 Research Article
Research Article
Impact of V2O5 and Gamma Ray Irradiation on The Optical and Structural FTIR Spectra of Lithium Phosphate Glass
MA Ouis* and FH El Batal
Glass Research Department, National Research Centre, ElBehoos Street, Cairo, Egypt
MA Ouis, Glass Research Department, National Research Centre, ElBehoos Street, Cairo, Egypt.
Received Date: October 22, 2019; Published Date: October 29, 2019
Abstract
Combined studies on prepared undoped lithium phosphate glass of the composition (Li2O330-P2O570 mol%) and derived samples containing additional dopants of V2O5 (0.4, 1, 2, 3, 4%). The glasses have been investigated using optical (UV-visible) and FTIR spectra before and after being gamma irradiated with a dose of 6 M rad (6x104 Gy). The collective spectral properties have been employed to explore the states of vanadium ions in this specific host phosphate glass. The study extends to identify the impact of gamma ray’s irradiation on the glasses with and without V2O5. The optical spectrum of the undoped lithium phosphate glass shows distinct UV absorption which is accepted by many researchers to be due to unavoidable contaminated trace ferric ions while the absorption of the V2O5- doped samples exhibit additional bands within the visible region which are correlated with combined presence of lower valences of trivalent and tetravalent vanadium ions. Gamma irradiation on the undoped glass increases the UV absorption and produces an induced visible broad band and this behavior is related to some suggested photochemical reaction and the formation and induced visible of phosphorus oxygen hole center (POHC) band while the vanadium ions show some shielding behavior with the maintenance of the spectral curves as before irradiation in the visible region. The FITR spectra show pronounced and condensed vibrational peaks within the mid region due to Q2 and Q3 phosphate groups beside few peaks due to OH, POH and water vibrations..
Keywords: Lithium phosphate glass; V2O5; Optical-FTIR spectra; Gamma irradiation
Introduction
Phosphate glasses are recognized through the review articles by Martin [1] and Brow [2] to be vitreous materials possessing polymeric structures based on tetrahedral phosphate (PO4) groups with a characteristic double bond to compensate for the Penta valency of phosphorous. These glasses have interesting UV-visible transmission which is useful for some optical applications [3]. The elevated thermal expansion coefficients and decrease in preparation temperatures make some phosphate glasses appropriate as solder and sealing glasses [4] beside extended applications of phosphate glasses as optical material, bio-glass and plant fertilizers [1-4].
Vanadium is an exceptional 3d transition metal which has the ability solely to present in glasses into three valence states, namely the trivalent, tetravalent and pentavalent states [5-10]. The high valence (V5+) ions belong to do configuration and hence show no characteristic visible absorption but only exhibit an UV band at 380 nm. The second tetravalent V4+ ions are accepted to be found as vanadyl (VO2-) ions showing four weak peaks at 420, 760-800, 1000 nm and a probable UV peak. The lowest valence V3+ ions exist in distorted octahedral coordination and revealing two prominent peaks at 350-400, 580-680 nm. Melting conditions and composition of the glasses control the percent of every valence. Alkali borate and alkali silicate glasses generally favor the higher valence state [V5+] but alkali phosphate glass promotes the low valence states (V3+, V4+) in noticeable percent.
When glasses are subjected to ionizing gamma irradiation, many glasses are darkened and reveal changes in their optical properties which can be followed and identified through optical and E.S.R spectral measurements [11,12].
The resultant induced defects and damages are accepted to originate from the presentation of free electrons and positive holes through the irradiation process and their reactions and trapping with intrinsic defects within the matrix of the glass being non periodic materials including nonbridging oxygens, voids, vacancies, and hence reveal or exhibit the formation of further damages in most cases.
Continuous studies on gamma irradiation effects of undoped and transition metals doped borate, silicate and phosphate glasses have reached to interesting and important conclusions [8,9,13-17] which can be summarized as follows:
• All transition metal ions are assumed to participate in the formation of induced defects through capturing electrons or positive holes and their valences are expected to change. Also, some specific transition metal ions (such as V3+, Cu2+, W6+, Mo6+) are assumed to cause some shielding behavior towards successive gamma irradiation.
• Distinct shielding effects were identified in glasses containing heavy metal oxides (e.g. PbO, Bi2O3) and the irradiated glasses showed stability of the irradiated optical spectral curves and the same effect was identified through the maintenance of the IR spectra after irradiation.
• The target of this work is to study different optical spectra of some prepared lithium phosphate glasses with and without addition of V2O5 before and after gamma irradiation.
Introduction
Preparation of the glasses
The glasses were prepared using laboratory chemicals of ammonium dihydrogen ortho-phosphate (NH4H2PO4) for P2O5 and lithium carbonate (Li2CO3) for Li2O and the dopant vanadium ions was added in the form of V2O5.
The weighed batches were heated at first in covered porcelain crucibles at 500 °C for 1/2 hour to expel the ammonia and water and the electric furnace ( SiC type , Vecstar, UK)was raised to 1000 °C and continued for 1 hour with frequent rotating the melts to reach complete mixing and homogeneity. Then the melts were poured into slightly warmed stainless steel with the required dimensions. The prepared glassy samples were transferred immediately to a muffle furnace regulated 280 °C. Then the muffle was switched off after 1 hour and left to cool to room temperature with a rate of 30 °C/hour.
Optical spectral measurements
Samples of undoped and varying V2O5-doped glasses were measured after polishing with equal thickness (2mm ±0.1 mm) to identify their UV-visible absorption characteristics using a recording spectrophotometer (Shimadzu UV-3660, Japan) to the wavelength ranges 200-2500 nm. The measurements were repeated after gamma irradiation with a dose 6 Mrad (6x104 Gy).
FT infrared absorption measurements
FTIR spectra were measured through the KBr disc technique. 2 mgs of fine powder of the glasses were mixed with 200mg of KBr and the mixtures were subjected to pressure in an evocable die to a load of 5 tons/cm2 to obtain transparent homogenous discs. The FTIR measurements were carried out after the preparation of the discs using FTIR spectrometer (type Nicolet i510, USA) within the range 400-4000 cm-1. Infrared spectra were corrected for the dark current noises and background using the two-point base line correction.
Gamma ray irradiation facility
An Indian 60Co gamma cell (2000 Ci) was used as a gamma source with a dose rate of 1.5 Gy/s (150 rad/s) at a temperature of 30 °C.
Results
Optical (UV-visible) absorption spectra of the undoped and varying V2O5-doped lithium phosphate glasses before and after irradiation
The undoped lithium phosphate glass reveals a spectrum (Figure 1) before irradiation consisting only of strong UV absorption with a distinct peak at about 260 nm and without any further absorption to the rest of measurement at 2500 nm. After gamma irradiation, the UV absorption increases in intensity together with the generation of another UV absorption band at about 320 nm and a broad visible band extending from about 500 to 1500 nm and centered at about 840 nm.
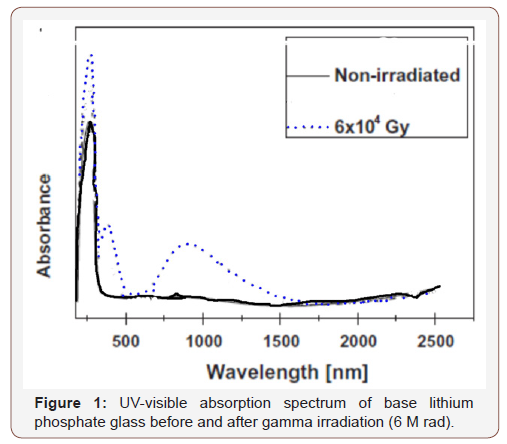
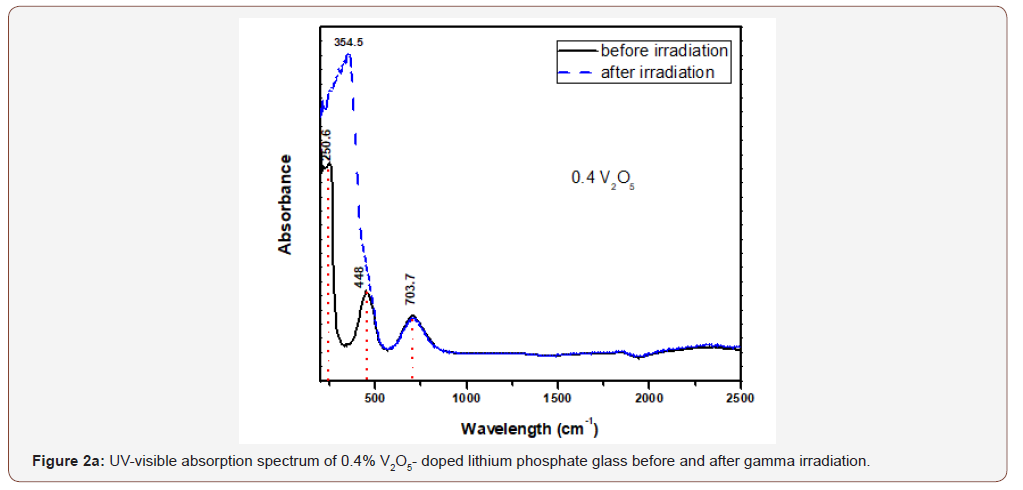
Figure 2 (a,b,c,d) illustrates the optical spectra of the four V2O5- doped glasses before and after gamma irradiation.
The optical spectrum of the 0.4 V2O5%-doped glass shows the same distinct and high intense UV absorption with two peaks at 220 and 250 nm and followed by two wide and distinct visible bands extending from about 350 to 900 nm and centered at 448 and 704 nm. Also, there are three small curvatures at about 1320, 1850 and 2270 nm. Upon gamma irradiation, the UV peaks increase in intensity together with the appearance of an UV strong band centered at 354 nm and attached to the first visible band and the rest absorption remains as before.
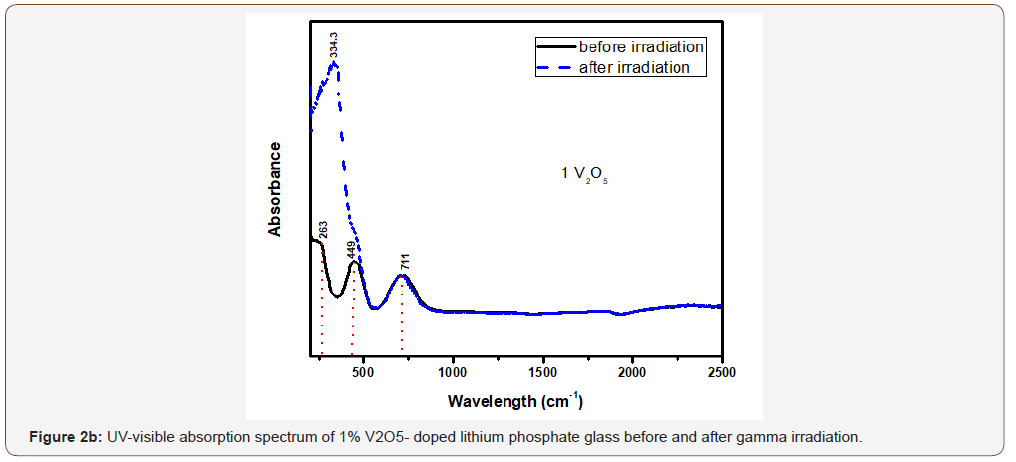
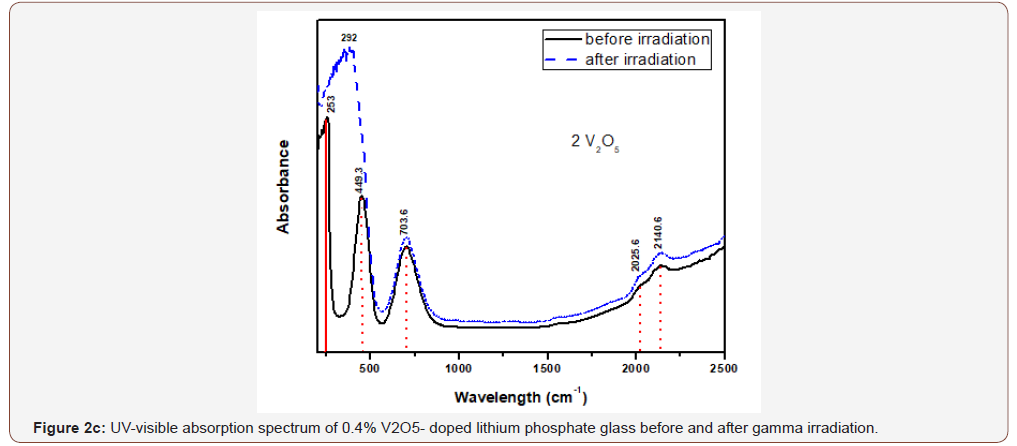
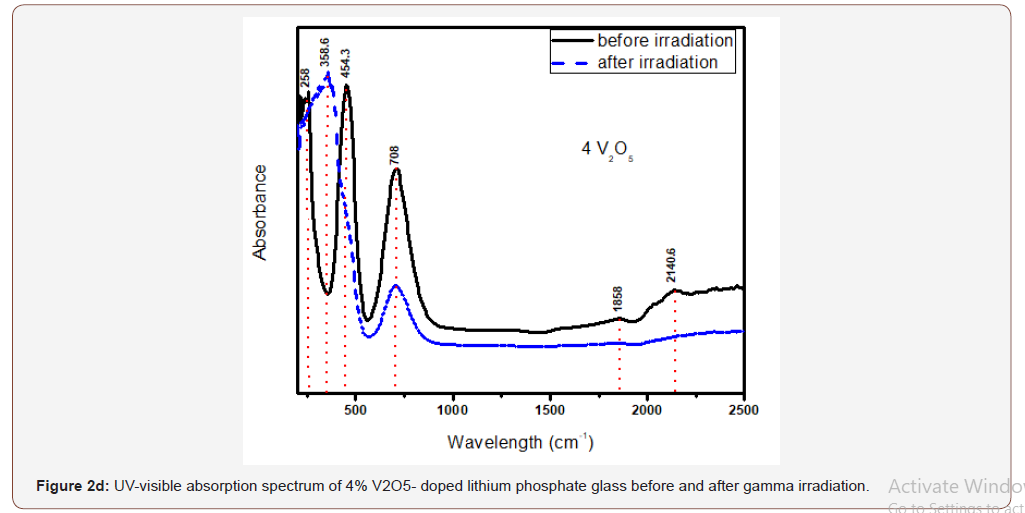
The glasses containing 1, 2 and 4% V2O5 reveal almost the same spectral characteristics as the low V2O5 0.4 doped glass spectrum and the two visible broad bands increase in intensity with the increase of V2O5 content and the intensity of the first broad band at 454 nm approaches that of the charge transfer UV band.
Upon gamma irradiation, the 1% V2O5 glass behaves like the previous glass with 0.4% V2O5, through the increase of UV and the appearance of a strong band at 334 nm linked to the first visible band at 449 nm and the rest spectrum remains unaffected. The 2% V2O5-doped behaves after irradiation as the two previous glasses. The same behavior repeated in the 4% V2O5 glass after irradiation.
Infrared absorption spectra of the studied glasses
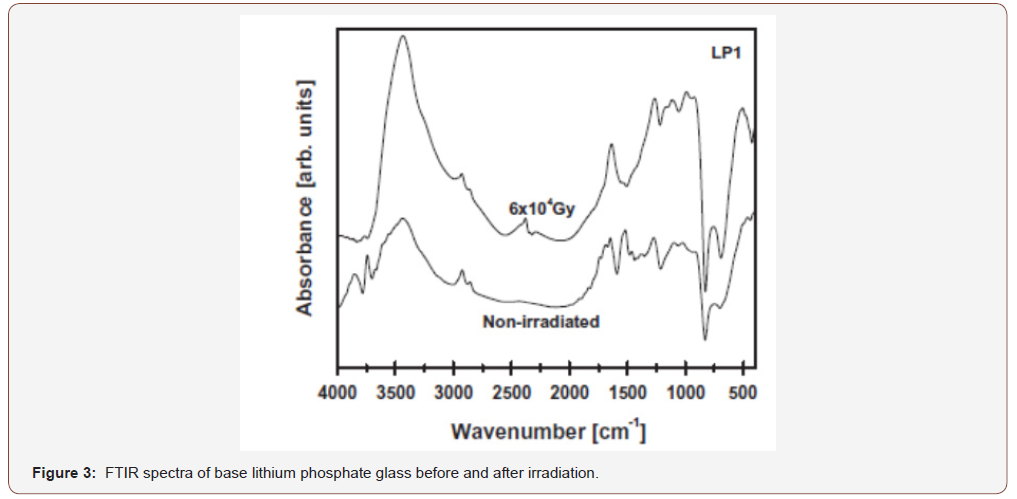
Figure (3) reveals the FTIR spectra of the undoped lithium phosphate (Li2O 30-P2O5 70 mol%) together with that for the samples with the same composition but doped with varying V2O5 contents (0.4, 1, 2, 4%), All the spectral curves are repetitive and the main vibrational peaks are concentrated in the mid region 400- 1600 cm-1. These IR peaks are indicating the structural building units of the considered glasses.
The IR spectral details of the base lithium phosphate glass can be shortened as follows:
a. The appearance of a distinct far-IR band at 531 cm-1.
b. A medium band is identified at about 730 cm-1
c. A strong and broad band is detected with two distinct peaks at 983 and 1083 cm-1
d. A sharp band is observed with a peak at about 1180cm-1.
e. A distinct band is identified at about 1264 cm-1.
f. Two small kinks are observed at about 2854 and 2928 cm-1.
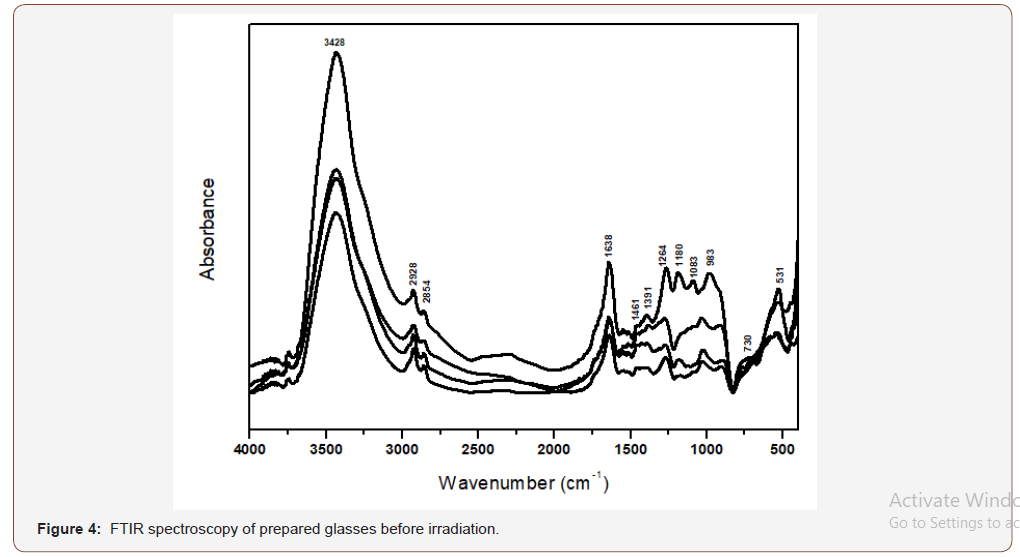
g. A final near IR broad band is observed centered at about 3428 cm-1.The IR spectra of the V2O5-doped glasses (Figure 4) disclose no distinct changes in the number of the absorption peaks than that for the undoped sample.
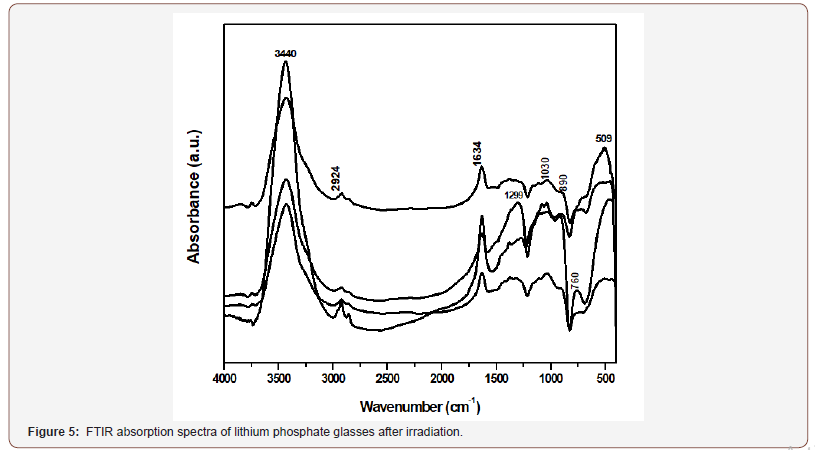
Figure 5 reveals the IR spectra of the studied glasses after gamma irradiation. Examination of the IR curves indicates the upkeep of the same structural features before gamma irradiation.
Discussion
Interpretation of the origin of UV absorption in the spectrum of the undoped glass
Numerous glass scientists have continuously identified distinct UV absorption bands within the optical spectra of undoped borate, silicate and phosphate glasses [14-19]. These authors have assumed that these UV bands within the range 200-320 nm mostly originated from unavoidable trace iron impurities (mainly ferric Fe3+ ions) contaminated within the raw materials or chemicals used for the preparation of these different glasses.
We agree to the forward attribution that the source of the UV absorption in the undoped glass is trace iron impurities within the chemicals used for the preparation of the studied glasses.
Optical spectra of V2O5 containing glasses
It is obvious from Figure (2) that the V2O5-doped glasses reveal optical spectra consisting from the same UV absorption as the undoped glass beside the appearance of two additional broad visible bands centered at about 450 and 710 nm and their intensities increase with the increase of V2O5 content.
The appearance of the previous two visible broad bands and their obvious increase in intensity to reach the intensity of the charge transfer UV absorption band can be understood and explained as follows:
• The positions of the strong two broad visible bands are almost the same cited wavelength for both collective V3+ ions (3d2 state) and V4+(3d1 state) as assumed by various authors [5, 8-10].
• The increase of the intensities of the two mentioned visible broad bands with the increase of V2O5 content indicates that this host lithium phosphate glass promotes very strongly the presence of vanadium ions in the trivalent and tetravalent states and leading to reflect high intense band at 450 nm approaching the intensity of the UV absorption peaks owing to trace iron (mainly Fe3+) present as trace impurities. This distinct behavior can be also related to the combined strengthening of the two low valence states possessing nearby absorption, within the wavelengths 400-500 nm.
Interpretation of the FTIR spectra of the undoped and V2O5 doped lithium phosphate glasses
The FTIR spectra are identified to be composed of condensed vibrational peaks extending mostly within the mid region (400- 1650 cm-1) and they can be realized and construed on the next basis [20-23]:
• It should be recognized that the observed IR vibrational peaks from the studied glasses are fingerprints of the constitutional network groups which are dependent on the detailed chemical composition of the host glass.
• The composition of the host glass consists of P2O5 (70%) and Li2O (30%) and hence the expected structural forming groups are phosphate groups (Q2 and Q3) and the Li2O acts as modifier oxide producing broken linkages and nonbridging oxygens, with the residence of Li+ ions in interstitial positions.
• The detailed assignments of the identified peaks can be existing as follows depending on the free independent concept of all vibrating groups irrespective of the presence of different units [20-23]:
(i) The far IR peaks at 400-450 cm-1 can be related to vibrations of Li+ cations in their sites.
(ii) The peaks identified at 460-480 cm-1 can be correlated with bending vibrations of O-P-O bond.
(iii) The peak observed at 730cm-1 is related to harmonics of P=O bending vibrations.
(iv) The peak at 983 cm-1 is related to vibrations of different metaphosphate groups.
(v) The peaks at 1083-1180 cm-1 are correlated with asymmetric stretching of P-O-P linkages.
(vi) The peaks at 1264cm-1 can be related to asymmetric stretching vibrations that contain double bonded oxygens O-P=O.
(vii) The peak at about 1630-1650 cm-1 is due to vibrations of OH, P-OH groups.
(viii) The near IR peak at about 3445 cm-1 is related to vibrations of OH, water, POH.
(ix) The same assignments are applied to the IR spectra of the studied glasses.
Effect of gamma irradiation on the measured optical and FTIR spectra
The understanding and interpretations of the changes exhibited by subjecting the glasses to gamma irradiation can be explained on the following basis [24-29]:
• When ionizing gamma irradiation impinges on glasses, most of them are darkened due to the generation of induced defects or damages which can be identified and classified through optical and electron spin resonance measurements. This response of most glasses can be related to their nonperiodic network structural arrangement and they do contain intrinsic defects before irradiation (such as nonbridging oxygens, vacancies, voids). These sites beside trace impurities can react with liberated electrons and positive holes during the irradiation process thus producing induced defects or damages.
• The resultant irradiation defects or damages are assumed to involve electronic process, radiolysis and sometimes photochemical reactions. Radiation induced defects are strongly dependent on glass type and compositions, trace impurities, the presence of polyvalent or heavy metal oxides and radiation sources.
• Many researchers [30-31] have reached the conclusion that the presence of heavy metal oxides such as PbO, Bi2O3, MoO3, WO3 causes obvious shielding behavior towards gamma irradiation and the optical structural curves remain unchanged or very close to that before irradiation. This effect is correlated with some blacking to the free passage of free electrons during the irradiation process due to the heavy masses of the mentioned cations.
• Some researchers [32-38] have identified some photochemical reactions between transition metals and generated electrons or positive holes and the net result is the increase or decrease of their valences. Consequently, some glasses reveal obvious shielding upon increasing the concentration of the specified transition metal content and the optical spectral curves remain unchanged or assume close parallel arrangement with that before gamma irradiation.
• The effect of gamma irradiation is quite identified on the undoped glass more than that on the V2O5 containing samples. The combined elevation of the strength of the UV absorption can be related to suggested photochemical reactions between some ferrous ions present as impurity due to the reducing action of the host phosphate glass, and positive holes and leading to the formation of additional ferric ions (Fe3+) or (Fe2+)+ as referred by some authors [12,16,17]. These new ions absorb in UV and hence the increase of its intensity. The second generated induced broad visible band in the spectrum of the irradiated undoped glass is linked to the effect of gamma irradiation on the phosphate network leading to the creation of phosphorous oxygen hole center (POHC) as assumed by some authors [17,24-29].
• The vanadium-doped glasses reveal quite maintenance of their spectral curves after gamma irradiation and no induced visible bands are identified as that observed in the spectrum of the undoped sample. This behavior is related to suggested shielding effect of vanadium ions and the same result was previously referred to by some authors [8-10, 32,33].
• The irradiated glasses reveal the same IR spectra as that before irradiation indicating the persistence of the structural building groups with number and position of the vibrational bands.
Conclusion
Binary lithium phosphate and V2O5- doped samples of the same composition have been prepared and characterized. Optical spectra of the undoped glass reveal pronounced UV absorption correlated with unavoidable traces of ferric ions present contaminated within the chemicals used for the preparation of the host glass. V2O5- doped glasses impart two additional characteristic broad visible bands, which are correlated with the presence of vanadium ions in both the trivalent and tetravalent states.
Gamma irradiation noticeably affects the spectrum of the undoped lithium phosphate glass increasing the intensity of the UV absorption and generating an induced visible band. These marked effects are correlated with proposed photochemical reaction and the formation of induced phosphorous-oxygen hole center (POHC) defect. Vanadium-doped samples show some shielding behavior towards gamma irradiation and the optical spectra remain very nearer. FTIR spectra show characteristic vibrational extended peaks within the mid region 600-1650 cm-1 due to the presence of Q2 and Q3 phosphate groups, while the far IR spectrum show vibrations due to Li+ cations and the near IR region reveals non-building groups vibrations related to OH, water, POH .Gamma irradiation is identified to cause no noticeable effects in the IR vibrational bands reflecting the stability of the structural building units.
Summary Statement
The prepared glasses have shielding effect towards gamma irradiation.
Acknowledgement
None.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- SW Martin (1991) Eur J Solid State Inorg Chem 28: 163-205.
- RK Brow (2000) J Non-Cryst. Solids, 263-264: 1-28.
- SF Khor, ZA Talib, WM (2012) Mat Yunus, Ceram Intern 38: 935-940.
- RM Morena (2000) J Non-Cryst. Solids, 263&264: 381-387.
- CR Bamford (1977) Colour Generation and Control in Glass, Glass Science and Technology, vol 2, Elsevier Publishers, Amsterda.
- A Paul (1990) Chemistry of Glasses, Second Edition, Elsevier, New York.
- AM Nassar, NA Ghoneim (1981) J Non-Cryst.Solids 46: 181.
- HA El Batal, NA Ghoneim (1997) Nucl Instr Meth Phys Res (B)124-81-90.
- AM Abdelghany, HA El Batal, LK Marei (2011) Rad Eff Def Solids.
- AM Abdelghany, HA El Batal (2016) Mater Des 89: 568-572.
- EJ Friebele (1991) DR Uhlmann, NJ Kreidl (Eds.), Optical Properties of Glass, American Ceramic Society, Westerville, OH, USA, pp. 205.262.
- D Möncke, DEhrt (2007) HP Glick (ed.) Handbook of Materials Science Research Horizons, Nova Science Publishers, Inc New York, pp. 1-56.
- FH El Batal (2009) Indian J Pure& Appl Phys 47: 631-642.
- GH Sigel, RJ Ginther (1968) Glass Technol 9: 66.
- JA Duffy (1997) Phys Chem Glasses 38: 289.
- D Ehrt, P Ebeling, U Natura, J Non-Cryst.Solids. 263-264: 240.
- D Moncke, D Ehrt (2004) Opt Mater. 25: 425.
- SY Marzouk, FH El Batal (2006) Nucl Instr Meth Phys Res (B) 248: 90-102.
- FH El Batal (2008) J Mater Sci 43: 1070-1079.
- p Tarte (1962) Spectrochim Acta 18: 16.
- AM Efimov (1997) J Non-Cryst Solids 209:209.
- YM Moustafa, K El Egili (1998) J Non-Cryst Solids 240: 144.
- HA El Batal, AM Abdelghany, FH El Batal, M El Badry, FM Moustafa (2011) Physica B 406: 3694.
- A Bishay (1970) J Non-Cryst Solids 3: 54-224.
- EJ Friebele (1990) Radiation effects. Optical properties of glass, Eds. DR Uhlmann, NJ KREIDL, American Ceramic Society, Westerville, OH, USA, pp. 205-262.
- D Ehrt, P Ebeling, U Natura (2000) J Non-Cryst Solids 263&264: 140-250.
- D Möncke, D Ehrt (2004) Opt Mater 25: 425-437.
- D Möncke, D Ehrt (2007) Photoionization of polyvalent ions. Materials Science Publishers, Inc, New York, pp 1-56.
- D Ehrt (2015) Review, Phosphate and fluoride phosphate glasses- properties, structure, and applications. Phys Chem Glasses, Eur J Glass Sci Technol (B) 56(6): 217-234.
- FH El Batal (2007) Nucl Instr Meth Phys Res, B 254: 243.
- FH El Batal, SY Marzouk, FM Ezz El Din (2010) J Non-Cryst Solids 356: 2750-2759.
- FH El Batal, YM Hamdy, SY Marzouk (2009) J Non-Cryst Solids 355: 2439.
- FH El Batal, MA Ouis, RM Morsi, SY Marzouk (2010) Phil Mag 90: 2905.
- FH El Batal (2008) J Mater Sci 43: 1070.
- HA El Batal, ZE El Mandouh, HA Zayed, SY Marzouk, GM El Komy, et al. (2013) J Mol Struct 1054-1055: 57-66.
- HE El Batal, NH Ghoneim (1997) Nucl Instr Meth Phys Res.
- FH El Batal, SY Marzouk, AM Abdelghany (2011) J Non-Cryst Solids 357: 1027-1036.
- AM Abdelghany, HA El Batal (2012) J Mol Struct 1024: 47-53.
-
MA Ouis, FH El Batal . Impact of V2O5 and Gamma Ray Irradiation on The Optical and Structural FTIR Spectra of Lithium Phosphate Glass. Glob J Eng Sci. 3(4): 2019. GJES.MS.ID.000570.
-
Lithium phosphate glass, V2O5, Optical-FTIR spectra, Gamma irradiation
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






