 Research Article
Research Article
Does Osteoporosis or Vitamin D Affect the Severity of Minimal Trauma Distal Radius Fractures? A Prospective Cohort Study
Ishvar Nedunchezhian1,2*, Dhruvil Oza2, Donald Ngo1, Luke McCarron1,3, Ann Robinson, Randipsingh Bindra1,2
1Gold Coast University Hospital, Southport QLD, Australia
2Griffith University School of Medicine and Dentistry, Southport QLD, Australia
3Bond University Institute of Health and Sport, Robina QLD, Australia
Corresponding AuthorIshvar Nedunchezhian, 1 Hospital Blvd, Southport QLD, 4215, Australia.
Received Date: January 24, 2022; Published Date: February 07, 2022
Abstract
Objectives: Distal radius fractures (DRF) account for the greatest percentage of upper limb fractures. Osteoporosis and hypovitaminosis D have
been associated with increased risk of minimal trauma DRF, however there is limited data regarding their impact on the severity of DRF. The aim of
this study was to evaluate whether osteoporosis and vitamin D levels influenced the severity of minimal trauma distal radius fracture as measured
by AO/OTA classification, treatment received and functional outcomes.
Methods: This is a prospective cohort study consisting of 45 patients over the age of 50 who presented with minimal trauma DRF. Bone mineral
density (BMD) and serum vitamin D levels were obtained in conjunction with routine patient care, whilst fractures were evaluated using the AO/OTA
classification on plain film. Patients completed the DASH and PRWE questionnaires at least 12 months post injury.
Result: 18 of the 45 patients were found to be osteoporotic, with only 3 being known osteoporotic prior to their DRF. There was no association
between BMD and serum vitamin D in this population (P = 0.257). Osteoporosis or hypovitaminosis D did not impact the classification of fracture,
treatment received or long-term functional outcomes.
Conclusion: BMD and serum vitamin D levels do not appear to influence the severity of DRF in patients with minimal trauma injury over the age
of 50. Vitamin D was not a strong predictor of BMD in this cohort, with socioenvironmental factors a potential reason. Minimal trauma DRF should
be utilised as an early opportunity to screen for osteoporosis.
Level of Evidence: Level II
Keywords: Osteoporosis; Vitamin D; Distal Radius Fracture; Severity; Functional Outcomes
Introduction
Osteoporosis is a metabolic skeletal disease hallmarked by low bone density and alteration of bone microarchitecture. This occurs due to the disruption in bone resorption and remodelling [1,2]. Despite being identified as a significant burden on the Australian health system, costing AUD $3.44 billion in 2017, osteoporosis remains largely ignored in primary health services [3]. Due to the reduced strength of bone in osteoporosis, the characteristic clinical manifestation of osteoporosis is minimal trauma fractures – fractures resulting from trauma equal to or less than a fall from standing height [4]. This is of particular concern in the elderly, with studies demonstrating osteoporotic fractures as major causes of hospitalisation and reduced quality of life [5,6]. Distal radius fractures (DRF) account for the greatest percentage of upper limb fractures, with trends highlighting an increase in incidence [7].
Despite DRF not being as strongly associated with osteoporosis as hip or vertebral fractures, they predict the risk of future fractures, especially in the elderly [2]. Despite overwhelming evidence of the cost effectiveness of implementing strategies to prevent future fractures, osteoporosis diagnosis and management following DRF is frequently overlooked [8].
DRF can be classified on the anatomical location of the fracture with involvement of the articular surface. The AO Foundation/ Orthopaedic Trauma Association (AO/OTA) classification categorises DRF into 3 major categories: completely extraarticular (type A), partially articular (type B) and completely intraarticular (type C) [9]. Intraarticular fractures, compared to extraarticular fractures, are associated with increased long-term complications including posttraumatic osteoarthritis due to articular surface disruption [10].
Management of DRF aims to reduce the fracture, promote bone healing, and restore function through either conservative or surgical options. Conservative management involves closed reduction and use of immobilization via casts and/or splints [11]. Surgical intervention, which includes open reduction and internal fixation (ORIF) or external fixation, has conventionally been viewed as more extreme due to the associated risks compared to conservative management [12]. Complication rates and a greater tendency for surgical intervention mean that intraarticular fractures are generally considered to be more severe than extraarticular fractures.
There are evident gaps in the literature in relation to osteoporosis status, serum vitamin D and their association with DRF classification, modality of treatment and functional outcomes. This study aims to analyse the correlation between bone health and severity of DRF in patients with minimal trauma injuries who present to a major tertiary hospital. Primarily, the objective is to evaluate osteoporosis as a risk factor for severity of DRF as measured by AO classification and modality of treatment received by patients. The secondary aim of this study is to evaluate the relationship of vitamin D status and severity of DRF, as well as its association with BMD in this cohort. Lastly, this study aims to explore any possible association of osteoporosis and hypovitaminosis D on long term functional outcomes.
Methods
Participants
This prospective cohort study examined patients presenting with a fracture of the distal radius to a tertiary level hospital. A total of 208 DRF patients were screened from June 2018 to April 2020. Patients over the age of 50 with a minimal trauma fracture – fractures resulting from trauma equal to or less than a fall from standing height [4] of the distal radius and subsequent BMD scan were included in this study. Exclusion criteria were fractures sustained from substantial trauma and those who had not consented to the study. 45 patients were included in this study. An age restriction of 50 was used as it is the primary recommendation for a presumptive diagnosis of osteoporosis [13]. Ethics was obtained for this study (Medical Research Council, HREC/17/QGC/162), with all patients providing informed consent to the participation in this study.
Bone Mineral Density
BMD was evaluated using dual-energy X-ray absorptiometry (DEXA scan). Patients were referred for DEXA scans as part of routine care following minimal trauma DRF. T-scores for the neck of femur and lumbar spine (L2-L4) were recorded. The World Health Organization (WHO) defines osteoporosis as a bone mineral density (BMD) of -2.5 standard deviations (SD) or less than that of a young healthy female; a T-score ≤-2.5 SD [14]. Osteopenia, the precursor of osteoporosis, is defined as a T-score that reads between -1 to -2.5 SD [14]. T-scores for BMD in the region of lowest density were utilized for statistical analysis.
Vitamin D
Serum 25-hydroxy vitamin D was measured at presentation. Serum Vitamin D level was used as a continuous variable, whilst also being categorised for further analysis. Patients with levels less than 50nmol/L were classified as having vitamin D deficiency, those from 50nmol/L to 150nmol/L were normal and those over 150nmol/L had high vitamin D [15].
AO Classification of fracture
Each patient received routine, standard plain radiography of their injured wrist upon presentation for diagnostic and treatment purposes. Both AP and lateral views were analysed by an orthopedic resident, in consultation with an upper limb orthopaedic surgeon. The fractures were categorised as per the AO/OTA classification of DRF into type A (extra-articular), type B (partially articular) and Type C (completely intraarticular).
Treatment Modality
All 45 participants underwent treatment of their DRF as clinically indicated. Treatment received by the patient’s included reduction and cast immobilisation in the emergency department, and where indicated, surgical fixation. Participants were grouped based on whether they received only cast immobilisation (conservative management) or ORIF (surgical intervention).
Functional Outcome
Functional outcomes were measured using patient-reported outcomes questionnaires, the Disabilities of the Arm, Shoulder and Hand (DASH) and Patient-Rated Wrist Evaluation (PRWE) instruments. These instruments were administered to participants at their last orthopaedic follow up, which occurred at least 12 months post injury.
Loss of Reduction
Loss of fracture reduction rates were captured through follow up clinics, in patients treated in casts. These participants subsequently required an ORIF to ensure appropriate alignment of their fracture.
Statistical Analyses
Data was stratified and categorised based on patient demographics, bone health status/BMD, vitamin D levels, AO/OTA classification of fractures, treatment received, functional outcomes and loss of reduction. Means and standard deviations were used to present continuous variables, whilst categorical data was presented using numbers and percentages. A significance level of p < 0.05 was used. Normality of continuous variables was visualised using histograms and checked using Shapiro-Wilk analysis.
Result
Among the defined 45 participants in this study, 43 were females and 2 were males. The mean patient age was 65.53 years (SD = 8.09), with a range of 52 to 82 years. As per their BMD, 7 participants (16%) were of normal bone health, 20 participants (44%) were osteopenic and 18 participants (40%) were osteoporotic. There was no significant difference (p=0.31) in the age of those who were osteoporotic (mean age = 67.06) and non-osteoporotic (mean age = 64.52).
As per plain film radiographs, 21 participants had a Type A fracture, 9 had a Type B fracture and 15 had a type C fracture. Conservative treatment was utilised for 17 participants and surgical intervention was utilised for 28 participants.
Vitamin D and Bone Mineral Density
40 of the 45 participants had vitamin D testing. Four were found to be vitamin D deficient, 35 were found to have normal vitamin D, whilst one was found to have high vitamin D. Of those who were osteoporotic, all participants were found to have normal vitamin D, whereas two osteopenic and two normal bone health participants had low vitamin D. Table 1 demonstrates this, as well as characteristics of patients in this cohort according to their osteoporosis status (Table 1).
Table 1: Characteristics of patients with DRF according to osteoporosis status.
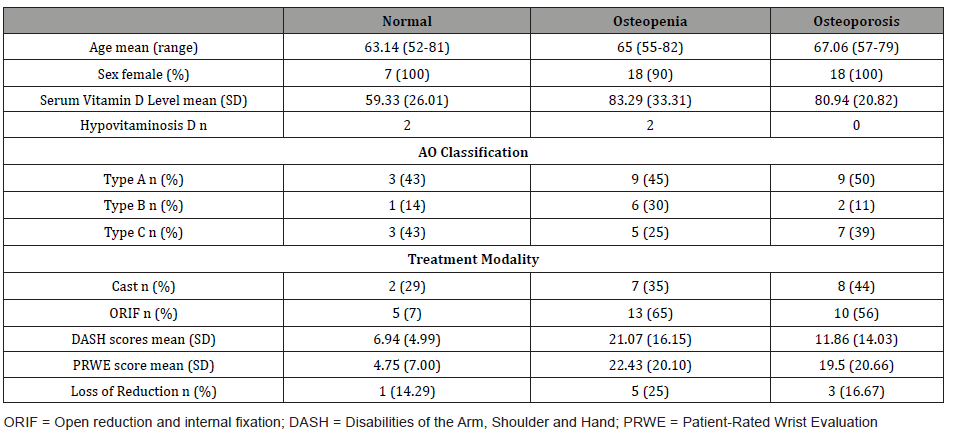
A Fisher’s exact analysis found no significant relation between vitamin D category and osteoporosis status, with the osteoporotic group mean vitamin D levels being 80.94nmol/L, whilst the osteopenic and non-osteoporotic (normal and osteopenic) group mean vitamin D levels being 83.29nmol/L and 77.04nmol/L respectively (p=0.123). Regression analysis found no correlation between vitamin D and T-score on BMD (p=0.257).
The association between bone health and classification of fracture.
(Figure 1) demonstrates the relationship between osteoporosis status and AO classification of fracture, with an apparent trend of type A fracture in patients with osteoporosis and osteopenia. A Fisher’s exact analysis showed no statistical difference between the three fracture types and those who were osteoporotic or nonosteoporotic status (p=0.530). No relation between BMD T-scores and AO classification of fracture on regression analysis was found (p > 0.05).
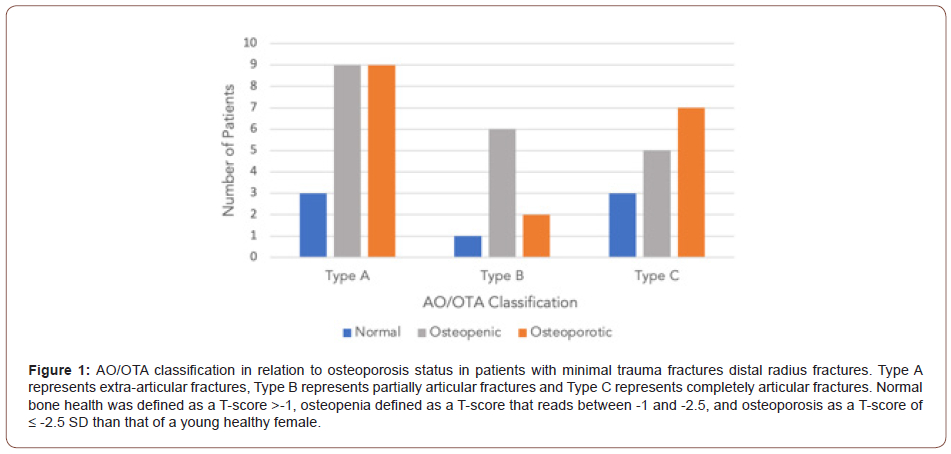
Group analysis through a t-test demonstrated no relationship between osteoporosis and extraarticular or intraarticular fractures (p=0.77). Regression analysis showed no statistical difference between extraarticular or intraarticular fractures in relation to BMD.
The Association Between Bone Health and Treatment Modality
Fisher’s exact analysis showed no statistical association was found between the modality of treatment received and osteoporosis status (p=0.537). A t-test showed no significant difference in the T-score means between treatment modalities.
The Association Between Bone Health and Functional Outcomes
Statistical analysis through ANOVA demonstrated no statistical difference in mean DASH scores (p = 0.09) or PWRE scores (p = 0.16) in relation to osteoporosis status.
The Association Between Vitamin D and Classification of Fracture
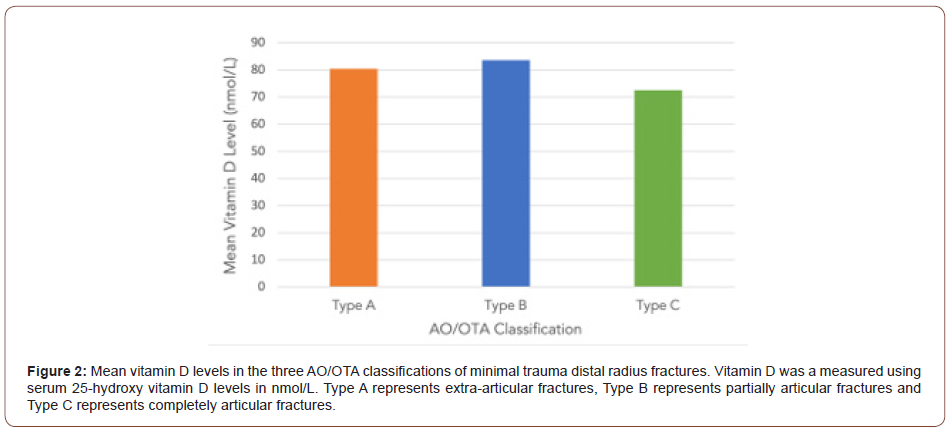
(Figure 2) demonstrates the relationship between mean vitamin D levels and AO classification of fracture. A Fisher’s exact analysis showed no statistical difference between the three fracture types and vitamin D category (p=0.223). There was no correlation between vitamin D levels and AO classification of fracture found on regression analysis (p > 0.05).
Group analysis through a t-test demonstrated no relationship between vitamin D levels and extraarticular or intraarticular fractures (p=0.70).
The Association Between Vitamin D and Treatment Modality
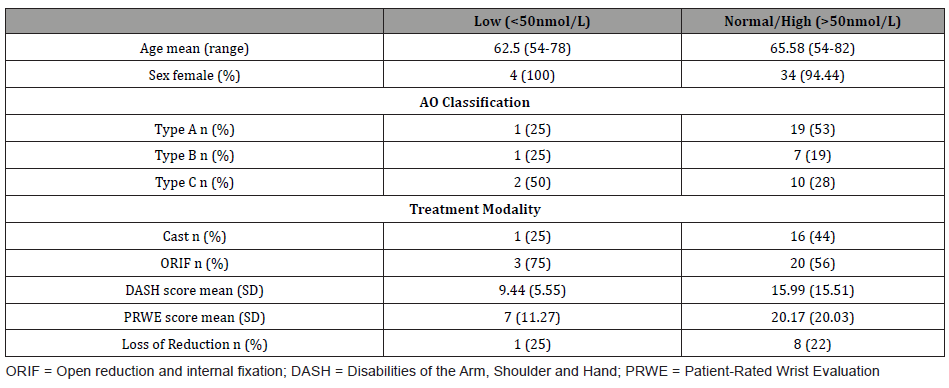
(Table 2) highlights the treatment modality received in relation to their serum vitamin D status, whilst also summarizing results of other pertinent findings. No statistical association was found between the modality of treatment received and vitamin D category (p=0.440) using Fisher’s exact analysis. T-test analysis showed no significant relation between vitamin D levels and treatment modality received.
Table 2: Characteristics of patients with DRF according to serum vitamin D status.
The Association Between Vitamin D and Functional Outcomes
Analysis through t-test showed no significant difference (p = 0.24) in the means of DASH scores in individuals with low vitamin D (mean = 9.44) and those with normal normal/high vitamin D (mean = 15.99). There was no significant difference (p = 0.14) in the means of PRWE scores in individuals with low vitamin D (mean = 7) and those with normal/high vitamin D (mean = 20.17).
Loss of Reduction
A Fisher’s exact analysis showed no statistical difference between loss of reduction in individuals with normal bone health, osteopenia or osteoporosis (p = 0.88). A t-test showed no significant difference between vitamin D levels of those with loss of reduction (mean = 77nmol/L) and those who did not (mean = 79.19nmol/L)
Discussion
Our study demonstrated that 40% of low energy fractures occurred in patients who were osteoporotic and 44% occurred in those who were osteopenic. This result aligns with studies globally that demonstrate variation of osteoporosis prevalence in DRF ranging from 34% to 60% [2]. Furthermore, 96% of our patients were female, and all osteoporotic patients in this study were female. This highlights the greater prevalence of minimal trauma DRF amongst the female population who are known to be at greater risk of osteoporosis [16].
Vitamin D did not appear to impact BMD in the studied cohort. Tahririan [17] found a direct relationship between vitamin D status and BMD. This was supported by Jang [18] and Lee [19]. However, there were reports in different populations which did not support this finding [20-22]. Along with this study conducted, it is hypothesised that there is no definitive relation between low vitamin D and BMD, with other factors including lack of physical activity, smoking and chronic corticosteroid use possibly playing a more important role in BMD [23,24].
This study did not identify any association between a decreased BMD and the nature of DRF as per the AO classification. This result concurs with the current literature, that indicates an inconsistent relationship regarding the severity of DRF in association with BMD.
A prospective cohort study conducted by Clayton [25] discovered that type A fractures were associated with a lower BMD than type B and type C (n=137, p=0.0026), yet found no significant correlation between BMD and the nine subgroups. This finding initially seems counterintuitive, as hypotheses suggest that osteoporosis would lead to more severe fractures, and thus an increased number of intraarticular fractures. Clayton [25], however, postulated that osteoporotic patients may have a greater reduction in BMD in the metaphyseal bone compared to the subchondral bone, leading to increased extraarticular fractures. Hollevoet and Verdonk [26] conducted a similar study which found no significant differences in BMD, clinical or radiological results between intraarticular and extraarticular fractures (n=40). Hjelle [27] conducted a study in Norway viewing patients who sustained both low and high energy DRF and found that those with osteoporosis did not sustain more complex fractures than those with osteopenia or normal BMD, as per the AO classification. Dhainaut [10] demonstrated no significant association between classical BMD and severity of fracture, whilst also finding no relationship between decreased hand cortical BMD and the nature of DRF. Our data correlates with these studies. Overall, this suggests that neither osteopenia not osteoporosis are major risk factors for the severity of minimal trauma DRF.
Likewise, studies analyzing the effect of BMD on fracture severity at other anatomical locations also yielded inconclusive findings. Spencer [24] analysed nine variables, including bone density, and reported no association between BMD and severity of hip fracture. In comparison, Cauley [28] found that reduced BMD led to less severe fractures of the hip. Studies analysing proximal humerus fractures concluded that complex fractures are not necessarily linked to poorer bone quality based on their insignificant association found on analysis of local bone structure, cortical index and severity of fracture [29,30].
Our study also found no significant difference in the treatment modality received based on patients’ bone health. This suggests that osteoporosis does not lead to an increased risk of requiring surgical intervention compared to those who have healthier bones. It is known that more severe fractures require greater surgical intervention. However, as this study found no association between the severity of fracture and bone health, it is expected that there was no association between treatment modalities received. Furthermore, surgical intervention is advised based on both radiographic and clinical indications. As there was no association between central BMD and treatment modality received, it was extrapolated that reduced BMD did not affect the radiographic parameters utilised to determine if surgical management of DRF is required. Xie and Barenholdt [31] investigated one such parameter, displacement of DRF, and found no relation in regard to central BMD. A study conducted by Hollevoet and Verdonk [26], analysing forearm BMD, found that reduced BMD at the distal forearm resulted in increased ulnar variance, however found no relation to palmar tilt or radial inclination.
Our study also found individuals with poorer BMD did not have an increased reduction loss compared to those with healthier bones, whilst serum vitamin D levels also did not affect loss of fracture reduction. This suggests that neither osteoporosis nor hypovitaminosis D led to poorer union of fractures that are initially managed conservatively.
In addition to this, there was no significant association between vitamin D level and the nature of DRF in the studied population. Osteomalacia has been described to be a risk factor for fractures, including DRF [32-34]. However, in the studied cohort, only 10% were found to be low in serum vitamin D (n=40). There is limited data regarding the prevalence of hypovitaminosis D in DRF compared to other fragility fractures. Despite this, the rates in our study are significantly less than rates found in other regions, which range from 18-49% [18, 34,35].
The region studied has historically low levels of hypovitaminosis D. Of particular note is that the study populations’ rate of 10% is almost identical to the general state population rate of 11% [36]. This highlights that vitamin D is not a major risk factor for minimal trauma DRF in Southeast Queensland. A study conducted by Awal et al. in the South-East Queensland region also identified low levels of vitamin D deficiency in proximal femur fractures compared to other regions globally [37]. This consolidate that the low rates of hypovitaminosis D can be attributed to other factors, including socioenvironmental factors such as a tropical environment, high UV index and an active outdoor lifestyle. This highlights that osteomalacia is not a strong predictor for DRF severity, especially in vitamin D replete populations.
A pertinent clinical finding from this study was that individuals’ long-term functional outcomes following DRF were not affected by bone mineral density or vitamin D levels. This keeps in line with the findings that BMD or serum vitamin D did not affect the severity of DRF, and thus do not affect the clinical and functional outcomes. Despite literature regarding functional outcomes in those suffering from osteoporosis being vague and indeterminate, there are arguments for early recognition of those suffering from osteoporosis and consideration of closer monitoring to ensure preventative measures are commenced earlier than later [26,38- 40].
Only three of the 38 participants with osteopenia or osteoporosis were known to have osteopenia or osteoporosis prior to their presenting injury. This emphasises that low energy DRF are often the first presentation of osteoporosis in patients. This supports the notion that referring for secondary fracture prevention care in DRF is an early opportunity to screen for osteoporosis to prevent more serious future fractures in the older population.
There are some limitations noted in this study. There was no control group without fractures for comparison. Due to the strict inclusion criteria, the study had a small number of participants. Furthermore, despite being underpowered, the number in this study is within the participant range used in similar studies and thus comparable to current literature [10, 25-27].
In summary, BMD and serum vitamin D do not appear to influence the severity of DRF or functional outcomes in patients with minimal trauma fracture over the age of 50. Vitamin D deficiency was not a strong predictor of BMD in this cohort. Additionally, minimal trauma DRF should be considered a sentinel event and an opportunity to screen for osteoporosis to prevent risk of future fracture.
Conflicts of Interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
Ethical approval for this study was obtained from Medical Research Council (HREC/17/QGC/162)
Acknowledgement
We would like to thank Ian Hughes for his guidance for this study.
References
- Miyamura S, Kuriyama K, Ebina K, Oka K, Kashii M et al. (2020) Utility of Distal Forearm DXA as a Screening Tool for Primary Osteoporotic Fragility Fractures of the Distal Radius: A Case-Control Study. JB JS Open Access 5(1): e0036.
- Niempoog S, Sukkarnkosol S, Boontanapibul K (2019) Prevalence of Osteoporosis in Patients with Distal Radius Fracture from Low-Energy Trauma. Malays Orthop J 13(3):15-20.
- Tatangelo G, Watts J, Lim K, Connaughton C, Abimanyi-Ochom J et al. (2019) The Cost of Osteoporosis, Osteopenia, and Associated Fractures in Australia in 2017. J Bone Miner Res 34(4):616-625.
- Warriner AH, Patkar NM, Curtis JR, Delzell E, Gary L et al. Which fractures are most attributable to osteoporosis? Journal of clinical epidemiology. 64(1):46-53.
- Singer A, Exuzides A, Spangler L, O'Malley C, Colby C et al. (2015) Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin Proc 90(1):53-62.
- Weycker D, Li X, Barron R, Bornheimer R, Chandler D (2016) Hospitalizations for osteoporosis-related fractures: Economic costs and clinical outcomes. Bone Rep 5:186-91.
- Ostergaard PJ, Hall MJ, Rozental TD (2019) Considerations in the Treatment of Osteoporotic Distal Radius Fractures in Elderly Patients. Curr Rev Musculoskelet Med 12(1):50-6.
- The Royal Australian College of General Practitioners. Clinical guideline for the prevention and treatment of osteoporosis in postmenopausal women and older men 2010 [cited 2021 March 13].
- Meinberg EG, Agel J, Roberts CS, Karam MD, Kellam JF (2018) Fracture and Dislocation Classification Compendium-2018. J Orthop Trauma 32 Suppl 1:S1-S170.
- Dhainaut A, Daibes K, Odinsson A, Hoff M, Syversen U, Haugeberg G (2014) Exploring the relationship between bone density and severity of distal radius fragility fracture in women. J Orthop Surg Res 9:57.
- Chung KC, Shauver MJ, Birkmeyer JD (2009) Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am 91(8):1868-73.
- Padegimas EM, Osei DA (2013) Evaluation and treatment of osetoporotic distal radius fracture in the elderly patient. Curr Rev Musculoskelet Med 6(1):41-46.
- The Royal Australian College of General Practitioners and Osteoporosis Australia. Osteoporosis prevention, diagnosis and management in postmenopausal women and men over 50 years of age 2017 [cited 2021 March 13].
- Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359(9321):1929-36.
- Lucas R (2014) What is the optimal level of vitamin D? Aust Fam Physician 43(10):670-1.
- Nellans KW, Kowalski E, Chung KC (2012) The epidemiology of distal radius fractures. Hand Clin 28(2):113-125.
- Tahririan MA, Motififard M, Omidian A, Aghdam HA, Esmaeali A (2017) Relationship between Bone Mineral Density and Serum Vitamin D with Low Energy Hip and Distal Radius Fractures: A Case-Control Study. Arch Bone Jt Surg 5(1):22-27.
- Jang WY, Chung MS, Baek GH, Song CH, Cho HE et al. (2012) Vitamin D levels in post-menopausal Korean women with a distal radius fracture. Injury 43(2):237-41.
- Lee JO, Chung MS, Baek GH, Oh JH, Lee YH et al. (2010) Age- and site-related bone mineral densities in Korean women with a distal radius fracture compared with the reference Korean female population. J Hand Surg Am 35(9):1435-41.
- Christodoulou S, Goula T, Ververidis A, Drosos G (2013) Vitamin D and bone disease. Biomed Res Int 2013:396541.
- Hosseinpanah F, Rambod M, Hossein nejad A, Larijani B, Azizi F (2008) Association between vitamin D and bone mineral density in Iranian postmenopausal women. J Bone Miner Metab 26(1):86-92.
- Tsai KS, Wahner HW, Offord KP, Melton LJ 3rd, Kumar R et al. (1987) Effect of aging on vitamin D stores and bone density in women. Calcif Tissue Int 40(5):241-3.
- Pouresmaeili F, Kamalidehghan B, Kamarehei M, Goh YM (2018) A comprehensive overview on osteoporosis and its risk factors. Ther Clin Risk Manag. 14:2029-49.
- Spencer SJ, Blyth MJ, Lovell F, Holt G (2012) Does bone mineral density affect hip fracture severity? Orthopedics 35(6): e945-9.
- Clayton RA, Gaston MS, Ralston SH, Court-Brown CM, McQueen MM (2009) Association between decreased bone mineral density and severity of distal radial fractures. J Bone Joint Surg Am 91(3):613-9.
- Hollevoet N, Verdonk R (2003) Outcome of distal radius fractures in relation to bone mineral density. Acta Orthop Belg 69(6):510-4.
- Hjelle AM, Gjertsen JE, Apalset EM, Nilsen RM, Lober A et al. (2020) No association between osteoporosis and AO classification of distal radius fractures: an observational study of 289 patients. BMC Musculoskelet Disord 21(1):811.
- Cauley JA, Lui LY, Genant HK, Salamone L, Browner W et al. (2009) Risk factors for severity and type of the hip fracture. J Bone Miner Res 24(5):943-55.
- Den Teuling J, Pauwels BS, Janssen L, Wyers CE, Janzing HMJ et al. (2017) The influence of bone mineral density and cortical index on the complexity of fractures of the proximal humerus. Bone Joint Res 6(10):584-9.
- Lee SY, Kwon SS, Kim TH, Shin SJ (2016) Is central skeleton bone quality a predictor of the severity of proximal humeral fractures? Injury 47(12):2777-82.
- Xie X, Barenholdt O (2001) Bone density and geometric properties of the distal radius in displaced and undisplaced Colles' fractures: quantitative CT in 70 women. Acta Orthop Scand 72(1):62-6.
- Larrosa M, Gomez A, Casado E, Moreno M, Vazquez I et al (2012) Hypovitaminosis D as a risk factor of hip fracture severity. Osteoporos Int 23(2):607-14.
- Muzzammil M, Kumar R, Minhas MS, Kumar V, Jahanzed S et al. (2019) Inadequate vitamin D level: association with low energy fractures of distal radius in young patients and its predictors in Karachi, Pakistan 2019 6(1):6.
- Oyen J, Apalset EM, Gjesdal CG, Brudvik C, Lie SA et al. (2011) Vitamin D inadequacy is associated with low-energy distal radius fractures: a case-control study. Bone. 48(5):1140-5.
- Wright S, Beringer T, Taggart H, Keegan D, Kelly J et al. (2007) A study of male patients with forearm fracture in Northern Ireland. Clin Rheumatol 26(2):191-5.
- Australian Bureau of Statistics. Australian Health Survey: Biomedical Results for Nutrients [Internet]. Commonwealth of Australia; 2013 [cited 2021 March 13]
- Awal W, Bindra R, Price N, Sadler A, Robinson A et al. (2020) Vitamin D deficiency in proximal femur fracture patients of South-East Queensland. Australas J Ageing 39(3): e271-e7.
- Buyukkurt CD, Bulbul M, Ayanoglu S, Esenyel CZ, Ozturk K et al. (2012) The effects of osteoporosis on functional outcome in patients with distal radius fracture treated with plate osteosynthesis. Acta Orthop Traumatol Turc 46(2):89-95.
- Choi WS, Lee HJ, Kim DY, Lee CH, Lee BG, Kim JH, et al. (2015) Does osteoporosis have a negative effect on the functional outcome of an osteoporotic distal radial fracture treated with a volar locking plate? Bone Joint J 97-B (2):229-34.
- Hollevoet N, Goemaere S, Mortier F, Van Bouchaute P, Kaufman JM et al. (2000) The role of osteoporosis in distal radius fractures. Acta Orthop Belg 66(2):163-8.
-
Ishvar Nedunchezhian, Dhruvil Oza, Donald Ngo, Luke McCarron, Ann Robinson, Randy Bindra. Does Osteoporosis or Vitamin D Affect the Severity of Minimal Trauma Distal Radius Fractures? A Prospective Cohort Study. Glob J Ortho Res. 3(4): 2022. GJOR.MS.ID.000570. DOI: 10.33552/GJOR.2022.03.000570.
-
Osteoporosis, Vitamin D, Distal Radius Fracture, Severity, Functional Outcomes, Metabolic Skeletal Disease, Trauma Fractures, Bone Mineral Density, Loss of Reduction
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






