 Original Article
Original Article
The Efficacy of Apple Mint Extract as a Nutraceutical Supplement for Skin Brightening: A Double-Blind, Randomized, Placebo-Controlled Clinical Study
Julia Baumann1*, Marta De Oliveira Ferreira2, Marta Monteiro2, Ines Mota2, Patrizia Alves2 and Torsten Grothe1
1Mibelle Group Biochemistry, Bolimattstrasse 1, 5033 Buchs, Switzerland
2Inovapotek, UPTEC, Science and Technology Park of University of Porto, 4200-135 Porto, Portugal
Julia Baumann, Mibelle Group Biochemistry, Bolimattstrasse 1, 5033 Buchs, Switzerland
Received Date: February 7, 2025; Published Date: February 19, 2025
Summary
Exposure to environmental stressors, such as UV radiation, pollution, and thermal stress, can lead to skin hyperpigmentation, resulting in uneven skin tone and dark spots. Nutraceutical skin lightening treatments have become a topic of increasing interest as individuals aim to reduce skin hyperpigmentation, particularly the use of botanical extracts. The current study investigated the potential of apple mint extract as a natural skinbrightening solution. In vitro experiments demonstrated that the extract stimulates genes involved in cellular stress response, including superoxide dismutase 3, and inhibits melanin synthesis in human melanocytes. A randomized, double-blind, placebo-controlled clinical trial involving 110 healthy female subjects showed that oral supplementation with apple mint powder significantly increased skin lightness, improved skin tone evenness, and reduced melanin variation after 84 days. These findings suggest that apple mint extract may be an effective and safe natural ingredient for skin brightening and depigmentation, offering a promising alternative to synthetic skin-lightening agents.
Keywords: Mentha suaveolens Erh; cell stress response; melanogenesis inhibition; skin lightening.
Introduction
Being continuously exposed to the environment, the human skin is particularly susceptible to external factors, which can lead to premature skin-aging such as skin hyperpigmentation and age spots [1]. As such, skin lightening treatments have become a topic of increasing interest as individuals aim to reduce skin hyperpigmentation to achieve a more radiant, even-toned complexion [2,3]. Skin pigmentation and melanin synthesis are influenced by various factors, including ultraviolet (UV) exposure, genetics, hormonal changes, and skin injuries (post-inflammatory hyperpigmentation) [4,5]. Melanin pigment production occurs in specialized cell organelles termed melanosomes, which are located within the melanocytes in the basal layer of the epidermis [6]. The melanogenesis process begins with the enzyme tyrosinase catalyzing the oxidation of the amino acid tyrosine to L-DOPA and then to dopaquinone [7]. Dopaquinone then undergoes further chemical reactions to form the two primary types of melanin: eumelanin (black and brown pigments) and pheomelanin (red and yellow pigments) [8]. Tyrosinase, being the rate-limiting enzyme in this pathway, plays a central role in melanin synthesis and skin pigmentation [9]. Once melanin is synthesized, melanosomes are transferred along the dendrites of melanocytes to neighboring keratinocytes where they are distributed around the nucleus, providing protection against (UV) radiation by absorbing and dissipating UV energy [10].
Increased melanin synthesis, driven primarily by the upregulation of tyrosinase activity, can lead to hyperpigmentation and the appearance of age spots or discoloration [1,9]. Dysregulation of this enzymatic pathway is often triggered by stress factors such as UV exposure [11] and inflammation [12]. Additionally, UV-induced ROS can contribute to oxidative stress, further exacerbating pigmentation issues and accelerating signs of aging [13]. Addressing both the regulation of melanin synthesis and the management of oxidative stress presents a promising strategy to combat skin hyperpigmentation. While topical skincare products have long been the focus of research and development in this area, there is growing recognition that nutritional supplements may also play a critical role in improving the appearance and overall health of the skin [2]. Dietary supplements, particularly those derived from natural sources, are being investigated to target skin pigmentation, counteract oxidative stress, and promote a more youthful, vibrant complexion from within [14-16]. These supplements can provide a complementary approach to topical treatments, potentially offering additional benefits for skin health and appearance. In addition to skin brightening, nutritional supplements have shown promise in addressing a wide range of skin concerns, such as signs of aging, inflammation, and overall skin barrier function [16-18]. As the scientific understanding of the skin-related benefits of dietary compounds continues to evolve, the use of nutraceuticals as part of a comprehensive skin care regimen is likely to become increasingly prevalent.
A bioactive compound that has garnered significant attention in this regard is glutathione, a powerful antioxidant that has been extensively studied for its potential to enhance skin brightness both topically and systemically [19-21]. However, the stability and bioavailability of glutathione can be challenging [22,23], leading researchers to explore alternative sources of skin-brightening actives, including botanical extracts. Such botanicals often contain an array of bioactive compounds that may target various aspects of skin health, including pigmentation, oxidative stress, and inflammation. One such botanical is the apple mint (Mentha suaveolens Ehrh.), a member of the mint family (Lamiaceae), native to Eurasia and Northern Africa [24]. It is further cultivated in various regions in Europe, including Switzerland and Austria. Mint species are generally rich in antioxidant polyphenols (rosmarinic acid, salvionalic acid) and flavonoids such as luteolin [25]. Particularly salvionalic acid is known for its antioxidant and anti-aging effects, moreover it has demonstrated brightening properties [26,27]. In line with this, apple mint has demonstrated a wide range of effects including antioxidant, anti-inflammatory and antimicrobial [28,29]. In vitro, protective effects of an aqueous extract of apple mint was observed against oxidative stress-induced damages in human keratinocyte cells [30]. Further, an apple mint extract protected human dermal fibroblasts from heat-shock induced damages and demonstrated anti-aging effects [28]. Given the welldocumented biological activities of mint phytochemicals, the apple mint is a promising candidate for a dietary supplement aimed at skin brightening. To address this, the following study investigated the effects of daily oral supplementation with apple mint extract on reducing skin pigmentation.
Materials and Methods
Apple mint extract preparation
The apple mint extract was obtained from the dried leaves of the plant (Österreichische Bergkräutergenossenschaft, Hirschbach, Austria). The process began with a water-ethanol extraction with 32.5% ethanol at 52–57°C for 2 hours by maceration. After, the solution was filtered and centrifuged to remove solid plant debris, and the clarified extract solution was subjected to vacuum evaporation, with 30% maltodextrin (Glucidex19, Roquette, France) added as a carrier. After ultra-high temperature (UHT) treatment at 120°C, the extract was spray-dried to obtain the finished plant extract powder. All studies were performed using the described powder extract (“apple mint extract”, MintyBright™ Nu, provided by Mibelle Group Biochemistry, Buchs, Switzerland).
Keratinocyte culture and treatment
Primary normal human epidermal keratinocytes (NHEK, (31)) were cultured in culture medium consisting of Keratinocyte- SFM (serum-free media, GibcoTM, Thermo Fischer Scientific, USA) supplemented with human recombinant epidermal growth factor (rEGF, 50 mg/L, GibcoTM) and bovine pituitary extract (5 μg/L, GibcoTM), in a humidified incubator at 37 °C and 5 % CO2. Prior to the gene expression analysis, the cells were seeded to 24-well plates and cultured for 24 hours in culture media. The cells were then cultured for further 24 hours in assay medium consisting of Keratinocyte-SFM without growth factors or antibiotics. The medium was then replaced by assay medium containing or not (control) 0.4 mg/mL apple mint extract and the cells incubated for 24 hours. After exposure, the cells were washed in phosphate buffer saline (PBS) and immediately frozen at – 80 °C.
Gene expression analysis
Gene expression was evaluated with reverse transcription quantitative real-time PCR (RT-qPCR). Total RNA was extracted from each cell sample using TriPureTM isolation reagent (Roche, Switzerland) according to the manufacturer’s instructions. RNA quality was assessed using capillary electrophoresis (Bioanalyzer 2100, Agilent Technologies, USA) and the quantity determined using a spectrophotometer (Synergy H1, BioTek Instruments, USA). cDNA was synthesized by reverse transcription of total RNA with oligo(dT) primers and Transcriptor Reverse Transcriptase (Roche). The SYBR Green I qPCR was performed with 25 ng cDNA per sample on a LightCycler® system (Roche Molecular Systems, USA) with primers for HSPB1, HSPA6, SOD3, DCN and GAPDH. Data was normalized to GAPDH, and fold changes were calculated based on the comparative ΔΔCt method [32].
Melanocyte culture and treatment
Primary human melanocytes were isolated from neonatal foreskin following circumcision surgery [33], seeded to 96-well plates and cultured in melanocyte growth medium (PromoCell, Germany) until confluency. For the melanin content evaluation, the cells were then incubated for 72 h in the presence of 0.02 mg/mL apple mint extract or glutathione (GSH, G6013-5G, Sigma-Aldrich, USA).
Melanin content assay
After the incubation period cells were lysed in 1M NaOH. A fraction of the lysate was used to determine total protein content [35]. The absorbance of the melanocyte lysates was then measured at 405 nm using a spectrophotometer (Agilent Technologies) and total melanin content was calculated against a melanin standard curve ranging from 1.25 μg/ml to 80 μg/mL. Total melanin content was then normalized to total protein content, and results expressed as ng / μg protein.
Clinical Study
Participants
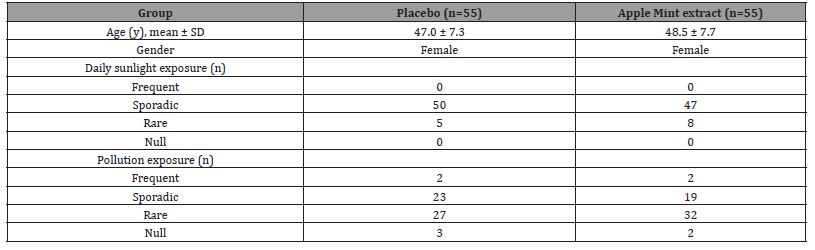
154 healthy female volunteers were screened of which 110 female healthy subjects, aged 25 – 60 years, presenting mild to moderate fine lines and wrinkles, mild to moderate skin spots and phototype III – V according to the Fitzpatrick phototyping scale, were included in the clinical study (Table 1). Main exclusion criteria were smoking, males, medical history of systemic diseases or dermatological conditions, damaged facial skin, performed major skin treatments (i.e. peelings, microderm abrasion, depigmenting treatments, retinoids) 30 days prior or with intent to perform during the trial, pregnancy, an occupation or lifestyle with excessive sun exposure, evidence of drug and/or alcohol abuse, intake of any kind nutritional supplements (vitamins, antioxidant, herbal), or intake of medication that might affect the study outcome. All participants were informed of the purpose of the study prior enrolment and included participants provided written and informed consent. The standard protocol and test conditions were submitted to and approved by the Ethical Committee of Inovapotek Pharmaceutical Research and Development Lda (Code number P016C23) and registered in the database for Protocol Registration and Results System ClinicalTrials.gov PRS (NCT06453837).
Table 1: Anemia.
Study design and product intake
The study was performed in a monocentric, double-blind, randomized, and placebo-controlled design. Participants attended an environmentally controlled facility for the evaluation of skin parameters. A 2-week washout period was performed if subjects had taken any supplements prior to study start (Figure 1). Following baseline determination (T0) of the skin parameters defined in the sections below, the subjects were randomly divided into two groups and instructed to ingest the test product (150 mg apple mint extract) or the corresponding placebo (150 mg maltodextrin) by oral intake once a day, in the morning, for 84 days. The treatments were provided as capsule preparation (hydroxypropylmethylcellulose) in neutral plastic flasks. Skin parameters were measured before treatment (T0) and after 84 days (T1). All participants were instructed to refrain from excessive sunlight exposure for the duration of the study and to apply sunscreen 15-30 mins before sun exposure. Subjects were requested to keep a diary and note sun exposure, SPF usage, protocol deviations, and any observed reactions or discomfort. The study was performed under dermatological surveillance.
Efficacy Evaluations
All skin parameters were measured before (T0) and after 84 (T1) consecutive days of product intake. Skin brightening was determined by measuring skin lightness (L*) with Antera 3D (Miravex, Ltd., Ireland). Skin colour was evaluated by measuring the Individual Typology Angle (ITA°) with a Colorimeter® CL400 (Courage+Khazaka electronic GmbH, Germany). Skin tone evenness was evaluated by assessing the melanin variation (MV) with Antera 3D (Miravex, Ltd., Ireland). The melanin variation is inversely proportional to the uniformity of the pigment, with a lower melanin variation corresponding to a higher degree of uniformity (more homogeneous skin tone). Further, standardized macrophotographs are taken with Visia-CR (Canfield Scientific Europe, Netherlands). Mean differences of each parameter were determined by subtracting the baseline results (T0) to the results obtained after 84 days (T1). Results were calculated as changes in absolute values and as % change compared to baseline, individually calculated for each participant. All study procedures were carried out under controlled conditions, with the room temperature set at 23.0 +/- 1.0 ºC and relative humidity at 50 +/- 10 %. A 20-minute acclimatization period of the subject’s exposed area was respected before the evaluations.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8 software (GraphPad, USA). Preclinical data was tested using unpaired Student’s T-test. Results are depicted as mean ± SD. The clinical data was tested for normality (Shapiro-Wilk) to assess normal distribution. If normal distribution, the data was verified with paired-sample T-tests to compare results obtained for each skin parameter before (baseline) and after treatment. Independent t-tests were applied to compare the differences to baseline obtained with the investigational product treatment versus the placebo. For non-normal distributions, Wilcoxon-tests were applied to compare the results obtained for each skin parameter before (baseline) and after treatment. Mann-Whitney U tests were applied to compare the differences to baseline obtained with the investigational product treatment versus placebo. Results are depicted as mean ± SEM. P values < 0.05 were considered statistically significant.
Results
Apple mint extract stimulates genes involved cellular stress response
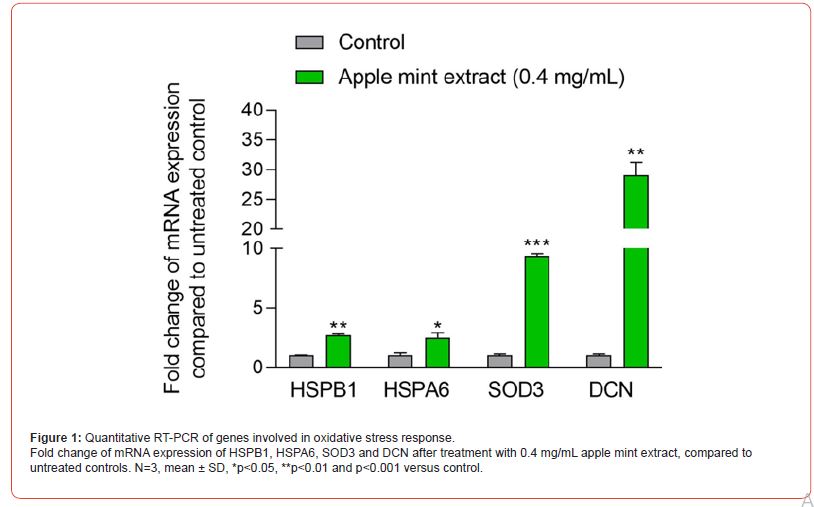
Treating normal human epidermal keratinocytes with the apple mint extract significantly upregulated genes critical in oxidative and cellular stress responses, such as heat shock protein family B (small) member 1 (HSPB1, 2.50, p<0.01), heat shock protein family A (Hsp70) member 6 (HSPA6, 2.27, p<0.05), and superoxide dismutase 3 (SOD3, +7.63, p<0.001) (Figure 2). SOD3 has been shown to inhibit UVB-induced melanogenesis (4), therefore its increased production by keratinocytes may have inhibitory effects on environmental stress-induced skin pigmentation. In addition, decorin (DCN), a proteoglycan of the extracellular matrix (ECM) with strong anti-inflammatory and antioxidant properties (5), was strongly upregulated (+ 25.65, p<0.01). As such, these findings highlight the potential of apple mint extract in systemic nutraceutical applications aimed at skin brightening.
Extract treatment reduces melanin synthesis in vitro
The inhibitory potential of the apple mint extract on melanin synthesis was assessed with a melanin content assay with primary human melanocytes. Glutathione (GSH) was used as a positive control at equivalent concentrations as apple mint extract. Compared to control, treatment with 0.02 mg/mL apple mint extract significantly inhibited melanin synthesis (p<0.01), comparable to the positive control kojic acid (Figure 3). In contrast, 0.02 mg/mL GSH moderately inhibited melanin content, the results were nonsignificant. This suggests that the apple mint extract demonstrates nearly equivalent potency in promoting skin brightening as compared kojic acid, and even outperformed the benchmark GSH in this assay.


Clinical compliance and subject characteristics
The clinical study was conducted from June 21st, 2023, until March 8th, 2024. After a two-week washout, the 110 enrolled subjects were randomized into two groups, with the verum group supplementing 150 mg apple mint extract (n=55) in a capsule preparation daily, or the placebo group with 150 mg maltodextrin (n=55). In the placebo group, 2 subjects were lost due to noncompliance, 1 withdrew due to mild erythema on the belly area, and 2 withdrew due to sleep problems. In the verum group with apple mint extract, 2 subjects were lost due to non-compliance, 3 subjects withdrew consent for personal reasons (travel plans, vacation, relocation) and 1 subject withdrew due to sleep problems (Figure 2). A comparison of the baseline characteristics (age, gender, daily sunlight and pollution exposure) and across treatment groups is summarized in the table (Table 1) below. No significant differences were observed between the treatment groups for the baseline characteristics. The product was in general well tolerated with no subjects reporting any serious adverse events.
Skin brightening effects of apple mint extract supplementation
Table 2: Changes in lightness (L*), individual typology angle (ITA°) and melanin variation (MV) after 84 days supplementation of placebo or apple mint extract



The nutritional supplementation of apple mint extract had beneficial effects on skin brightening. The numerical values of the evaluated skin parameters at baseline, after treatments, as well as the delta and % changes are depicted in Table 2. After 84 days, a significant increase in L* was observed, in comparison to both baseline (p<0.001) and placebo (p<0.05) (Figure 4). Furthermore, the apple mint extract supplementation led to a significant increase in mean ITA° (p<0.001), whereas the placebo showed no effect. Compared to baseline, apple mint extract supplementation led to a mean increase of ITA° of 15.78 ± 3.94 % (Figure 4). Melanin variation (MV), a parameter to determine skin tone evenness, was evaluated to yield additional insight into the skin depigmenting effects. After 84 days of apple mint extract treatment, a significant decrease of melanin variation was observed compared to baseline (p<0.05). In contrast, the placebo treatment showed no effect. This indicates that the apple mint extract can improve skin tone evenness, leading to a more even complexion. The skin brightening clinical effects were also visible in the images taken (Figure 5).
Discussion
Environmental stress, including UV irradiation, oxidative stress and pollution, can lead to increased skin pigmentation [35]. This is accomplished in part through the activation of melanogenic proteins such as the enzyme tyrosinase or oxidative-stress induced skin damage, which can further affect skin colour and tone [11,13,36]. This study highlights the anti-oxidative and antimelanogenetic effects of an apple mint extract in cell models. The observed antioxidant properties demonstrated by the extract suggest it may be effective in neutralizing free radicals and reactive oxygen species, thereby reducing oxidative stress-induced skin damage and discoloration. As such, the preclinical observations were confirmed in the placebo-controlled clinical study, where the apple mint supplementation resulted in decreased melanin variation and overall increased skin lightness in volunteers with Fitzpatrick III-V skin tones. These findings align with previous research on the potential skin-related benefits of botanical extracts, which have been attributed to their diverse phytochemical profiles. Several studies have implicated that providing natural extracts rich in antioxidants can combat oxidative stress and help prevent melanin synthesis [14,15,37,38]. For example, previous research on oral supplementation with a pine bark extract (Pinus pinaster) demonstrated its skin lightening effects after 56 days [39]. Further, a formulation containing liquorice and grape (seed and pomace) extracts has been shown to inhibit tyrosinase activity and reduce melanin production, leading to improved skin lightness and reduced dark spots over 12 weeks [37]. Similarly, the carotenoids lutein and zeaxanthin, extracted from dried marigold flowers, have been found to increase skin tone and lightness (L*), after 12 weeks daily supplementation [14]. Furthermore, colourless carotenoids like phytofluene and phytoene, known to be present in white tomato extracts, are reported for their potential to reduce skin pigmentation and melasma [40], but at higher applicated dosages in comparison to the flavonoid-based apple mint extract investigated in this study. Flavonoids are very promising nutraceuticals for melanogenesis inhibition, based on detailed knowledge about potentially underlying molecular mechanisms and their multitarget properties [41]. These findings emphasize the strength of natural botanicals in addressing skin hyperpigmentation, offering a promising alternative to synthetic skin-brightening actives.
Both intrinsic and extrinsic factors can drive pigmentary changes, especially in the aging skin. Increasing evidence is establishing a role of skin aging in hyperpigmentation [42]. Interestingly, despite decreased melanocyte activity during aging, older exposed skin is predominantly characterised by increased pigmentation, including localized hyperpigmentation such as age spots (lentigo senile, solar lentigo) and melasma [43,44]. Such localized areas of hyperpigmentation are related to increased epidermal melanin synthesis and deposition, most commonly triggered by prolonged UV exposure [45]. Furthermore, melasma have distinct histopathologic features that are similar to photoaged skin, such as decreased elasticity, increased vascularization and basement membrane changes [46]. UV-induced oxidative stress and cellular damage can in part be mitigated by antioxidant enzymes, such as superoxide dismutase (SOD). Among them, the extracellular superoxide dismutase (SOD3) has been implicated in regulating melanocyte homeostasis. In a murine melanocyte cell line, SOD3 inhibited UVB induced ROS production and melanogenesis [47]. Furthermore, overexpression of SOD3 in mice prevented UVBinduced skin pigmentation [47]. As such, the observed significant stimulation of SOD3 by the apple mint extract may promote the skin’s stress response against UV-exposure, thereby preventing excessive skin pigmentation.
In addition to UV radiation, thermal stress plays a significant role in skin aging. The human skin is frequently exposed to considerable amounts of infrared (IR) radiation and visible light from sunlight, which can increase the skin temperature and induce skin aging [48,49]. As such, heat stress due to IR and visible light from sunlight has been shown to affect melanogenesis in vitro [50] and pigmentation clinically [51,52]. Several in vitro and ex vivo have demonstrated that exposure to increased temperature can affect skin pigmentation. A study on human skin explants found enhanced pigmentation when cultured at 39 °C and 41 °C, compared to 37 °C [53]. Furthermore, conditioned media from heat-treated HaCaT cells (39 °C, 41 °C, 43 °C versus 37 °C control) promoted melanogenesis in a melanoma cell line MNT1 [53]. Heat shock proteins (HSPs) play a crucial role in the cellular response to thermal stress, impacting both thermal aging and pigmentation. HSPs, such as HSP70 (heat shock 70kDa protein) and HSPB1 (heat shock protein beta-1, also known as HSP27), are induced by heat and other stressors, functioning as molecular chaperones to maintain protein homeostasis and protect cells from damage [54]. In the context of thermal aging, HSPs help reduce the harmful effects of heat on skin cells by preventing protein denaturation and aggregation, thereby maintaining cellular integrity and function [55]. Additionally, HSPs are involved in the regulation of melanogenesis. For instance, HSP70 has been shown to influence the activity of melanocytes, the cells responsible for pigment production, thereby affecting skin pigmentation [56]. The dual role of HSPs in protecting against thermal damage and regulating pigmentation underscores their importance in maintaining skin health under thermal stress conditions.
Our in vitro evidence demonstrated that the apple mint extract stimulated the gene expression of HSPB1 (heat shock protein beta- 1) and HSPA6 (heat HSP70 member 6). In response to environmental stress, the encoded protein of HSPB1 translocates to the nucleus and functions as a molecular chaperone to prevent aggregates and promote correct protein folding [57]. Similarly, HSPA6 is essential for cell survival when exposed to increased temperatures [58]. By enhancing the skin’s natural defence mechanisms through the upregulation of HSPs, the apple mint extract may offer a novel approach to prevent heat stress-induced skin pigmentation. Indeed, a study of Son et al. demonstrated that apple mint leaf extract has activity against thermal aging in human dermal fibroblasts [28]. The extract demonstrated significant antioxidant activities, prevented the expression of MMPs, as well as the formation of ROS, and suppressed the activity of the mitogen-activated proteins kinases (MAPKs) that were induced by heat shock treatment. These findings highlight the importance of considering thermal stress responses in developing effective skin-brightening solutions and further position the apple mint extract as a promising candidate for this approach.
Conclusion
In conclusion, this study highlights that apple mint extract could be an effective skin-brightening solution due to its antioxidant and anti-melanogenic properties. Aside from sun exposure and UV radiation, the impact of thermal stress on skin pigmentation is an interesting and emerging avenue to explore when addressing skin brightening pathways. Exploring the molecular mechanisms behind the extract’s action further could provide additional insights into its skin brightening potential. This study evidences that botanical extracts can be utilized as natural, safe and effective actives to combat hyperpigmentation and enhance overall skin health.
Conflicts of interest
Julia Baumann and Torsten Grothe are employed by Mibelle Group Biochemistry. The authors declare that no conflict of interest exists.
Acknowledgements
We acknowledge our colleagues Florian Hofer, Cornelia Schürch, Fred Zülli, Franziska Wandrey and Kathrin Nowak for scientific support and sample preparation. Furthermore, we acknowledge our colleagues Juliana Gouveia, Cândida Kringel, Claudia Pinto e Vera Pimenta for protocol design and elaboration, data analysis and subjects’ recruitment.
Funding
The research was funded by Mibelle Group Biochemistry.
References
- Lee AY (2021) Skin Pigmentation Abnormalities and Their Possible Relationship with Skin Aging. Int J Mol Sci 22(7): 3727.
- Juhasz MLW, Levin MK (2018) The role of systemic treatments for skin lightening. J Cosmet Dermatol 17(6): 1144-1157.
- Parente JBM, Silva GS, Gotschall JW, Ferreira AL, Grant-Kels JM (2024) Cosmetic skin lightening: Contextualizing biomedical and ethical issues. Clin Dermatol 42(5): 513-514.
- Brenner M, Hearing VJ (2008) The Protective Role of Melanin Against UV Damage in Human Skin. Photochem Photobiol 84(3): 539-49.
- Costin GE, Hearing VJ (2007) Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J 21(4): 976-94.
- Slominski A, Tobin Dj Fau Shibahara S, Shibahara S Fau - Wortsman J, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. (0031-9333 (Print)).
- Park HY, Kosmadaki M, Yaar M, Gilchrest BA (2009) Cellular mechanisms regulating human melanogenesis. Cell Mol Life Sci 66(9): 1493-506.
- Ito S, Wakamatsu K (2003) Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res 16(5): 523-531.
- Hearing VJ, Jiménez M (1987) Mammalian tyrosinase--the critical regulatory control point in melanocyte pigmentation. Int J Biochem 19(12): 1141-7.
- Ando H, Niki Y, Ito M, Akiyama K, Matsui MS, Yarosh DB, et al (2012) Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol 132(4): 1222-9.
- Gilchrest BA, Eller MS (1999) DNA photodamage stimulates melanogenesis and other photoprotective responses. J Investig Dermatol Symp Proc 4(1): 35-40.
- Fu C, Chen J, Lu J, Yi L, Tong X, et al (2020) Roles of inflammation factors in melanogenesis (Review). Mol Med Rep 21(3): 1421-1430.
- Schallreuter KU, Kothari S, Chavan B, Spencer JD (2008) Regulation of melanogenesis –controversies and new concepts. Exp Dermato l7(5): 395-404.
- Juturu V, Bowman JP, Deshpande J (2016) Overall skin tone and skin-lightening-improving effects with oral supplementation of lutein and zeaxanthin isomers: a double-blind, placebo-controlled clinical trial. Clin Cosmet Investig Dermatol 9: 325-332.
- Kee Kim J, Hyun Park N, Sung Hwang J, Hoon Baek J, Woo Jeong H (2018) Effect of Oral Intake of A Mixture Containing Natural Citrus Peel Extract, Vitamin C, and L-Cystine on Skin Brightness in Healthy Women. Journal of Food and Nutrition Research 6(10): 655-659.
- Tarshish E, Hermoni K (2023) Beauty from within: Improvement of skin health and appearance with Lycomato a tomato-derived oral supplement. J Cosmet Dermatol 22(6):1786-1798.
- Baumann J, Wandrey F, Nowak K, Grothe T (2024) Nutritional supplementation of an apple callus extract to target epidermal aging. Journal of Food Nutrition and Diet Science 2(1): 18-28.
- Wandrey F, Pickel C, Jongsma E, Ewald CY, Grothe T (2021) Evaluation of the collagen-boosting effects of a Moldavian dragonhead extract. Journal of Community Medicine and Public Health Reports.1-9.
- Weschawalit S, Thongthip S, Phutrakool P, Asawanonda P (2017) Glutathione and its antiaging and antimelanogenic effects. Clin Cosmet Investig Dermatol 10: 147-153.
- Watanabe F, Hashizume E, Chan GP, Kamimura A (2014) Skin-whitening and skin-condition-improving effects of topical oxidized glutathione: a double-blind and placebo-controlled clinical trial in healthy women. Clin Cosmet Investig Dermatol 7: 267-74.
- Arjinpathana N, Asawanonda P (2012) Glutathione as an oral whitening agent: a randomized, double-blind, placebo-controlled study. J Dermatolog Treat 23(2): 97-102.
- Witschi A, Reddy S, Stofer B, Lauterburg BH (1992) The systemic availability of oral glutathione. Eur J Clin Pharmacol 43(6): 667-9.
- Chung BY, Choi SR, Moon IJ, Park CW, Kim YH, et al (2016) The Glutathione Derivative, GSH Monoethyl Ester, May Effectively Whiten Skin but GSH Does Not. Int J Mol Sci 17(5): 629.
- Bozovic M, Pirolli A, Ragno R (2015) Mentha suaveolens Ehrh. (Lamiaceae) Essential Oil and Its Main Constituent Piperitenone Oxide: Biological Activities and Chemistry. Molecules 20(5): 8605-33.
- Tafrihi M, Imran M, Tufail T, Gondal TA, Caruso G, et al (2021) The Wonderful Activities of the Genus Mentha: Not Only Antioxidant Properties. Molecules 26(4): 1118.
- Zuo Z, He S, Qiu Y, Guo R, He Y, Jiao C, et al. (2024) Salvianolic acid A prevents UV-induced skin damage by inhibiting the cGAS-STING pathway. Int Immunopharmacol 10: 132:111971.
- Sun H, Shen Y, Zhang Y, Zhou L (2024) Potential Beneficial Effects of Salvianic Acid A and Salvianolic Acid B in Skin Whitening. Natural Product Communications 19(1):1934578X231219604.
- Son D, Kim M, Woo H, Park D, Jung E (2018) Anti-Thermal Skin Aging Activity of Aqueous Extracts Derived from Apple Mint (Mentha suaveolens Ehrh.) in Human Dermal Fibroblasts. Evid Based Complement Alternat Med. 4595982.
- Moreno L, Bello R, Primo-Yufera E, Esplugues J (2002) Pharmacological properties of the methanol extract from Mentha suaveolens Ehrh. Phytother Res. 16 Suppl 1:S10-3.
- Lee H, Yeom M, Shin S, Jeon K, Park D, et al. (2019) Protective Effects of Aqueous Extract of Mentha suaveolens against Oxidative Stress-Induced Damages in Human Keratinocyte HaCaT Cells. Evid Based Complement Alternat Med. 5045491.
- Bernard FX, Pedretti N, Rosdy M, Deguercy A (2002) Comparison of gene expression profiles in human keratinocyte mono-layer cultures, reconstituted epidermis and normal human skin; transcriptional effects of retinoid treatments in reconstituted human epidermis. Exp Dermatol 11(1): 59-74.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4): 402-408.
- Gilchrest BA, Vrabel MA, Flynn E, Szabo G (1984) Selective Cultivation of Human Melanocytes from Newborn and Adult Epidermis. Journal of Investigative Dermatology 83(5): 370-376.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72: 248-254.
- Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T (2017) The skin aging exposome. J Dermatol Sci 85(3):152-161.
- Papaccio F, A DA, Caputo S, Bellei B (2022) Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants (Basel) 11(6).
- Pouchieu C, Pourtau L, Gaudout D, Gille I, Chalothorn K, Perin F (2023) Effect of an Oral Formulation on Skin Lightening: Results from In Vitro Tyrosinase Inhibition to a Double-Blind Randomized Placebo-Controlled Clinical Study in Healthy Asian Participants. Cosmetics. 10(5).
- Nahhas AF, Abdel-Malek ZA, Kohli I, Braunberger TL, Lim HW, et al. (2019) The potential role of antioxidants in mitigating skin hyperpigmentation resulting from ultraviolet and visible light-induced oxidative stress. Photodermatol Photoimmunol Photomed. 35(6): 420-428.
- Piriou Y, Sirvent A, Natalizio A, Girard-ory F (2014) Skin-lightening and anti-ageing effect of a food supplement containing Pinus pinaster extract. Nutrafoods 13(3):123-131.
- Von Oppen-Bezalel L, Havas F, Ramot O, Kalo E, Fishbein D, et al. (2014) Phytoene and phytofluene for (photo) protection, anti aging, lightening and evening of skin tone. SOFW J. 140: 8-12.
- Liu-Smith F, Meyskens FL (2016) Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol Nutr Food Res. 60(6):1264-1274.
- Yang Y, Wu Y, Xiang L, Picardo M, Zhang C (2025) Deciphering the role of skin aging in pigmentary disorders. Free Radic Biol Med. 227: 638-655.
- Kang HY, Lee JW, Papaccio F, Bellei B, Picardo M (2021) Alterations of the pigmentation system in the aging process. Pigment Cell Melanoma Res. 34(4): 800-813.
- Unver N, Freyschmidt-Paul P, Horster S, Wenck H, Stab F, et al. (2006) Alterations in the epidermal-dermal melanin axis and factor XIIIa melanophages in senile lentigo and ageing skin. Br J Dermatol. 155(1): 119-128.
- Lee AY (2015) Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res 28(6): 648-660.
- Passeron T, Picardo M (2018) Melasma a photoaging disorder. Pigment Cell Melanoma Res. 31(4): 461-465.
- Kim H-Y, Sah SK, Choi SS, Kim T-Y (2018) Inhibitory effects of extracellular superoxide dismutase on ultraviolet B-induced melanogenesis in murine skin and melanocytes. Life Sciences 210: 201-218.
- Cho S, Shin MH, Kim YK, Seo JE, Lee YM, Park CH, et al. (2009) Effects of infrared radiation and heat on human skin aging in vivo. J Investig Dermatol Symp Proc 14(1): 15-19.
- Schieke SM, Schroeder P, Krutmann J (2003) Cutaneous effects of infrared radiation: from clinical observations to molecular response mechanisms. Photodermatol Photoimmunol Photomed. 19(5):228-34.
- Nakazawa K, Sahuc F, Damour O, Collombel C, Nakazawa H (1998) Regulatory effects of heat on normal human melanocyte growth and melanogenesis: comparative study with UVB. J Invest Dermatol. 110(6): 972-977.
- Ramasubramaniam R, Roy A, Sharma B, Nagalakshmi S (2011) Are there mechanistic differences between ultraviolet and visible radiation induced skin pigmentation? Photochem Photobiol Sci. 10(12):1887-1893.
- Singh N, Wigmann C, Vijay P, Phuleria HC, Kress S, Majmudar G, et al. (2024) Combined Effect of Ambient Temperature and Relative Humidity on Skin Aging Phenotypes in the Era of Climate Change: Results From an Indian Cohort Study. Dermatitis.
- Zhang L, Zeng H, Jiang L, Fu C, Zhang Y, Hu Y, et al. (2023) Heat promotes melanogenesis by increasing the paracrine effects in keratinocytes via the TRPV3/Ca(2+)/Hh signaling pathway. iScience. 26(5):106749.
- Gomez CR (2021) Role of heat shock proteins in aging and chronic inflammatory diseases. Geroscience. 43(5): 2515-2532.
- Scieglinska D, Krawczyk Z, Sojka DR, Gogler-Piglowska A (2019) Heat shock proteins in the physiology and pathophysiology of epidermal keratinocytes. Cell Stress Chaperones. 24(6):1027-1044.
- Hoshino T, Matsuda M, Yamashita Y, Takehara M, Fukuya M, Mineda K, et al. (2010) Suppression of melanin production by expression of HSP70. J Biol Chem 285(17):13254-13263.
- Phan TM, Berkeley RF, Plonski AP, Debelouchina GT, Mittal J (2024) Small heat shock protein HSPB1: Molecular insights into its structural complexity and chaperone activity. Biophysical Journal. 123(3):58a.
- Ramirez VP, Stamatis M, Shmukler A, Aneskievich BJ (2015) Basal and stress-inducible expression of HSPA6 in human keratinocytes is regulated by negative and positive promoter regions. Cell Stress Chaperones 20(1): 95-107
-
Julia Baumann*, Marta De Oliveira Ferreira, Marta Monteiro, Ines Mota, Patrizia Alves and Torsten Grothe. The Efficacy of Apple Mint Extract as a Nutraceutical Supplement for Skin Brightening: A Double-Blind, Randomized, Placebo-Controlled Clinical Study. 5(3): 2025. GJNFS.MS.ID.000615.
-
Mentha suaveolens Erh; cell stress response; melanogenesis inhibition; skin lightening
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






