 Review Article
Review Article
Use of Hydrogels in Brain Applications
Haider Butt*
Department of Mechanical Engineering, Associate Professor, Khalifa University of Science and Technology, Abu Dhabi, UAE
Haider Butt, Department of Mechanical Engineering, Associate Professor, Khalifa University of Science and Technology, Abu Dhabi, UAE.
Received Date: January 12, 2020; Published Date: January 16, 2020
Abstract
In the last decades, an exponential and rapid evolution has been seen in hydrogel studies. This all started when for the very first-time hydrogels were used in eye surgery. Hydrogels made their way in the medical application because of its biodegradable nature. Now a day’s hydrogels applications can be found in many fields including contact lenses, wearable sensors for muscular activities, biodegradables hydrogels for reconstructive surgery and many more. All of these classes have been continuous studies with time to improve the efficiency and filed of applications. Beside all these applications, recently hydrogels were used for tissue engineering especially as a scaffold for stem cells. It was found that hydrogels play an important role in brain application including the treatment of brain tumors. This review covers the use of hydrogels in brain applications.
Introduction
Hydrogel is a three-dimensional network composed of polymers that are hydrophilic in nature. As a result, hydrogel structures can retain a substantial quantity of water. Functional groups like -NH2, -SO3H, -OH, -CONH2, -COOH provide hydrophilicity to hydrogels. Wichterle & Lím [1] reported hydrogels for the very first time in 1960. These structures are flexible and contain at least 10% of water (by volume or weight). Sometimes they are also named hungry networks. Hydrogels respond to external stimuli (physical or chemical) and undergo a phase transition. The external stimuli consist of pressure, magnetic field, light intensity, temperature, chemical composition, and pH and more. But the transition is usually reversible which means hydrogels return to their initial state after the stimuli are removed. It should be noted that not all the monomers or hydrogels react similarly to different stimuli. The functional groups present in the hydrogels make them responsive to various stimuli [2-4]. Hydrogels have the potential to find their uses in many fields; oil recovery, agriculture, biosensors, biotechnology, pharmaceutical, to name a few. Other applications involve their use as superabsorbent diapers [5], drug delivery [6], tissue regeneration [7], cell cultures [8] and contact lenses [9].
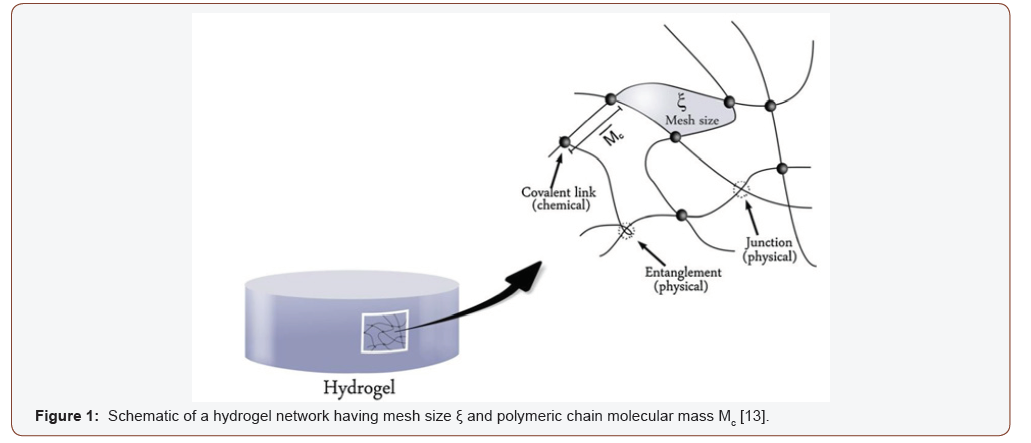
Hydrogels can be classified into two categories; i) physically cross-linked, ii) chemically cross-linked. Chemically cross-linked hydrogels are insoluble in water unless the covalent bond in the network is broken first [10]. The network of physical hydrogels is formed by the non-covalent cross-linking of monomers, e.g., electrostatic interaction [11]. Usually, hydrogels are inert in nature which makes hydrogels one of the best candidates to be used as biomaterials [12]. The response of hydrogels depends on their composition, degree of cross-linking and type of cross-linking. Tailoring these properties may allow us to synthesize applicationbased hydrogels. Mesh size is an important factor that determines the space available for external particles to enter or leave the hydrogel network and is controlled by the degree of cross-linking [13] (see Figure 1).
There are certain properties of hydrogel structures that are hunted when being used for sensing applications; i) the degree of wetness and inertness of the structure that makes it qualified to be a hydrogel, ii) the degree of sensitivity or response to the external stimuli like change in swelling, and iii) the diffusion control of particles through their polymeric structures [14-16]. The theory of swelling needs to be understood which is essential for sensing applications of hydrogels. The following section will describe the swelling theory of hydrogels.
In hydrogel network, osmotic pressure is created by the hydrophilicity of monomers which results in the swelling of structure upon contact with water [17]. The swelling occurs in three stages; i) first the water diffuses into the structure, ii) after hydration, the polymer chains relax, and iii) lastly, they expand upon relation [17,18]. There is a limit to which hydrogels can absorb water. This limit or so-called equilibrium is attained when the osmotic pressure becomes equal to the elastic counteractive forces of polymeric chains in hydrogel structure. The expansion stops at this equilibrium state. When osmotic pressure changes due to any kind of activity, for example by breakage of bond or change in pH value, the equilibrium is disturbed which results in the swelling or shrinking of hydrogel structure to stabilize. Flory & Rehner [19,20] have described the theory of swelling in detail using the free energy and chemical potential of hydrogels.
The response of hydrogels, when they encounter external stimuli, is in the form of volume-phase transitions (VPT) which occurs due to sudden changes in the hydrogel network. These sudden changes are the product of molecular interactions with the external particles or stimuli. Therefore, it can be stated that the stimulus determines the response mechanism of hydrogels. There are several types of hydrogels that respond differently to different stimuli. Most researched hydrogels are those that respond to pH changes leading to volume-phase transitions. Consider having a system where we have a hydrogel that has a pH value equal to pKa (acidic nature) and an environment having a pH value greater than pKa. This difference results in the deprotonation of the hydrogel, causing a negative charge to appear on polymeric chains or functional groups attached to the monomers to be more accurate which leads to the repulsion of polymeric chains and hence the swelling of the network. An example of this mechanism can be found with poly (methacrylic acid) (PMAA) structure [21]. However, if this ionized acidic network is protonated or the pH of the environment is lowered than the pH of the network, the structure returns from the swollen state to its initial state because of the reduction of electrostatic repulsive forces between the ions on the polymeric chains. Similar behavior exists in the hydrogel structures with cationic functional groups (basic nature), but in an opposite fashion [22].
Status of Development
Hydrogel sensors were reported for the detection of Glutamate in vivo [23]. Glutamate is one of the most important excitatory neurotransmitters in the brain. Osmium is recycled through a chemical reaction and acts as an electron transfer mediator. These sensors were hydrogel-based second generation sensors. They were obtained using carbon fiber which used redox mediator wired coating. They were reported previously reported by Kulagina et al. [24]. Anesthetized rats were used for this experiment. It was found that a very less amount of glutamate oxides was used in these sensors when compared to the first-generation sensors.
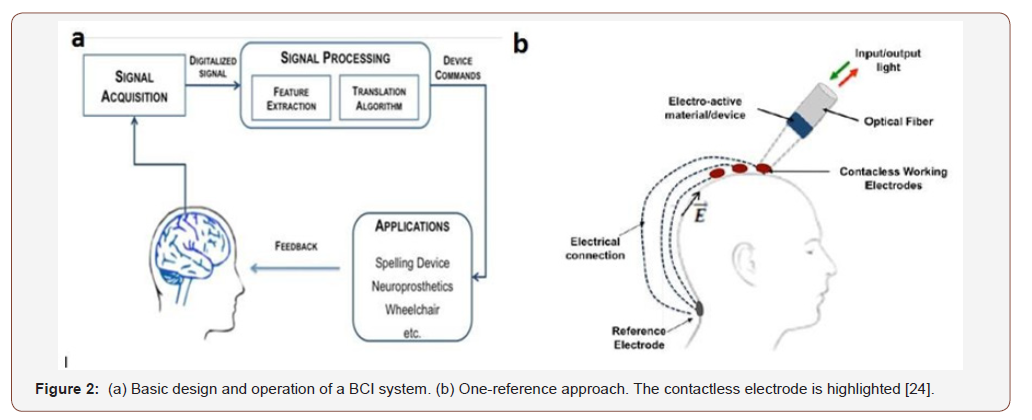
In 2009, a wearable brain cap was reported in the 4th International IEEE EMBS Conference on Neural Engineering [25]. This cap showed the ability to measure electroencephalogram (EEG) signals. Unlike other caps that use electric contact, this cap doesn’t require any electric contact with the head. Hydrogel materials were used for cap manufacturing. These contacts do not need any electric contact between its surface and scalp. On the other hand, these contacts are flexible, and their integration is easy in the wearable cap. This cap plays a vital role in the basic braincomputer interfaces (BCI) system (See Error! Reference source not found (a). On the other hand, Error! Reference source not found (b). highlighted one reference approach using contactless electrodes. The electrode used an electro-active hydrogel, polyacrylamide hydrogel (PAAM), for sensing and modulation of the sensor. This work provided a solution to the uncomfortable attachment of caps for the BCI system to monitor the EEG signal by reducing the attachment procedure and time consumption. Right after this, another detailed report was published by the same authors with the same results in 2010 [26].
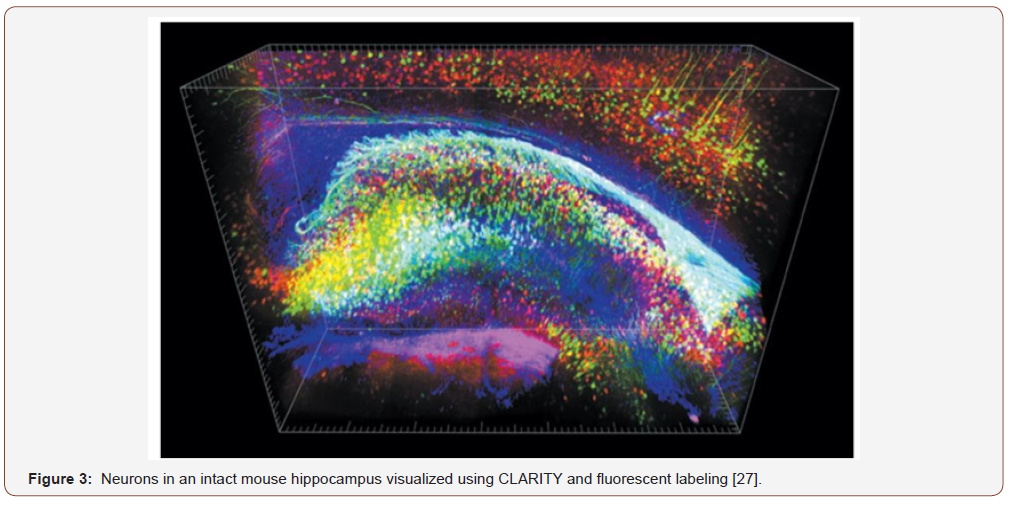
One of the milestones for the usage of hydrogel for brain applications was reported by Shen [27]. A new technique for the imaging of a brain, called Clear Lipid-exchanged, Anatomically Rigid, Imaging/immunostaining compatible, Tissue hydrogel (CLARITY), was developed in Stanford University. This technique made it possible to get the whole image of a mouse brain and make it possible to label a lot of molecules. This technique involved the use of hydrogel to remove fat lipid layer without disrupting the cell structure and hold the rest of the components in place. This process is called hydrogel-hybrid tissue formation. This is done by the formation of formaldehyde-acrylamide fixative-generated hydrogel mesh. By using this cellular lipid is removed using sodium dodecyl sulfate [28]. This makes the whole brain transparent to light but also permeable to molecules (Figure 2).
This leads scientists to highlight specific features by adding molecular markers. By doing this one can create a brain library that can be studied by others. Figure 3 shows the 1mm block of the hippocampus where green, red and blue represent excitatory neuron, inhibitory neuron, and astrocytes, respectively. This was said that this technique works in the human brain too. By looking at someone’s brain and match those with molecular information make it possible for scientists to know how a disease can make changes to the brain. Many other groups have presented different reports talking about how to find an ideal solution for the detection of fluorescent-labeled signal and tissue clearing [28,29].
Later, CLARITY was used together with magnetic resonance imaging (MRI) [30] to investigate myelination contribution to measurable from diffusion tensor imaging. In neuroscience, diffusion tensor imaging (DTI) is widely used to estimate the white matter microarchitecture (WM). It was found that, in WM regions, fractional anisotropy is sensitive to myelination. The relationship between the diffusion signal and its biological underpinnings revealed for the very first time in the framework of brain-wide immunolabeling of WM targets using combined DTI-CLARITY.
Different case studies using CLARITY has been done on Parkinson’s mouse model [31], Alzheimer’s disease patients (postmortem brain tissue) [32], post-mortem human brain tissue (died 6 years ago) [33] and to visualize Lewy pathology of Parkinson’s disease patient [34] (Figure 3).
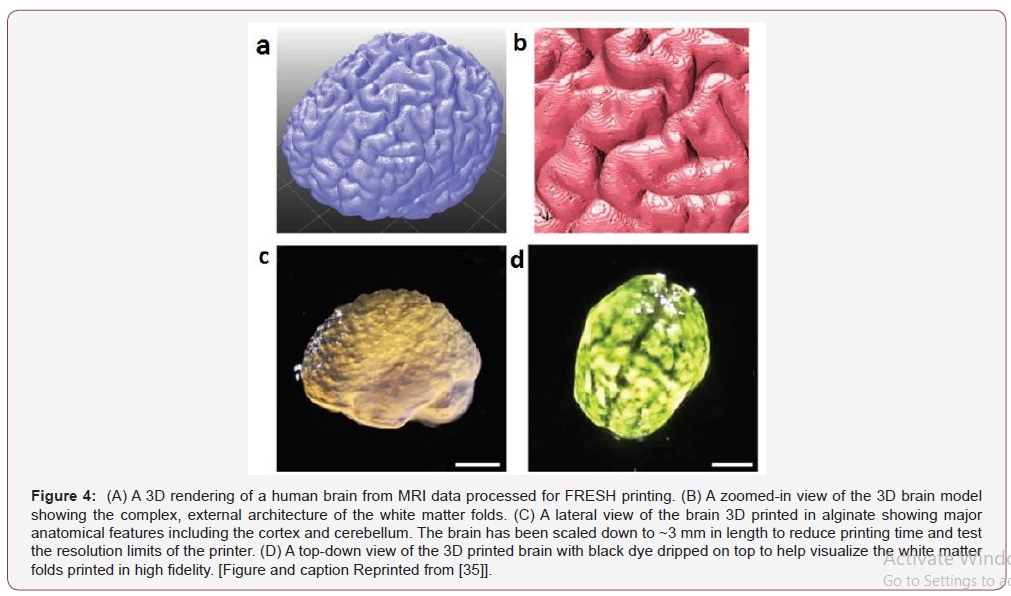
A report published in 2015 presented the hydrogel for the 3D printing applications [35]. This work reported the printing of complex external structures of the human brain using alginate. A high-quality 3D brain model was obtained using an MRI image of the brain. This model defined the different parts of the brain including temporal and frontal lobes of the cortex and the cerebellum. This printing technique using hydrogel has presented the unique ability to print complex structures.
In 2018, an injectable hydrogel was reported for the human umbilical cord mesenchymal stem cells (hUC-MSCs) implantation [36]. This hydrogel contained hyaluronic acid and sodium alginate. This provided a microenvironment for the implant by acting as a scaffold. This research was carried out using SD rats. The injecting performance was satisfied by the gelation time of more than 6 min. This hydrogel scaffold presented suitable rheological behavior for matching of brain tissue and decent compatibility for stem cells (Figure 4).
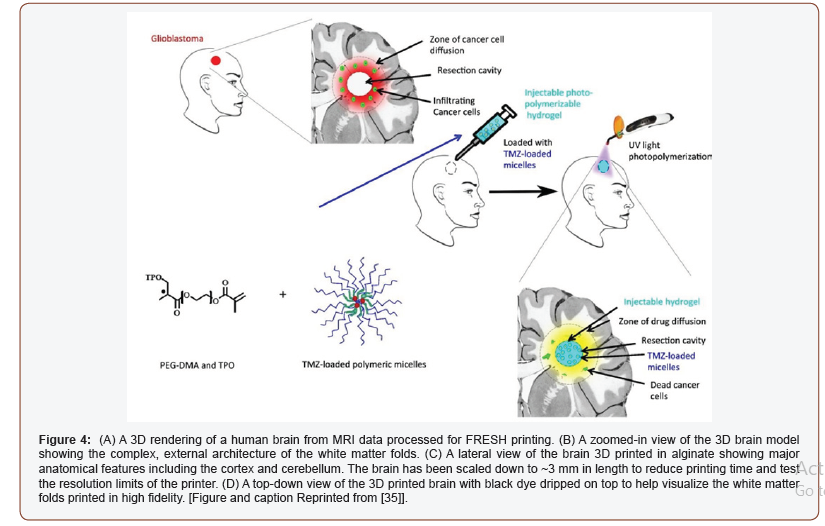
In the advancement of hydrogel application for brain application, a published report demonstrated the use of hydrogel for the treatment of brain tumors (glioblastoma) [37]. Local drug delivery Temozolomide (TMZ) into the brain was done using photopolymerizable PEG-DMA-based hydrogel. The systematic of injectable hydrogel drug delivery can be seen in Figure 5. The figure is self-explanatory where glioblastoma was treated with injectable polymerizable hydrogel loaded with TMZ- loaded micelles. Photopolymerization was done using UV light which ends up killing cancer cells. In another study, the hydrogel was combined with paclitaxel (PTX) nanoparticles for the treatment of brain tumors (glioma) [38]. An MRI image was used to prepare the 3D structure of the tumor for the local delivery of paclitaxel. In the end, it was found that nanocomposite hydrogel can be used as a potential system in the improvement of the therapeutic efficacy of PTX (Figure 5).
Ultra-thermosensitive hydrogel was reported in 2017 which helps in brain tumor recurrence by developing a local drug delivery system [39]. Because of the ultra thermosensitivity (operational temperature ≤28 ᵒC) of these hydrogels make their use possible after surgical resection (temperature of operation theater is always ≤30 ᵒC). This solved the problem of conventional thermosensitive hydrogel which operates on temperature ≥32 ᵒC that can cause the gel formation failure in tissue.
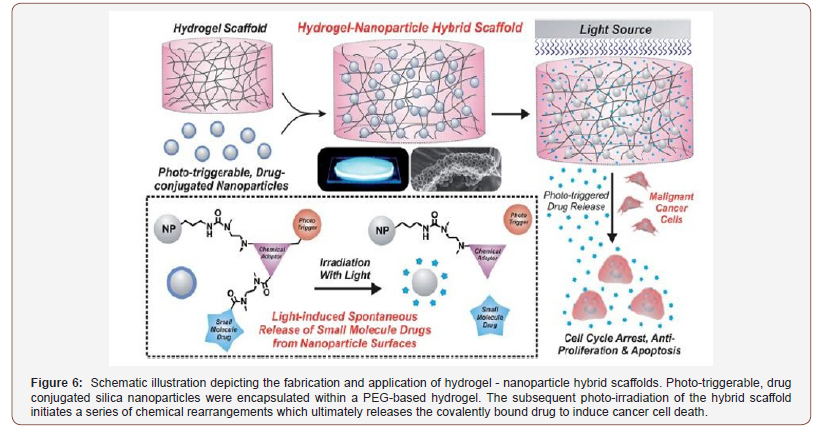
Besides the local drug delivery system, a remote drug delivery system is also important for clinical needs and the patient’s physiological response. A new nanoparticle hydrogel system was reported that can control the on-demand drug delivery of chemically defined small drug molecules [40]. The use of phototriggerable chemical compounds makes it a remotely-triggerable system. Induced light triggers the photo- triggerable material to start intermolecular rearrangements. This leads to the separation and release of the drug from the hydrogel. The systematic of this process can be seen in Figure 6.
For the temperature/pH sensitivity brain tumors can be monitored by magnetic nanogels [41]. Magnetic nanogel was combined with Cy5.5-labeled lactoferrin (Cy5.5-Lf- MPNA nanogels). This nanogel presented the ability to change its size and hydrophilic/hydrophobic properties depending upon the surrounding temperature and pH. This property makes it possible to understand the surrounding environment of nanogel, whether it’s physiological (normal) or acidic (tumor). This enhances the targeting ability for drug delivery.
Many other studies have also presented the use of hydrogels in for brain applications in different forms: magnetic nanoparticles [42-45], polymeric nanoparticle [46] and lipid-based DDS [47-49]. All of them are not possible to cover in this short review.
Conclusion
Hydrogels have attracted a lot of attraction in the medical field because of their easy processing, nano size, viscoelasticity, swelling capability, high drug encapsulation efficiency, and minimal toxicity [50,51]. In this review, we have tried to cover the max of hydrogel brain application including 3D printing, local and remote drug delivery, treatment methods for brain tumors and brain mapping. It was found that hydrogel is a promising material for brain applications. The research in this filed is still going on at full speed to explore future opportunities in the field of medicine.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- O Wichterle, D Lím (1960) Hydrophilic Gels for Biological Use. Nature 185(4706): 117-118.
- TZ Grove, CO Osuji, JD Forster, ER Dufresne, L Regan (2010) Stimuli- Responsive Smart Gels Realized via Modular Protein Design. J Am Chem Soc 132(40): 14024-14026.
- S Garty, Kimelman-Bleich N, Hayouka Z, Cohn D, Friedler A, et al. (2010) Peptide-Modified Smart Hydrogels and Genetically Engineered Stem Cells for Skeletal Tissue Engineering. Biomacromolecules 11(6): 1516-1526.
- V Alzari, O Monticelli, D Nuvoli, JM Kenny, A Mariani (2009) Stimuli Responsive Hydrogels Prepared by Frontal Polymerization. Biomacromolecules 10(9): 2672-2677.
- SA Dubrovskii, MV Afanaseva, MA Lagutina, KS Kazanskii (1990) Comprehensive characterization of superabsorbent polymer hydrogels. Polym Bull 24(1): 107-113.
- AS Hoffman (2002) Hydrogels for biomedical applications. Adv Drug Deliv Rev 54(1): 3-12.
- J Teßmar, F Brandl, A Göpferich (2009) Hydrogels for tissue engineering. in Fundamentals of Tissue Engineering and Regenerative Medicine. Springer Berlin Heidelberg, pp. 495-517.
- J Thiele, Y Ma, SMC Bruekers, S Ma, WTS Huck (2014) 25th Anniversary Article: Designer Hydrogels for Cell Cultures: A Materials Selection Guide. Adv Mater 26(1): 125-48.
- O Wichterle, D Lim, M Dreifus (1961) [On the problem of contact lenses]. Cesk Oftalmol 17: 70-75.
- DA Ossipov, J Hilborn (2006) Poly (vinyl alcohol)-Based Hydrogels Formed by Click Chemistry. Macromolecules 39(5): 1709-1718.
- N Sanabria-DeLong, AJ Crosby, GN Tew (2008) Photo-Cross-Linked PLA-PEO-PLA Hydrogels from Self-Assembled Physical Networks: Mechanical Properties and Influence of Assumed Constitutive Relationships. Biomacromolecules 9(10): 2784-2791.
- P Gasteier, Reska A, Schulte P, Salber J, Offenhäusser A, et al. (2007) Surface Grafting of PEO-Based Star-Shaped Molecules for Bioanalytical and Biomedical Applications. Macromol Biosci 7(8): 1010-1023.
- GD Nicodemus, SJ Bryant (2008) Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Engineering - Part B: Reviews 14(2): 149-165.
- RV Ulijn, NurguseBibi, VineethaJayawarna, Paul D Thornton, Simon J Todd, et al. (2007) Bioresponsive hydrogels. Mater Today 10(4): 40-48.
- S Venkatesh, J Saha, S Pass, ME Byrne (2008) Transport and structural analysis of molecular imprinted hydrogels for controlled drug delivery. Eur J Pharm Biopharm 69(3): 852-860.
- H El-Sherif, M El-Masry, MFA Taleb (2010) pH-sensitive hydrogels based on bovine serum albumin for anticancer drug delivery. J Appl Polym Sci 115(4): 2050-2059.
- S Eichler, O Ramon, Y Cohen, S Mizrahi (2002) Swelling and contraction driven mass transfer processes during osmotic dehydration of uncharged hydrogels. Int J Food Sci Technol 37(3): 245-253.
- RA Gemeinhart, C Guo (2004) 3 Fast Swelling Hydrogel Systems.
- PJ Flory, J Rehner (1943) Statistical mechanics of cross-linked polymer networks II. Swelling. J Chem Phys 11(11): 521-526.
- PJ Flory (1950) Statistical mechanics of swelling of network structures. J Chem Phys 18(1): 108-111.
- JZ Hilt, AK Gupta, R Bashir, NA Peppas (2003) Regular Paper: Ultrasensitive Biome’s Sensors Based on Microcantilevers Patterned with Environmentally Responsive Hydrogels. pp. 177-184.
- NP Bayramgil (2008) Synthesis and characterization of new methacrylate-type hydrogels containing 2-tert-butylamino ethyl groups for sorption purposes. J Appl Polym Sci 109(2): 1205-1211.
- WH Oldenziel, BHC Westerink (2005) Improving glutamate microsensors by optimizing the composition of the redox hydrogel. Anal Chem 77(17): 5520-5528.
- NV Kulagina, L Shankar, AC Michael (1999) Monitoring Glutamate and Ascorbate in the Extracellular Space of Brain Tissue with Electrochemical Microsensors. Anal Chem 71(22): 5093-5100.
- M Fernandes, NS Dias, J Serrado Nunes, M El Tahchi, S Lanceros-Mendez, et al. (2009) Wearable brain cap with contactless electroencephalogram measurement for brain-computer interface applications. in 2009 4th International IEEE/EMBS Conference on Neural Engineering, pp. 387-390.
- MS Fernandes, Dias NS, Silva AF, Nunes JS, Lanceros-Méndez S, et al. (2010) Hydrogel-based photonic sensor for a biopotential wearable recording system. Biosens Bioelectron 26(1): 80-86.
- H Shen (2013) See-through brains clarify connections. Nature 496(7444): 151.
- DM Krolewski, Kumar V, Martin B, Tomer R, Deisseroth K, et al. (2018) Quantitative validation of immunofluorescence and lectin staining using reduced CLARITY acrylamide formulations. Brain Struct Funct 223(2): 987-999.
- R Tomer, L Ye, B Hsueh, K Deisseroth (2014) Advanced CLARITY for rapid and high- resolution imaging of intact tissues. Nat Protoc 9(7): 1682-1697.
- EH Chang, Argyelan M, Aggarwal M, Chandon TS, Karlsgodt KH, et al. (2017) The role of myelination in measures of white matter integrity: Combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. Neuroimage 147: 253-261.
- U Nordström, Beauvais G, Ghosh A, Pulikkaparambil Sasidharan BC, Lundblad M, et al. (2015) Progressive nigrostriatal terminal dysfunction and degeneration in the engrailed1 heterozygous mouse model of Parkinson’s disease. Neurobiol Dis 73: 70-82.
- K Ando, Quentin Laborde, Adina Lazar, David Godefroy, Ihsen Youssef, et al. (2014) Inside Alzheimer brain with CLARITY: Senile plaques, neurofibrillary tangles and axons in 3-D. Acta Neuropathologica 128(3): 457-459.
- K Chung, Jenelle Wallace, Sung-Yon Kim, Sandhiya Kalyanasundaram, Aaron S Andalman, et al. (2013) Structural and molecular interrogation of intact biological systems. Nature 497(7449): 332-337.
- AKL Liu, Hurry ME, Ng OT, DeFelice J, Lai HM, et al. (2016) Bringing CLARITY to the human brain: visualization of Lewy pathology in three dimensions. Neuropathol Appl Neurobiol 42(6): 573-587.
- TJ Hinton, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, et al. (2015) Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv 1(9).
- K Zhang, Zhenqing Shi, Jiankang Zhou, Qu Xing, Shanshan M, et al. (2018) Potential application of an injectable hydrogel scaffold loaded with mesenchymal stem cells for treating traumatic brain injury. J Mater Chem B 6(19): 2982-2992.
- T Fourniols, Randolph LD, Staub A, Vanvarenberg K, Leprince JG, et al. (2015) Temozolomide-loaded photopolymerizable PEG-DMA-based hydrogel for the treatment of glioblastoma. J Control Release 210: 95-104.
- J Tao, Zhang J, Hu Y, Yang Y, Gou Z, et al. (2017) A conformal hydrogel nanocomposite for local delivery of paclitaxel. J Biomater Sci Polym Ed 28(1): 107-118.
- FW Lin, PY Chen, KC Wei, CY Huang, CK Wang, et al. (2017) Rapid in situ MRI traceable gel-forming dual-drug delivery for synergistic therapy of brain tumor. Theranostics 7(9): 2524-2536.
- S Shah, PK Sasmal, KB Lee (2014) Photo-triggerable hydrogel-nanoparticle hybrid scaffolds for remotely controlled drug delivery. J Mater Chem B 2(44): 7685-7693.
- L Jiang, Zhou Q, Mu K, Xie H, Zhu Y, et al. (2013) PH/temperature sensitive magnetic nanogels conjugated with Cy5.5- labled lactoferrin for MR and fluorescence imaging of glioma in rats. Biomaterials 34(30): 7418-7428.
- SA Meenach, JZ Hilt, KW Anderson (2010) Poly (ethylene glycol)-based magnetic hydrogel nanocomposites for hyperthermia cancer therapy. Acta Biomater 6(3): 1039-1046.
- SA Meenach, JM Shapiro, JZ Hilt, KW Anderson (2013) Characterization of PEG- iron oxide hydrogel nanocomposites for dual hyperthermia and paclitaxel delivery. J Biomater Sci Polym Ed 24(9): 1112-1126.
- J Il Kim, B Kim, CJ Chun, SH Lee, SC Song (2012) MRI-monitored long-term therapeutic hydrogel system for brain tumors without surgical resection. Biomaterials 33(19): 4836-4842.
- J Il Kim, BS Lee, CJ Chun, JK Cho, SY Kim, et al. (2012) Long-term theranostic hydrogel system for solid tumors. Biomaterials 33(7): 2251- 2259.
- Y Xu, M Shen, Y Sun, P Gao, Y Duan (2015) Polymer nanocomposites based thermo- sensitive gel for paclitaxel and temozolomide co-delivery to glioblastoma cells. J Nanosci Nanotechnol 15(12): 9777-9787.
- T Arai, et al. (2010) Novel local drug delivery system using thermoreversible gel in combination with polymeric microspheres or liposomes. Anticancer Res 30(4): 1057-1064.
- C Bastiancich, Vanvarenberg K, Ucakar B, Pitorre M, Bastiat G, et al. (2016) Lauroyl-gemcitabine-loaded lipid nanocapsule hydrogel for the treatment of glioblastoma. J Control Release 225: 283-293.
- C Bastiancich, Bianco J, Vanvarenberg K, Ucakar B, Joudiou N, et al. (2017) Injectable nanomedicine hydrogel for local chemotherapy of glioblastoma after surgical resection. J Control Release 264: 45-54.
- DM Eckmann, RJ Composto, A Tsourkas, VR Muzykantov (2014) Nanogel carrier design for targeted drug delivery. J Mater Chem B 2(46): 8085-8097.
- I Neamtu, AG Rusu, A Diaconu, LE Nita, AP Chiriac (2017) Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv 24(1): 539-557.
-
Hyungjung Kim. A Brief Analysis of Recent Trends in Collaborative Robots. Glob J Eng Sci. 4(3): 2020. GJES.MS.ID.000587.
-
Hydrogels, Brain applications, Polymers, Water, Hydrophilicity, Oil recovery, Agriculture, Biosensors, Biotechnology, Pharmaceutical
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






