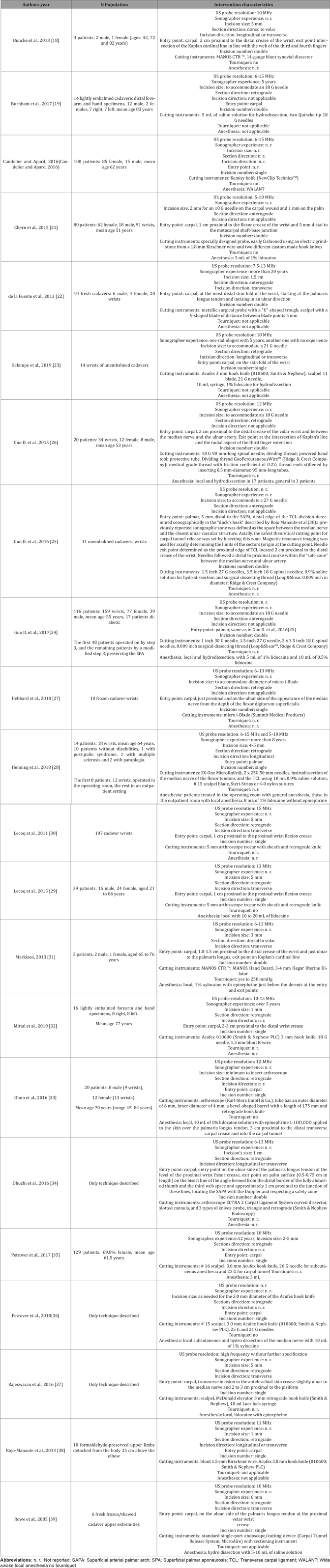
The technique encompassing the most used characteristics of the interventions described in the articles reviewed would be one carried out with a 13 MHz transducer, involving a 3 mm longitudinal incision, with a single carpal entry point, involving a retrograde section, and performed under local anesthesia (Figure 2).

The instruments used to section the FR were essentially different in each group and included different types of scalpels with various tips and blades, sectioning instruments of the endoscopes used, sectioning instruments designed ad hoc for this purpose, even with a built-in electro stimulator, surgical section threads and different types of needles.
Comparison intervention in the randomized controlled trials
Four of the RCTs included [11-14] used a comparison group subjected to one open technique. In contrast, in their comparison group, Zhang et al. [15] infiltrated betamethasone under USguidance.
Outcomes of the randomized controlled trials
The outcome measure in the two RCTs by Rojo-Manautes’ group [13,14] was the Quick-Disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH). In their reports, they described improved results of this questionnaire, which were more significant in the first weeks after surgery. We are unaware of the clinical relevance of these improvements, as minimal clinically important differences (MCID) were not reported [41]. In both papers, a greater number of complications were described for the group subjected to the open surgery technique.
Nakamichi and Tachibana [11] observed no significant differences in numbness, paresthesia, electrophysiological, or sensory test results between their PUSG and open technique groups. Although small in magnitude, they found significant differences in pain and scar sensitivity, both measured on a 4-point Likert-type scale (maximum difference 0.7 points at 13 weeks for both variables). Significant differences also emerged in hand grip strength (maximum difference 3.1 kg at 3 weeks) and key-pinch strength (maximum difference 0.53 kg at 3 weeks). No complications were detected in either of the two groups.
To assess symptoms and functionality, Zhang et al. [15] used the Boston Carpal Tunnel Syndrome Questionnaire (BCTQ). These investigators noted significant differences, albeit small in magnitude, in symptoms, function, compound muscle action potentials, sensory conduction velocity, and median nerve crosssectional area, all in favor of the PUSG technique.
In our RCT [12], we detected no significant differences in symptom severity as assessed through the BCTQ. However, the number of patients showing a minimal clinically important difference (MCID) [42] following surgery in the PUSG surgery group was significantly higher. In contrast, when we assessed functionality also using the BCTQ, the differences were significantly greater in the PUSG group. However, numbers of patients showing MCID were not different between the two groups, and neither were their differences in complications between the groups.
Discussion
To our knowledge, this is the first study to describe and classify all PUSG techniques of CTR. Of the 30 articles reviewed, 22 are descriptive works with no comparison group, three are nonrandomized controlled studies, and five are randomized controlled trials. This paper allows a quick review and update of the PUSG techniques of CTR described in the literature and should help surgeons choose the technique that best suits their needs, skills, and preferences. Contrary to the case of endoscopic CTR techniques, for which numerous publications exist allowing for detailed SRs of more than 20 original studies [43,44], we were unable to find any SR that analyzes and synthesizes the efficacy of PUSG techniques for CTR.
Besides enabling the localization of possible anatomical variants, US offers the possibility of marking the safety zones of the CT. In effect, US visualization can be adapted to all percutaneous techniques, providing an expanded field of view and therefore offering greater safety. According to the study by Dekimpe et al. [23], the learning curve for the US-guided CTR is short. In this cadaver study, two radiologists with different experience were able to perform a complete section of the FR after only two previous attempts. We would thus encourage young physicians to acquire skills in this technique, which, as already mentioned, has benefits both in terms of diagnosis and treatment.
Every medical procedure has its contraindications. Henning et al. proposed the following for the PUSG technique of CTR: 1) inability to adequately visualize structures at risk, including the thenar motor branch and recurrent motor branch of the median nerve, the palmar cutaneous branch of the median nerve, ulnar vessels, superficial palmar arch, and median and ulnar palmar digital nerves; 2) presence of anatomical variants that would make it impossible to create a safe transverse zone; and 3) presence of a mass or other event that would require treatment other than a FR section [28].
A limitation of our review is that its design does not allow for any conclusions regarding the effectiveness of the different PUSG techniques for CT section. While we provide the results of the five RCTs described to date, there is still a need for a comprehensive SR, including a more complete bibliographic review, peer assessment of the relevance and quality of the original articles, an analysis of heterogeneity and, if appropriate, a meta-analysis of the extracted data.
Considering the reports by Huisstede et al. and Zhang et al. (Huisstede et al., 2010; Zhang et al., 2019), another topic of future investigation would be to explore whether irrigation or steroid infiltration of the median nerve before skin closure after percutaneous RF section has any added effect to exclusively that of mechanically releasing the CT. To establish whether any of the techniques described here is more effective than another, there is also a need for adequately designed RCTs, preferably considering MCID. The conclusions to be drawn from our descriptive review are that: 1) PUSG surgery for CTR offers multiple technical possibilities; 2) no technique has been shown to be more effective than another in a RCT; 3) SRs are needed to establish whether PUSG are better than open techniques in reducing symptoms and improving hand functionality in patients with CTS; and 4) more RCTs are needed to establish the most effective PUSG techniques.
Conclusion
The present review shows that minimally invasive percutaneous US-guided surgery for CTR offers multiple technical possibilities. As only four of these techniques have been the focus of RTCs, more work is needed to assess the efficacy of this approach in improving the pain and hand functionality problems experienced by patients with CTS.
Acknowledgement
The authors thank Maria del Mar Ubeda and Eukene Ansuategi from the Donostia University Hospital Library for their help in the literature search.
Conflicts of Interest
The authors declare that they have neither conflict of interest nor any disclosure to declare.
References
- Latinovic R (2006) Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry 77(2): 263-265.
- Carpal-tunnel.net. The Basics - CTS in a nutshell.
- Erickson M, Lawrence M, Jansen CWS, Coker D, Amadio P, et al. (2019) Hand Pain and Sensory Deficits: Carpal Tunnel Syndrome: Clinical Practice Guidelines Linked to the International Classification of Functioning, Disability and Health from the Academy of Hand and Upper Extremity Physical Therapy and the Academy of Orthopaedic Physical Therapy of the American Physical Therapy Association. J Orthop Sports Phys Ther 49(5): CPG1-CPG85.
- American Academy of Orthopaedic Surgeons (2016) Evidence-Based Clinical Practice Guideline on the Management of Carpal Tunnel Syndrome.
- Werner RA, Andary M (2002) Carpal tunnel syndrome: pathophysiology and clinical neurophysiology. Clin Neurophysiol 113(9):1373-1381.
- Duetzmann S, Tas S, Seifert V, Marquardt G, Dombert T, et al. (2018) Cross-sectional Area of the Median Nerve Before Revision Carpal Tunnel Release-A Cross-sectional Study. Oper Neurosurg Hagerstown 14(1): 20-25.
- Aslani HR, Alizadeh K, Eajazi A, Karimi A, Karimi MH, et al. (2012) Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg 114(7): 965-968.
- Apard T, Candelier G (2017) Surgical ultrasound-guided carpal tunnel release. Hand Surg Rehabil 36(5): 333-337.
- Louis DS, Greene TL, Noellert RC (1985) Complications of carpal tunnel surgery. J Neurosurg 62(3): 352-356.
- Nakamichi K, Tachibana S, Yamamoto S, Ida M (2010) Percutaneous carpal tunnel release compared with mini-open release using ultrasonographic guidance for both techniques. J Hand Surg 35(3):437-445.
- Nakamichi K, Tachibana S (1997) Ultra sonographically assisted carpal tunnel release. J Hand Surg 22(5): 853-862.
- J de la Fuente, Aramendi JF, Ibanez JM, Blasi M, Vazquez A, et al. (2021) Minimally invasive ultrasound-guided vs open release for carpal tunnel syndrome in working population: A randomized controlled trial. J Clin Ultrasound 49(7): 693-703.
- Capa-Grasa A, Rojo-Manaute JM, Rodriguez FC, Martin JV (2014) Ultra-minimally invasive sonographically guided carpal tunnel release: an external pilot study. Orthop Traumatol Surg Res OTSR 100(3): 287-292.
- Rojo-Manaute JM, Capa-Grasa A, Chana-Rodriguez F, Perez-Mananes R, Rodriguez-Maruri G, et al. Ultra-Minimally Invasive Ultrasound-Guided Carpal Tunnel Release: A Randomized Clinical Trial. J Ultrasound Med Off J Am Inst Ultrasound Med 35(6): 1149-1157.
- Zhang S, Wang F, Ke S, Lin C, Liu C, et al. (2019) The Effectiveness of Ultrasound-Guided Steroid Injection Combined with Miniscalpel-Needle Release in the Treatment of Carpal Tunnel Syndrome vs. Steroid Injection Alone: A Randomized Controlled Study. BioMed Res Int 1-9.
- Guo X-Y, Xiong M-X, Lu M, Cheng X-Q, Wu Y-Y, et al. (2018) Ultrasound-guided needle release of the transverse carpal ligament with and without corticosteroid injection for the treatment of carpal tunnel syndrome. J Orthop Surg 13(1): 69.
- Guo X-Y, Xiong M-X, Zhao Y, He F-D, Cheng X-Q, et al. (2017) Comparison of the Clinical Effectiveness of Ultrasound-Guided Corticosteroid Injection with and without Needle Release of the Transverse Carpal Ligament in Carpal Tunnel Syndrome. Eur Neurol 78(1-2): 33-40.
- Buncke G, McCormack B, Bodor M (2013) Ultrasound-guided carpal tunnel release using the manos CTR system. Microsurgery 33(5): 362-366.
- Burnham R, Playfair L, Loh E, Roberts S, Agur A (2017) Evaluation of the Effectiveness and Safety of Ultrasound-Guided Percutaneous Carpal Tunnel Release: A Cadaveric Study. Am J Phys Med Rehabil 96(7): 457-463.
- Candelier G, Apard T (2016) Contribution of Walant in ultrasound-guided surgery for carpal tunnel syndrome, a new hyper-ambulatory approach. Hand Surg Rehabil 35(6): 438-439.
- Chern T-C, Kuo L-C, Shao C-J, Wu T-T, Wu K-C, et al. (2015) Ultrasonographically Guided Percutaneous Carpal Tunnel Release: Early Clinical Experiences and Outcomes. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc 31(12): 2400-2410.
- J de la Fuente, Miguel-Perez MI, Balius R, Guerrero V, Michaud J, et al. (2013) Minimally invasive ultrasound-guided carpal tunnel release: a cadaver study. J Clin Ultrasound JCU 41(2): 101-107.
- Dekimpe C, Andreani O, Camuzard O, Raffaelli C, Petrover D, et al. Ultrasound-guided percutaneous release of the carpal tunnel: comparison of the learning curves of a senior versus a junior operator. A cadaveric study. Skeletal Radiol 48(11): 1803-1809.
- Guo D, Guo D, Guo J, Schmidt SC, Lytie RM (2017) A Clinical Study of the Modified Thread Carpal Tunnel Release. Hand NYN 12(5): 453-460.
- Guo D, Guo D, Guo J, Malone DG, Wei N, et al. (2016) A Cadaveric Study for the Improvement of Thread Carpal Tunnel Release. J Hand Surg 41(10): e351-e357.
- Guo D, Yu T, Ji Y, Sun T, Guo J, et al. (2015) A Non-Scalpel Technique for Minimally Invasive Surgery: Percutaneously Looped Thread Transection of the Transverse Carpal Ligament. Hand 10(1):40-48.
- Hebbard PD, Hebbard AIT, Tomka J, Appleyard R (2018) Ultrasound-Guided Microinvasive Carpal Tunnel Release Using a Novel Retractable Needle-Mounted Blade: A Cadaveric Study. J Ultrasound Med Off J Am Inst Ultrasound Med 37(8): 2075-2081.
- Henning PT, Yang L, Awan T, Lueders D, Pourcho AM (2018) Minimally Invasive Ultrasound-Guided Carpal Tunnel Release: Preliminary Clinical Results. J Ultrasound Med Off J Am Inst Ultrasound Med 37(11): 2699-2706.
- Lecoq B, Hanouz N, Morello R, Jean-Jacques P-Y, Dutheil J-J, et al. (2015) Ultrasound-assisted surgical release of carpal tunnel syndrome: Results of a pilot open-label uncontrolled trial conducted outside the operating theatre. Joint Bone Spine 82(6): 442-445.
- Lecoq B, Hanouz N, Vielpeau C, Marcelli C (2011) Ultrasound-guided percutaneous surgery for carpal tunnel syndrome: a cadaver study. Joint Bone Spine 78(5): 516-518.
- Markison RE (2013) Percutaneous Ultrasound-Guided MANOS Carpal Tunnel Release Technique. Hand 8(4): 445-449.
- Mittal N, Sangha H, Flannery J, Robinson LR, Agur A (2019) Ultrasound‐Guided Incisionless Carpal Tunnel Release Using a Hook Knife: A Cadaveric Study. PM&R 11(10): 1101-1106.
- Ohno K, Hirofuji S, Fujino K, Ishidu T, Kira S, et al. (2016) Sonographic monitoring of endoscopic carpal tunnel release. United States 597-599.
- Ohuchi H, Hattori S, Shinga K, Ichikawa K, Yamada S (2016) Ultrasound-Assisted Endoscopic Carpal Tunnel Release. Arthrosc Tech 5(3): e483-e487.
- Petrover D, Silvera J, De Baere T, Vigan M, Hakime A (2017) Percutaneous Ultrasound-Guided Carpal Tunnel Release: Study Upon Clinical Efficacy and Safety. Cardiovasc Intervent Radiol 40(4): 568-575.
- Petrover D, Richette P Treatment of carpal tunnel syndrome: from ultrasonography to ultrasound guided carpal tunnel release. Joint Bone Spine 85(5): 545-552.
- Rajeswaran G, Healy JC, Lee JC (2016) Percutaneous Release Procedures: Trigger Finger and Carpal Tunnel. Semin Musculoskelet Radiol 20(5): 432-440.
- Rojo-Manaute JM, Capa-Grasa A, Rodriguez-Maruri GE, Moran LM, Martinez MV, et al. (2013) Ultra-minimally invasive sonographically guided carpal tunnel release: anatomic study of a new technique. J Ultrasound Med Off J Am Inst Ultrasound Med 32(1): 131-142.
- Rowe NM, Michaels J, Soltanian H, Dobryansky M, Peimer CA, et al. (2005) Sonographically guided percutaneous carpal tunnel release: an anatomic and cadaveric study. Ann Plast Surg 55(1): 52-56.
- Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM (2002) Single-Portal Endoscopic Carpal Tunnel Release Compared with Open Release: A Prospective, Randomized Trial. J Bone Jt Surg 84(7): 1107-1115.
- Franchignoni F, Vercelli S, Giordano A, Sartorio F, Bravini E, et al. (2014) Minimal Clinically Important Difference of the Disabilities of the Arm, Shoulder and Hand Outcome Measure (DASH) and Its Shortened Version (QuickDASH). J Orthop Sports Phys Ther 44(1): 30-39.
- De Kleermaeker FGCM, Boogaarts HD, Meulstee J, Verhagen WIM. Minimal clinically important difference for the Boston Carpal Tunnel Questionnaire: new insights and review of literature. J Hand Surg Eur 44(3): 283-289.
- Sayegh ET, Strauch RJ (2015) Open versus Endoscopic Carpal Tunnel Release: A Meta-analysis of Randomized Controlled Trials. Clin Orthop Relat Res 473(3): 1120-1132.
- Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC (2007) Surgical treatment options for carpal tunnel syndrome. Cochrane Neuromuscular Group, editor. Cochrane Database Syst Rev.
- Huisstede BM, Randsdorp MS, Coert JH, Glerum S, van Middelkoop M, et al. (2010) Carpal Tunnel Syndrome. Part II: Effectiveness of Surgical Treatments-A Systematic Review. Arch Phys Med Rehabil 91(7): 1005-1024.
-
Javier de la Fuente*, Jose F Aramendi, Davila Fernando, Balius Ramon and Marc Blasi. Percutaneous Ultrasound-Guided Techniques for Carpal Tunnel Release: A Descriptive Review. Glob J Ortho Res. 3(4): 2021. GJOR.MS.ID.000568. DOI: 10.33552/GJOR.2021.03.000568.
-
Minimally invasive surgery, interventional ultrasound, percutaneous technique, carpal tunnel syndrome, Boston carpal tunnel syndrome questionnaire, Carpal tunnel, Carpal tunnel release, Carpal tunnel syndrome, Daily life activities, Flexor retinaculum
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






