 Case Report
Case Report
Technological Characterization of Bigadic Boron Works Waste Clay: A Case Report
Ilker Ozkan, Torbali Vocational School, Industrial Glass and Ceramics Department, Torbali, Izmir, Turkey.
Received Date: September 01, 2018; Published Date: October 04, 2018
Abstract
The aim of this study is to characterize the technical properties of Bigadic Boron Works Waste Clay (BWC) fired at various temperatures. For this purpose, the clay sample was first characterized by chemical and X-ray diffraction (XRD) analyses. The mineralogical composition of BWC was composed of ulexite, smectite, illite, quartz, calcite and magnesite phases. To evaluate technological behaviors, pressed clay samples were fired separately at temperatures between 900, 950 and 1000° C. Fired specimens were evaluated by water absorption, linear shrinkage, bending strength, scanning electron microscopy (SEM) and color measurements. The results showed that there became a significant densification at temperatures especially above 950° C. Based on the technological characteristics, BWC could be used as an additive raw material in the manufacture of structural ceramics.
Keywords: Bigadic waste clay; Characterization; Firing behavior; Ceramic properties
Introduction
Industrial development over the last decades has generated large amounts of inorganic waste. The problems related to waste production are becoming more and more significant in relation to the improvement of economic conditions, the progress of industrial development and the population and urban increase. [1] The recycling of by-products and wastes is a hot research topic for industrial and environmental uses [2-3]. Traditionally, waste products are disposed of as soil conditioners or in land filling. However, reusing or recycling alternatives should be investigated [4].
Turkey has about 800 million tons of boron reserves, which consist of 70% of the world boron reserves. The borate deposits of Turkey occur in western Anatolia, south of the Sea of Marmara, within an area roughly 300 km east-west by 150 km north-south. The main borate districts are Bigadic, Kestelek, Sultancayir, Emet and Kirka. The Bigadic borate deposits are among the largest colemanite and ulexite deposits in the world. The high-grade colemanite and ulexite ores are adequate to supply the world’s B needs for many years. During the processing of boron ores, concentrator wastes occur, and these wastes are stored in tailing dams [5-9].
Although very limited studies on the characterization of BWC have been reported in the literature, evaluating technological properties of fired samples has not been studied. The aim of this research is to characterize the BWC and this study also will be useful to minimize the environmental impact of BWC disposal.
Material and Methods
The waste clay investigated in this work was obtained from Eti Maden Bigadic Boron Works, which is located in Balikesir, Turkey. The waste clay was characterized by chemical and X–ray diffraction (XRD) analyses. The chemical composition of the waste clay was analyzed by atomic absorption spectroscopy (GBC). B2O3 component was determined by titrimetric method at Eti Maden laboratories. The phases present in the samples were identified by X-ray diffraction (XRD) using a Rigaku Model diffractometer with monochromatic CuKα radiation.
In order to enlighten the influence of firing temperature on ceramic properties on a laboratory scale, pellet clay samples were used. To produce pellet samples, clay sample was dried and ground. The ground agglomerates were then humidified up to 6 wt.% water. The humid powders were pressed under 150kg/cm2 pressure to obtain 100×50×8 mm prismatic samples. The shaped samples were dried at 110 °C for 24 h and fired at 900, 950 and 1000 °C using a laboratory kiln (Nabertherm LH 15/14). Fired samples were used to characterize the firing properties of the material. The physical properties were determined according to the standards suggested by ISO 10545-3 and ISO 10545-4.
For the SEM characterization studies, 0.5×0.5×0.5mm cubic samples were cut from the fired specimens and their surfaces were cleaned first, with 400, 800 and 1200 grade emery papers, then with acetone ultrasonically. Phase changes with increasing temperature were investigated by X-ray diffraction. The microstructures of the fired samples were analyzed by a scanning electron microscope; SEM (JEOL-JSM 6060). The samples were sputtered with Au-Pd alloy in order to obtain conductivity to prevent charging under electron beam. Images were taken under the conditions of 20 kV beam voltage for back-scattered electron (BE) mode and 10 kV beam voltage for secondary electron (SE) mode with a tungsten filament. Color measurements of the samples were performed using a portable colorimeter 3NH (model NH-310).
Results and Discussion
Table 1 lists the chemical compositions of BWC. The main characteristic of the clay is its high MgO, CaO, and B2O3 content, and its low Fe2O3 content. Also, BWC contain low amounts of Na2O and K2O content that act as fluxing agent in ceramics. XRD analysis showed that BWC powder sample contains ulexite, smectite, illite, quartz, calcite and magnesite phases (Figure 1).
Table 1: Chemical analysis results of the BWC sample [10].

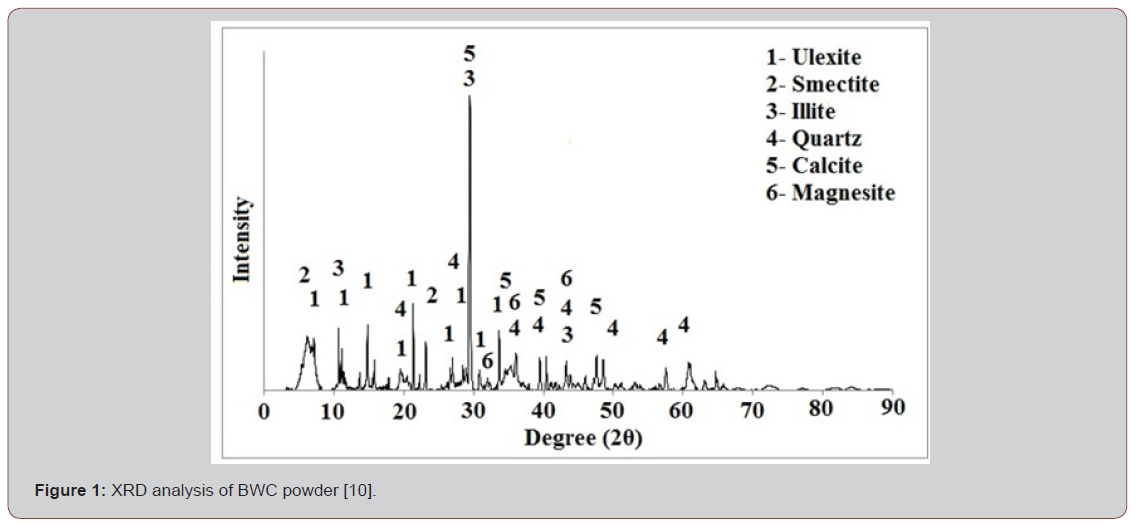
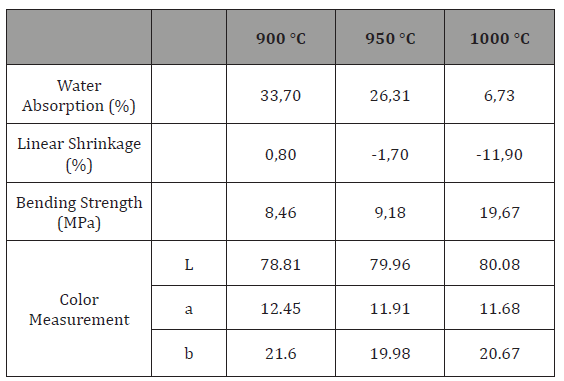
Table 2 lists the physical properties of the BWC samples fired at different temperatures. According to this data, with increasing temperature, the water absorption values decreased where linear shrinkage values and strength values increased. Especially at 1000˚C, significant changes were observed in the values. As the deformation began to occur, no measurements were taken over 1000˚C.Also color measurements are given in Table 2. Color measurements along the chromatic coordinates show that L values increased while a and b values decreased with increasing temperature (L value indicates the lightness scale where 0 is black; 100 is white, a value indicates the red–green scale where positive values are red; negative values are green and 0 is neutral, b value indicates the blue yellow scale where positive values are yellow; negative values are blue and 0 is neutral). It is reasonable to think that high CaO content caused whitening effect.
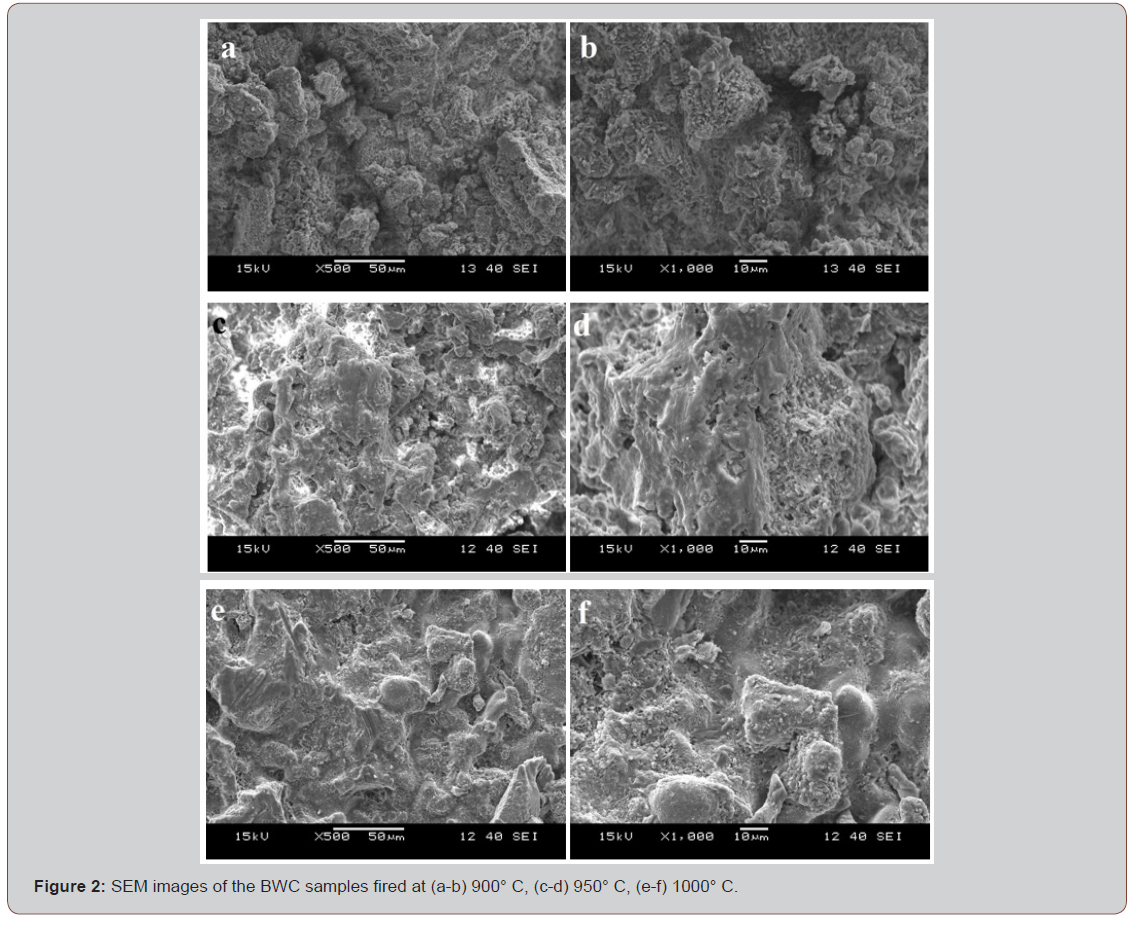
The microstructures of the waste clay samples fired at different temperatures are shown in Figure 2. Microstructures are consistent with the physical analysis results given in Table 2. Porous structures are observed at 900˚C and 950˚C. In addition, the sample fired at 1000˚C is much more compact and the number of pores is considerably reduced.
Table 2: Chemical analysis results of the BWC sample [10].
Conclusion
In this study, the characteristics and technological properties of Bigadic Boron Works waste clay (BWC) were investigated. From the results obtained, the following conclusions can be drawn:
The predominant oxides in BWC are MgO, CaO, and B2O3. ulexite, smectite, illite, quartz, calcite and magnesite are found as the main phases.
The technological properties of BWC were evaluated by water absorption, linear shrinkage, bending strength and scanning electron microscopy (SEM). The changes in the physical properties were moderate up to 950˚C. Especially at 1000°C, significant increase in linear shrinkage and bending strength was observed whereas water absorption values decreased. SEM micrographs, taken at increasing firing temperatures, show the reduction of porosity and the progression of enhanced densification with increasing temperature. In view of these evaluations, this raw material is suitable for porous structural ceramics.
It is expected that this study will help to improve the knowledge about BWC and contribute correct assessment of the deposits.
Acknowledgments
This work was supported by the Dokuz Eylul University Scientific Research Project within the project number 2015. KB.FEN.027..
References
- A Karamberi, K Orkopoulos, A Moutsatsou (2007) Synthesis of glassceramics using glass cullet and vitrified industrial by-products. J Eur Ceram Soc 27(2-3): 629-636.
- NV Boltakova, GR Faseeva, RR Kabirov, RM Nafikov, Yu A Zakharov (2017) Utilization of inorganic industrial wastes in producing construction ceramics. Review of Russian experience for the years 2000-2015. Waste Manage 60: 230-246.
- A Olgun, Y Erdogan, Y Ayhan, B Zeybek (2005) Development of ceramic tiles from coal fly ash and tincal ore waste. Ceram Int 31(1): 153-158.
- Raupp Pereira, D Hotza, AM Segadaes, JA Labrincha (2006) Ceramic formulations prepared with industrial wastes and natural sub-products. Ceram Int 32(2): 173-179.
- B Oto, A Gur (2013) Determination of mass attenuation coefficients of concretes containing ulexite and ulexite concentrator waste. Ann Nucl Energy 59: 72-74.
- U Gemici, G Tarcan, C Helvaci, AM Somay (2008) High arsenic and boron concentrations in groundwaters related to mining activity in the Bigadic borate deposits (Western Turkey). Appl Geochem 23(8): 2462-2476.
- C Helvacı, H Mordogan, M Colak, I Gundogan (2004) Presence and Distribution of Lithium in Borate Deposits and Some Recent Lake Waters of West-Central Turkey. Int Geol Rev 46(2): 177-190.
- C Helvaci (1995) Stratigraphy, Mineralogy and Genesis of the Bigadic Borate Deposits, Western Turkey. Econ Geol 90(5): 1237-1260.
- C Helvaci, RN Alonso (2000) Borate Deposits of Turkey and Argentina; A Summary and Geological Comparison. Turk J Earth Sci 9: 1-27.
- I Ozkan (2017) Utilization of Bigadic Boron Works Waste Clay in Wall Tile Production. Acta Phys Pol A 132(2): 427-429.
-
Ilker Ozkan. Technological Characterization of Bigadic Boron Works Waste Clay: A Case Report. Glob J Eng Sci. 1(2): 2018. GJES.MS.ID.000507.
-
Bigadic waste clay, Characterization, Firing behavior, Ceramic properties, Industrial, Soil conditioners, Boron, Tailing dams, Eti Maden Bigadic, Spectroscopy, Humid powder, Ulexite, Smectite, Illite, Quartz, Calcite, Magnesite phase, Microstructure, Linear shrinkage, Bending strength, Scanning electron microscopy
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






