 Research Article
Research Article
Silver Alloy Protection by Applying Nanomaterial Coat after Tarnish Removal
AA El-Meligi*
Physical Chemistry Dept., Advanced Materials Technology & Mineral Resources Research Institute, National Research Centre, Egypt
AA El-Meligi, Physical Chemistry Dept., Advanced Materials Technology & Mineral Resources Research Institute, National Research Centre, Egypt
Received Date: November 23, 2022; Published Date: December 06, 2022
Abstract
This research focuses on the tarnish removal and protection of silver alloy by using nanoalumina coating. In the presence of a high concentration of sodium sulphide (0.1 M Na2S) at room temperature, a dark tarnish forms on the silver surface. Tarnish removal proceeds by coupling with aluminum sheets of various areas in a solution of 10% (1:1) sodium carbonate-bicarbonate at room temperature. Nanoalumina (Al2O3) is used to protect silver alloy. A silver alloy specimen was immersed in a suspension of nano-Al2O3, acetone and paraloid under an ultrasonic shaker. An efficient, invisible and protective layer against the tarnishing appearance was formed on the silver alloy surface. The protectiveness of the nanoalumina coating was tested by electrochemical measurement during immersion in a concentrated sulphide solution and by visual observation. The potential shifts to a more positive potential (anodic) in the presence of low concentrations of Na2S and shifts to a more negative potential in the presence of high concentrations of Na2S. The potential shifts to less negative values in the presence of the nanoalumina coating. This means that the nanoalumina coating increases the protective behaviour of the silver alloy. Visual observation indicates that the surface is bright and there is no tarnish formation,especially with the homogenous distribution of the nanoalumina layer on the surface.
Keywords:Silver alloy; Silver tarnish; Tarnish removal; Nanoalumina coating
Introduction
Silver tarnishing is a natural process that occurs on the surface of silver jewelry or artifacts. When silver artefacts are exposed to sulphide-polluted atmospheres, they tarnish. The tarnish begins with a change in the colour of silver to light yellow; then it begins to turn into a darker brown shade as the spots become more intense. In extreme cases, silver luster may appear very dark, almost black. Silver artefacts fade over time by merging with the polluting elements of the atmosphere. The blackish film thus formed makes these artefacts inappropriate for display. Silver tarnish or corrosion is a real industrial and archaeological problem [1,2]. The tarnishing process is influenced by the environment in which the silver is exposed, such as hydrogen sulphide (H2S), sulphur dioxide (SO2), and organic sulphur compounds; liquid phases containing sulphide; and solid phases, such as elemental sulphur, egg, yolk, onions, wood, and vulcanized rubber. The tarnishing of silver is also accelerated by high humidity in the environment. When silver is exposed to a sulphur-contaminated environment, it tarnishes. Self-assembled monolayers are a tarnishing-resistant, invisible, and protective coating [3]. When you consider the elements that cause silver to tarnish, you’ll see that it’s difficult to avoid tarnishing, and it’s not a simple process that’s not always the same in different museums. Before tarnishing, the presence of sulphur species, oxygen, and water vapour is required [4].
The most frequently used methods to remove tarnish are mechanical polishing or chemical stripping of the silver surface. An electrochemical reduction by galvanic coupling with an aluminum piece (anode) or an external current source is also employed. The polishing or chemical tarnish removal method removes a certain amount of underlying silver as well as the surface layer of tarnish [5]. But the ethics of curators and conservators require them to keep the initial aesthetics harmless and stable so that the applied treatment does not alter the silverwork’s integrity of materials, that is, not to remove any material from the object. The modified parts should be readily recognizable, and the reversibility of treatments allows the recovery of the initial state. Therefore, it is recommended that tarnish removal should not be done frequently on museum objects. Also, it is important to protect the surface after tarnish removal. Formation of film on the silver surface by applying different chemical compounds was applied. Two methods for preventing the formation of a dark deposit, for example, were tested: an electrodeposited poly(amino-triazole) film and a surface treatment in hexadecane-thiol [6]. The protection by poly(aminotriazole) is not reliable for all nuances of silver. In contrast, the film formed with hexadecane-thiol showed satisfactory properties. The plasma polymer coating system developed by the Fraunhofer Institute (IFAM) in Bremen is a suitable tarnish protection process for silver jewels [7]. Tarnish protection of silver by octadecanethiol self-assembled monolayers prepared in aqueous micellar solution was investigated [8]. The film controlled the transfer of ions markedly and the sulfuration rate of silver was reduced substantially. Ag-based alloy samples are protected by a Paraloid B72 coating containing different percentages of Al2O3 nano pigments and treated by plasma to improve corrosion protection. The plasma treatments are carried out by deposition of SiOx thin films in plasma fed with tetraethylorthosilicate (TEOS), Ar and O2 [9]. A number of patents have been addressed for silver protection against corrosion [10,11]. A paint and method are disclosed for protecting the silver film on mirrors. The paint in its preferred embodiment includes a metal carboxylate which will contribute metal ions, namely stannous octoate for the contribution of stannous (II) ions in an amount of 0.5% or greater by weight. Silver is protected against tarnishing using an Atomic Layer Deposition method. In the Atomic Layer Deposition method, a thin film coating is formed on the surface of silver by depositing successive molecular layers of the coating material. Coating materials such as Al2O3 or zirconium oxide, for example, can be used [12]. Silver surfaces were coated with atomic layer deposition alumina/titania nanolayers [13].
The aim of this work is to study the formation and removal of silver tarnish, and to apply the nanoalumina coating to protect silver after the removal of tarnish.
Experimental Procedures
Materials and electrolytes
Silver alloy disc samples are used to run the experiments, and its composition is about 92.50% silver and 7.50% copper. The area of the sample is 2.54 cm2 and it is connected on one side by Ni- Cr wire for electric connection. The surface of the silver alloy was polished with 1000 and 1200 grade emery paper to get a wellfinished surface.
Aluminum (Al) sheets was used for galvanic coupling with silver alloy samples.
Different concentrations of sodium sulfide (0.1, .01, 0001, 6.4 × 10-6 M Na2S) were used to form the tarnish on the surface of the silver alloy. Nano-alumina (Al2O3) powder, acetone and paraloid were used to prepare the bath to protect the silver alloy.
Electrochemical measurements
The free corrosion potential of silver alloy samples was recorded in different concentrations of sodium sulfide at room temperature. Electrochemical reduction was performed by galvanic coupling to an aluminum sample (anode) to remove tarnish from silver alloys. Also, external cathodic current was used on silver alloy samples to remove tarnish. A 10% (1:1) solution of sodium carbonate (Na2CO3) and sodium bicarbonate (NaHCO3) was used during cleaning of silver alloy. Electrochemical measurements were operated using AMEL Potentiostat-Model 553. The reference electrode was saturated calomel electrode (SCE) and the counter electrode was platinum.
Gloss measurements
The reflectometer is a measuring unit for determining the gloss value of paint coatings, plastics, ceramics and metal surfaces. The light is directed at the sample surface at a specific angle and the reflected light is measured photoelectrically (reflectometer). Depending on the typical gloss value of the test object, reflectometers that direct light onto the surface at different angles (geometry) can be used. Highly reflective surfaces give high gloss values and mute surfaces give low gloss value. Gloss measurements was operated using PicoGloss 503-Erichsen GMBH & Co KG-Germany. The images were taken by using LUMIX Camera-8.1 MEGAPIXLES.
Results and Discussion
Free corrosion potential measurements
Various concentrations of Na2S are used to tarnish silver alloys at room temperature. In the presence of a high concentration of sodium sulphide (0.1 M), a dark tarnish is formed on the surface. Also, the tarnish forms in the presence of 0.01M Na2S, but it is slower than in the case of 0.1M of Na2S. Light tarnish on the silver surface forms in low concentrations of 0.0001 M and 06.4 × 10-6 M of Na2S at RT. Free corrosion potential follows during the tarnish process, as shown in Figure 1. As soon as a silver electrode is immersed in a solution of sodium sulfide, the potential becomes -840 mVSCE in the case of 0.1M of Na2S, -750 mVSCE in 0.01M of Na2S, and -210 mVSCE in 0.0001M of Na2S. In the case of 6.4 × 10-6 M Na2S, the potential becomes +50 mV. In all concentrations, the potential remained essentially constant, though fluctuations of a few mV were maintained all through the experiment The potential shifts to anodic direction (positive potential) in the presence of low concentrations of Na2S (0.0001M and 6.4x10-6M). This observation means that there is no tarnish formation and the positive potential is due to silver metal. As known, silver is one of the noble metals and its free corrosion potential always shifts to positive direction. Visual observation shows very light orange color. This color may be due to formation of the copper oxide on the silver surface. In case of high Na2S concentrations (0.1 and 0.01M of Na2S), the potential shifts to cathodic direction (more negative potential). Visual observation shows dark color in presence of the 0.1 and 0.01M of Na2S. The dark color is due to formation of Ag2O (equation 2), which transforms to Ag2S (equation 3). The potential difference between low and high concentrations is nearly -600 mV. Based on Pourbaix Diagram [14], the big difference in open circuit potential between low and high concentrations is due to formation of the tarnish layer of silver sulfide (Ag2S) on silver surface. The standard equilibrium potential of the Ag2S formation is -890 mV, as in equation 1. Also, it has been noted that the potential of silver decreases to more cathodic value due to hydrogen evolution reaction [1]. This is consistent with the proposed discharge of hydrogen, since the hydrogen overvoltage on silver is about 700 mV [15]. This means that more tarnish will form on the surface in presence of high concentrations of Na2S. Accordingly, it can be concluded that tarnish rarely occurs with increasing potential to more positive and heavy tarnish occurs with more negative potential.
Figure 1:Open circuit potential during silver tarnishing in various concentrations of Na2S at RT.
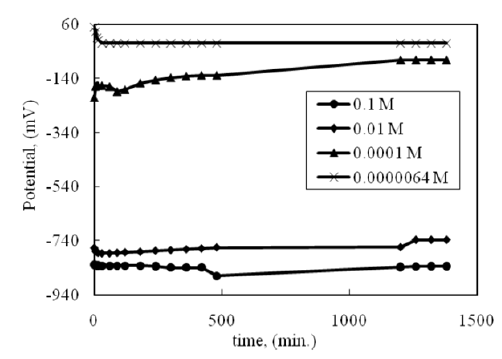

The mechanism of tarnishing in atmospheric environments is explained by Liagna et. al [16]. In case of liquid phase, the mechanism has been suggested as follows: it consists of a slow induction process by the slow formation of Ag2O on the silver surface followed by the reaction with H2S, as in equations 2 and 3.

In fact, silver has a very low affinity for oxygen, therefor, H2S may function in two directions: • oxygen in Ag2O can be replaced by sulfur to form Ag2S, as in equation 3.

H2S may react with oxygen, as in equation 4, to form sulfur, which reacts with metallic silver and the film growth occurs at a more rapid rate, as in equation 5.

As stated, for more tarnished silver, the rate-determining step is diffusion through the tarnish layer itself [1]. Therefore, the diffusion of H2S through the tarnish layer will be the rate determining step, as in equation 3.
There is a study to explain silver tarnishing process by using molecular dynamic simulation [17]. The research team proposed a technique called “ReaxFF (reactive field force)”. The simulation process of the technique explained the mechanisms behind silversulfur and silver-oxygen reactions at the atomic level. As explained, silver oxide forms much slower than silver sulfide because when the S8 molecules get close to the silver, they quickly dissociate into individual atoms and react with the silver. Conversely, O2 dissociates more slowly and has a lower “sticking” strength due to a higher kinetic barrier.
Electrochemical measurement of tarnish removal
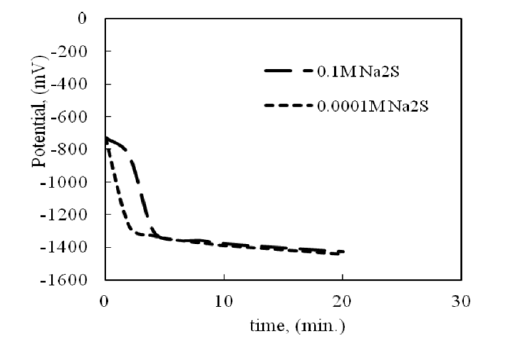
Cathodic current and galvanic reduction:The tarnish removal proceeds by applying a cathodic current density of 0.5 mA/cm2 in 10 % (1:1) of sodium carbonates and sodium bicarbonate solution. Applying the cathodic current shows a satisfactory result for the removal of tarnish, which forms in presence of 0.1M and 0.0001M of Na2S. Figure 2 shows the free corrosion potential variation during tarnish removal. The potential tends to more negative value and becomes stable after completing tarnish removal.
Figure 2:Potential variation during cathodic reduction of silver tarnish in 10% (1:1) sodium carbonate-bicarbonate solution.
Three silver samples were immersed in 0.1 M Na2S for 1 hour at room temperature. The dark tarnish layer was formed on the surface. These samples were coupled with aluminum sheets of an area ratio 1 (Ag):10 (Al), 1:50 and 1:100 cm2. Tarnish removal was operated in solution of 10 % (1:1) sodium carbonate-bicarbonate at room temperature. As observed in Figure (3), the gloss value increases with increasing time of immersion. Also, Figure (4) represents the potential variation during tarnish removal. The potential decreases from -700 to -950 within 20 minutes of tarnish reduction.
Figure 3:Effect of Al anode area (cm2) on silver tarnish removal in 10% (1:1) sodium carbonate-bicarbonate solution.
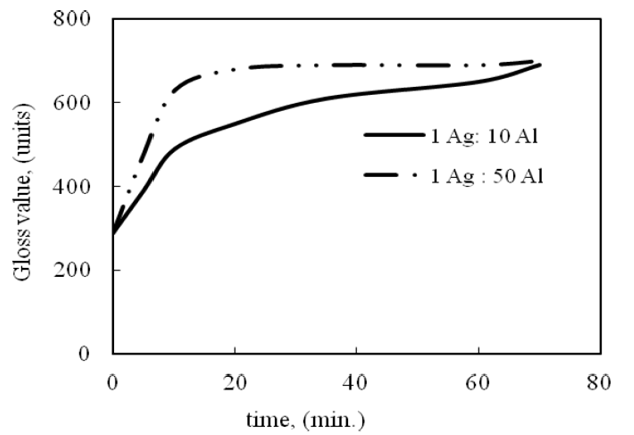
Figure 4:Free corrosion potential variation of galvanic coupling of silver and Al during tarnish removal in 10% (1:1) sodium carbonatebicarbonate solution.
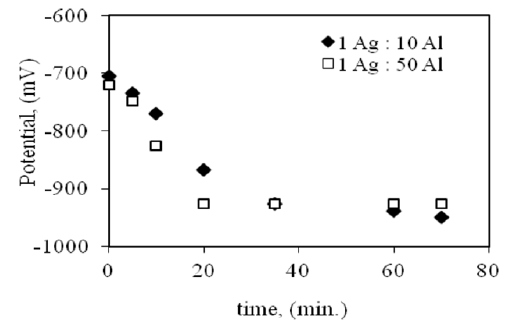
The effect of the aluminum area is observed in the first 10 minutes of immersion. The gloss value increases from 29 % to 63 % in case of an area ration 1:50 cm2, where it increases from 29 % to 49 % in case of an area ration 1:10 cm2. This means that the increase of Al area increases tarnish removal. The area ratio of silver and Al, 1:100 cm2, has the same effect as in case of an area ratio 1:50 cm2, but the time of cleaning decreases (5-7 min). Visual observation indicates that the surface cleans after 10 minutes of immersion in case of an area ratio 1: 50 cm2. After 60 minutes of immersion, the gloss value is nearly the same both in case of an area of 1:10 cm2 and 1:50 cm2. This means that after certain time the effect of an Al area on tarnish removal is diminished. It can be explained on the basis that silver coupling with Al sheet leads to clean the tarnish, but the surface still covers with mute and dull layer. This can be removed by gentle polishing with a silver polishing cloth or cotton wool dipped in suitable solvent, e.g., acetone. The galvanic coupling method is suitable for certain artifacts, especially which have flat surface. This is to ensure good contact between the objects and the aluminum surface. The good contact leads to better result of cleaning the tarnish. This method can be used with other objects of uneven surface, but the bends and holes cannot be cleaned properly.
Silver protection by nanolayer of Al2O3
Nanolayer preparation:The 0.015M Al2O3 nanoparticles suspends in certain volume of acetone in ultrasonic bath for 30 min in order to achieve better pigment dispersion. Certain weight of paraloid adds to the suspension and stirs with a magnetic stirrer in a closed container. The suspension of Al2O3 and paraloid mix in an ultrasonic bath again for 15 minutes. The suspension brushes on the silver surface. The coat thickness is from 5 to 10 microns, depending on the viscosity of the vehicle and brushing times. It is important to brush the surface properly to make homogeneous layer.
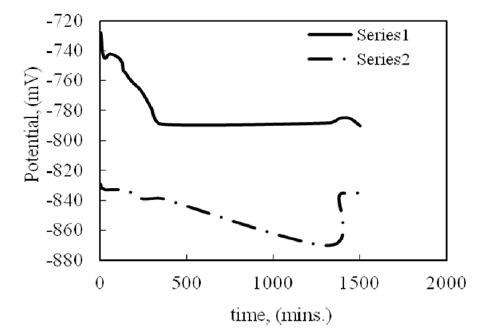
Effect of the aggressive environment on the nanolayer: The homogeneity of Al2O3 nanolayer and its resistance against aggressive environment were tested. Silver sample covered with the nanolayer immerses in 0.1 M Na2S. Figure 5 represents the relation between the open circuit potential and time of immersion in 0.1M Na2S. As observed, the potential shifts to less negative values in presence of the nanolayer. This means that the layer increases the protective behavior of silver. Visual observation indicates that the surface is bright but black spots appear on the surface after two days of immersion. The appearance of the black spots means that the coat is not homogenous on the surface and the thickness of the coat is lower than 5 microns. This behavior may be due to surface contamination during coating process. Solvent and deionized water is apparently a source of surface contamination by way of drying stains. The contaminants are either present in the solvents or dissolved from material on the surface itself and re-distributed when the fluid evaporates [18]. Another silver sample covers with the same coat, but the thickness of the coat is increased to 10– 15 microns. This sample immerses in the same solution of 0.1M Na2S for the same period of time. It is noted that the sample maintains its appearance as it was before immersion. There are no black spots on the surface. This means that the coating is homogeneously distributed over the surface. Nanoalumina protection is up to 90% in terms of reflectivity, that is, no alteration by visual inspection. Also, the presence of Polaroid, which is made from a crystalline material called herapathite and can be extruded into polymers to make polarizing filters, may help to form the homogenous film on the silver surface [19,20]. This behavior can be explained on the basis that this film markedly limits the diffusion of dissolved oxygen on the silver surface, and thus slows down the rate of silver sulfide (Ag2S) formation., as in equation 5 [15].
Figure 5:Relation between potential and time of immersion of silver alloy sample in 0.1M Na2S: series 1: with Al2O3 nancoat and series 2: without nanocoat.
Conclusion
Silver tarnishing occurs in the presence of various concentrations of Na2S. Free corrosion potential shifts to more positive potential with a decrease of Na2S concentrations. Shifting to a more negative potential, increasing Na2S concentrations, increasing gloss values, and increasing immersion time are all factors in the formation of tarnish at medium to heavy layer levels. The tarnish removal could be done electrochemically by applying cathodic current, but this method is not highly efficient with heavy tarnish. Galvanic coupling with an aluminum anode is also used for tarnish removal. This method is effective with different degrees of tarnish. The area of the aluminum anode has influenced the tarnish removal. The tarnish removal is improved by increasing the area of Al. Nanoalumina coating is applied on the silver surface. The homogeneous distribution and thickening of the coating on the surface prevent the appearance of dulling spots when immersed in a concentrated solution of Na2S. The transparency of the coating is achieved by gloss measurements.
Acknowledgement
All authors, whether or not they are cited in the study, are gratefully acknowledged by the author. In addition to all the National Research Centre, universities, firms, and organizations mentioned or cited in the paper, as well as those not included.
Conflict of Interest
No conflict of interest.
References
- Hubertus A Ankersmita, Norman H Tennentb, Simon F Watts (2005) Hydrogen sulfide and carbonyl sulfide in the museum environment - Part 1, Atmospheric Environment 39: 695.
- Hallet K, Thickett D, McPhail DS, Chater RJ (2003) Application of SIMS to silver tarnish at the British Museum. Applied Surface Science 203-204; 789.
- Chenghao Lianga, Changjiang Yangb, Naibao Huanga (2009) Tarnishprotection of silver by octadecanethiol self-assembled monolayers prepared in aqueous micellar solution. Surface and Coatings Technology 203(8): 1034-1044.
- McEwan JJ, Scott M, FE Goodwin FE (2005) 16th International Corrosion Congress, Beijing, China.
- Bernard MC, Dauvergne E, Evesque M, Keddam M, Takenouti H (2005) Reduction of silver tarnishing and protection against subsequent corrosion. Corrosion Science 47: 663.
- Magali Evesque, Michel Keddam, Hisasi Takenouti (2004) The formation of self-assembling membrane of hexadecane-thiol on silver to prevent the tarnishing. Electrochimica Acta 49(17): 2937-2943.
- Frey T, Kögel M (2003) Tarnish protection of silver jewels by plasmapolymer coatings. Surface and Coatings Technology 174-175: 902-904.
- Chenghao Lianga, Changjiang Yang, Naibao Huang (2009) Surface and Coatings Technology 20: 1034.
- Sabrina Grassinia, Emma Angelini, Yangwu Mao, Jelica Novakovic, Panayota Vassiliou (2011) Aesthetic coatings for silver-based alloys with improved protection efficiency. Progress in Organic Coating 72: 131.
- DeNuccio DJ (2005) Paint for Silver Film Protection and Method, US Patent no. 6,979,478 B1.
- Newman IJ, Washburn D (2005) Ionic Silver Complex. U.S. Patent no. 6,838,095.
- Milja M, Soininen, Pekka S, Sami S (2009) Protective Coating of Silver. US Patent no. 20090004386.
- Paussaa L, Guzmana L, Marina E, Isomakib N, Fedrizzi L (2011) Protection of silver surfaces against tarnishing by means of alumina/titania-nanolayers. Surface and Coatings Technology 206: 976.
- Pourbaix M (1974) Atlas of Electrochemical Equilibria in Aqueous Solutions. National Association of Corrosion Engineers, Houston, TX, pp. 19-89, 313-20 and 115-17.
- Warren GW, Drouven B, Price DW (1984) Relationships between the Pourbaix Diagram for Ag-S-H2O and Electrochemical Oxidation and Reduction of Ag2S. Metallurgical Transactions B, 15B: 235.
- Chenghao Lianga, Changjiang Yangb, Naibao Huanga (2009) Tarnish protection of silver by octadecanethiol self-assembled monolayers prepared in aqueous micellar solution. Surface and Coatings Technology 203(8): 1034.
- G Saleh, C Xu, S Sanvito (2019) Silver Tarnishing Mechanism Revealed by Molecular Dynamics Simulations. Angew Chem Int Ed 58(18): 6017-6021.
- MG Dowsett, A Adriaens, M Soares, H Wouters, VVN Palitsin, et al. (2005) Nuclear Instruments and Methods in Physics Research B 239: 51.
- Kahr B (2009) Uncover the Polaroid Structure. Science 324: 1407.
- Kemsley J (2009) Chemical and Engineering News.
-
AA El-Meligi*. Silver Alloy Protection by Applying Nanomaterial Coat after Tarnish Removal. Glob J Eng Sci. 10(3): 2022. GJES.MS.ID.000739.
-
Silver alloy, Silver tarnish, Tarnish removal, Nano alumina coating
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






