 Review Article
Review Article
Recent Advances and Emerging Challenges in Parkinson’s Disease Management: Diagnostic Innovations, Therapies, and Complementary Approaches
Shivani A Dave1*, Dhruv J Shah2,a, Khushi V Pandya1 and Kunal Vora3
1School of Pharmacy, Massachusetts College of Pharmacy and Health Sciences, USA
2School of Arts and Sciences, Massachusetts College of Pharmacy and Health Sciences, USA
3College of Professional Studies, Northeastern University, USA
aEqual first author
Shivani A Dave, Massachusetts College of Pharmacy and Health Sciences, 179 Longwood Ave, Boston, Massachusetts, USA
Received Date: March 28, 2025; Published Date: April 02, 2025
Abstract
As of 2019, more than 8.5 million persons worldwide were affected with Parkinson’s disease (PD), the second most prevalent neurological disorder among older adults. Despite advances in understanding disease pathology, PD remains incurable, with existing treatments predominantly being symptomatic. Recent advancements in diagnostic precision have emerged through innovative biomarkers, including neuron-derived extracellular vesicles containing α-synuclein, volatile organic compounds (VOCs) analysis, and fluid biomarkers such as serum autoantibodies and cerebrospinal fluid seed amplification assays (SAAs). Neuroimaging has also advanced, with techniques like neuromelanin-sensitive MRI and quantitative susceptibility mapping (QSM) offering enhanced visualization of brain pathology. The complexity of diagnosis is further increased by overlapping symptoms between PD, Alzheimer’s disease, and Lewy body dementia, requiring precise biomarkers and clinical supervision. Psychiatric comorbidities such as depression, psychosis, and apathy significantly influence PD prognosis, indicating aggressive disease progression. Complementary and alternative medicine (CAM) interventions, notably acupuncture, probiotics, traditional Chinese exercises (TCEs) such as Tai Chi, and adherence to the Mediterranean diet, have shown significant effectiveness in reducing both motor and non-motor symptoms, thereby improving quality of life. Future treatment approaches include investigating potential disease-modifying treatments like α-synuclein aggregation inhibitors, gene therapies, and mitochondrial enhancers; focusing on neuroinflammation and changes in the gut microbiome; and using artificial intelligence for early PD diagnosis and progression monitoring. However, significant challenges remain, particularly in ensuring the inclusion of older adults in clinical trials, optimizing geriatric-focused research, and translating promising therapies from experimental stages into clinically viable interventions. This review summarizes the current PD management, highlighting recent diagnostic, therapeutic, and complementary treatment advancements while outlining emerging opportunities and critical challenges in treating this increasingly prevalent neurodegenerative disease.
Keywords: Parkinson’s Disease; Biomarkers; Neuroimaging; Disease-Modifying Therapies; Complementary and Alternative Medicine; Artificial Intelligence; Pharmacological Treatment
List of Abbreviations: PD: Parkinson’s disease; AD: Alzheimer’s disease; HDI: Human Development Index; SDI: Sociodemographic Index; EV: Extracellular Vesicle; AI: Artificial intelligence; CAM: Complementary and alternative medicine; DBS: Deep brain stimulation; VOCs: Volatile Organic Compounds; AUC: Area Under the Curve; UPDRS: Unified Parkinson’s Disease Rating Scale; SMD: Standard mean difference; MD: Mean Difference; CSF: Cerebrospinal fluid; MRI: Magnetic resonance imaging; DAT: Dopamine transporter; SAAs: Seed amplification assays; TCEs: Traditional Chinese exercises; GBA: Glucocerebrosidase; LRRK2: Leucine-rich repeat kinase 2; α- syn: Alpha-synuclein; GCase: Glucocerebrosidase enzyme; PT: Physical Therapy; OT: Occupational Therapy; CBT: Cognitive behavioral therapy; SLP: Speech-Language Pathology; NfL: Neurofilament light chain; SCFA: Short-chain fatty acids; AAV: Adeno-associated virus; OR: Odds Ratio; MoCA: Montreal Cognitive Assessment; PAC-QOL: Patient Assessment of Constipation Quality of Life; CCS: Chronic Constipation Severity; RRP: Rectal Resting Pressure; QSM: Quantitative Susceptibility Mapping; MAO-B: Monoamine oxidase B; COMT: Catechol-O-methyltransferase; NMDA: N-methyl-D-aspartate; AUC: Area under the curve; TNF-α: Tumor necrosis factor-alpha; TCE: Traditional Chinese Exercise; IL: Interleukin; CRP: C-reactive protein; MAPT: Microtubule-associated protein tau; SNpc: Substantia nigra pars compacta; DaT: Dopamine transporter; GDNF: Glial cell line-derived neurotrophic factor; UDCA: Ursodeoxycholic acid
Introduction
Parkinson’s disease is the second most common neurodegenerative disorder, primarily affecting individuals over the age of 60, with incidence rates increasing with age [1,2]. Parkinson’s disease is a neurological disorder with motor symptoms like tremors, bradykinesia, stiffness in their limbs, and impaired balance and non-motor symptoms such as cognitive impairment, mood and behavior problems, and sleep disorder. A meta-analysis encompassing 83 studies from 37 countries highlighted a global pooled prevalence of 1.51 cases per 1000 people.3 This analysis further highlighted a significant increase in Parkinson’s disease prevalence since the 1980s, with a pronounced rise in the past two decades.3 Prevalence was also shown to increase with both age and Human Development Index (HDI) and Sociodemographic Index (SDI), which are comprehensive measures of a country’s level of socioeconomic development, reflecting the growing number of older people in the population and differences in awareness and access to healthcare [3].
Globally, the prevalence of Parkinson’s disease has doubled in the past 25 years, with global estimates in 2019 showing over 8.5 million individuals living with Parkinson’s disease [4]. Approximately 500,000 Americans are diagnosed with Parkinson’s disease, and the number of people diagnosed with Parkinson’s disease in the United States is expected to double by 2040 [5]. The Unified Parkinson’s Disease Rating Scale (UPDRS) is a clinician’s tool to assess the severity of Parkinson’s symptoms-scores ranging from 0 (no impairment) to 199 (severe impairment). Meta-analysis indicated an average annual increase of 3.9 points on the UPDRS, indicating a gradual worsening of disease severity over time. This progression occurs independently of placebo effects and symptomatic treatments [6]. In the longitudinal model-based meta-analysis of 66 arms from 4 observational studies and 17 interventional trials, the occurrence of temporary placebo effects in clinical trials complicates the evaluation of treatment by mimicking genuine drug efficacy and highlights the need for trustworthy progression biomarkers to assess treatment effects more accurately beyond symptomatic relief [6].
Parkinson’s disease results from the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) in the midbrain. This leads to dopamine deficiency and disruption of the neural network of basal ganglia that impairs motor movement [7]. The deposition of alpha-synuclein protein forming Lewy bod ies disrupts cellular function, which, with aging, brings problems like mitochondrial dysfunction, oxidative stress, loss of protein, and neuroinflammation, accelerating neurodegeneration in the SNpc. The interaction of these age-related changes with the characteristics of Parkinson’s disease suggests that aging intensifies the vulnerability of dopaminergic neurons to the damaging processes in Parkinson’s disease [8]. Although, the exact cause of Parkinson’s disease remains unknown, age, pathogenic variants in genes (such as LRRK2, CHCHD2, VPS35, SNCA, PARKIN, DJ1, and PINK1), exposure to neurotoxic agents (pesticides such as paraquat, rotenone, 2,4-D, and several organochlorides), lifestyle behavior (such as smoking habits), and physical factors have been postulated to play a role in Parkinson’s disease [9].
Recent Advances and Factors Influencing the Diagnosis
Recent advancements in diagnostic criteria and biomarkers for Parkinson’s disease (PD) emphasize a growing precision in distinguishing PD from healthy controls and other neurodegenerative diseases. Biomarkers have notably transitioned from traditional imaging methods to innovative fluid-based assays and extracellular vesicles (EVs). Neuron-derived EV α-synuclein from plasma has emerged as a significant biomarker, demonstrating marked elevation in PD patients (standard mean difference [SMD] = 1.84, 95% CI [0.76–2.93], p = 0.0009), particularly when enriched using neuron- specific L1CAM immunocapture methods, yielding substantial diagnostic accuracy [10]. Notably, oligomeric forms of α-synuclein within EVs exhibit even greater diagnostic power (SMD = 3.36, 95% CI [1.69–5.58], p = 0.0003), suggesting their role as strong markers of PD pathology.11 Likewise, volatile organic compounds (VOCs) analysis from breath and sebum represents a promising, non-invasive method with pooled sensitivity and specificity of approximately 81% and 76%, respectively, and an impressive area under the ROC curve (AUC = 0.85) and with a diagnostic odds ratio of approximately 14 [11]. Fluid biomarkers further enhance diagnostic specificity, particularly serum autoantibodies and cerebrospinal fluid (CSF) proteins. Serum autoantibody panels yielded exceptional sensitivity (93.1%) and specificity (100%) in differentiating early PD from controls [12].
Innovative neuroimaging methods significantly enhanced PD diagnosis accuracy. Dopamine transporter (DAT) SPECT imaging remains standard for confirming dopaminergic neuron loss in PD but is limited in progression tracking.10 Recent techniques, such as seed amplification assays (SAAs) detecting misfolded α-synuclein in cerebrospinal fluid (CSF), represent a transformative diagnostic advancement. SAAs achieved remarkable pooled sensitivity (88%) and specificity (95%) for distinguishing PD patients, including prodromal stages, from controls [10]. Furthermore, emerging MRI modalities, including neuromelanin-sensitive imaging and quantitative susceptibility mapping (QSM), offer valuable structural insights by detecting substantia nigra changes and brain iron accumulation linked directly to disease pathology [10]. Additionally, inflammatory markers like IL-6, TNF-α, and CRP are significantly elevated in PD CSF, reinforcing inflammation’s role in PD pathogenesis [13].
PD frequently exhibits overlapping symptoms with Alzheimer’s disease (AD) and Lewy body dementia (DLB), complicating differential diagnoses. Approximately 30-50% of advanced AD patients manifest extrapyramidal symptoms like rigidity and bradykinesia [14]. Conversely, dementia is prevalent in advanced PD, highlighting clinical and pathological intersections, particularly evident through common genetic vulnerabilities [14]. For instance, the MAPT gene polymorphism elevates PD dementia risk, while co-occurrence of α-synuclein and tau pathologies indicates cross-seeding phenomena that amplify neuropathological progression [14].
Moreover, age-related psychiatric comorbidities significantly influence PD diagnosis and prognosis. Depression, psychosis, and apathy strongly correlate with worsened cognitive impairment and accelerated motor progression. Depression alone is associated with substantial cognitive decline (SMD = 0.37, p = 0.0085) and accelerated motor impairment (SMD = 0.46, p = 0.0011) [15]. Psychosis similarly predicts pronounced cognitive deterioration (SMD = 0.44, p < 0.0001), implying that these psychiatric symptoms are potent indicators of aggressive disease progression.15 In contrast, anxiety and impulse control disorders show minimal prognostic impact, underscoring the specificity of depression, psychosis, and apathy as key markers of poorer PD outcomes [15].
Current Approach in PD Treatment
This section presents the pharmacological and non-pharmacological treatments for Parkinson’s disease. The tables below summarize the key therapeutic strategies aimed at managing symptoms and enhancing patients’ quality of life [16-18].
Pharmacological Treatment
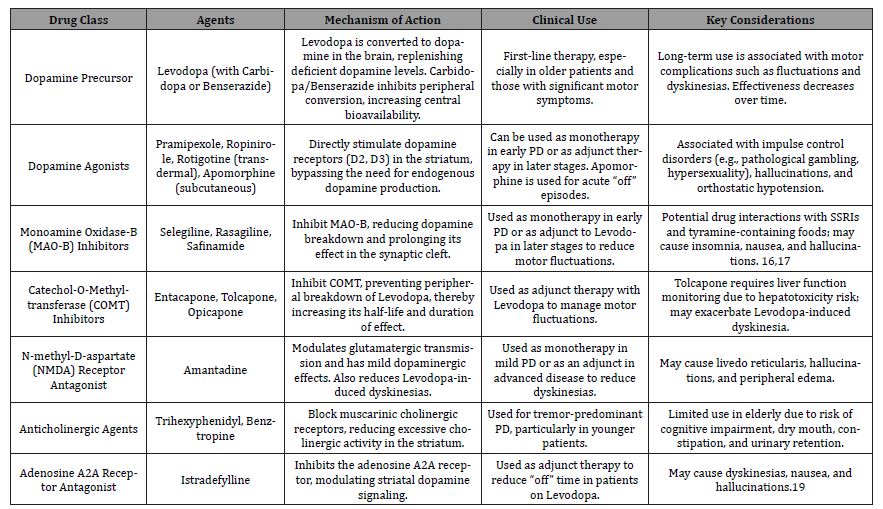
Table 1 represents pharmacological management of Parkinson’s disease which includes levodopa-based therapy, dopamine agonists, MAO-B inhibitors, COMT inhibitors, anticholinergics, and amantadine, each targeting different aspects of symptom control.
Table 1: Pharmacological treatment for Parkinson’s Disease Management.
Non-Pharmacological Treatment
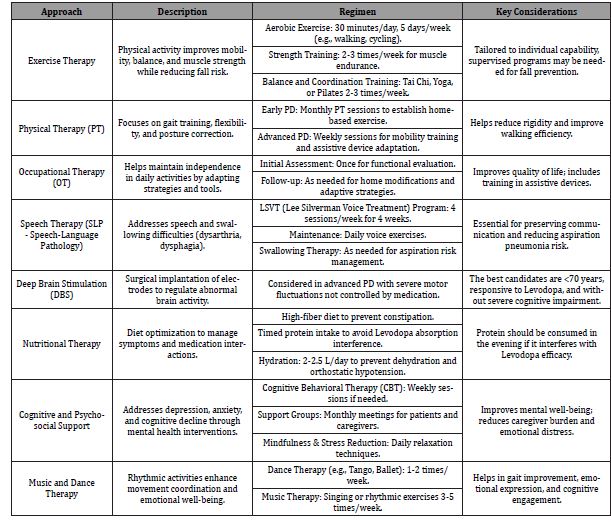
Table 2 represents non-pharmacological interventions such as physical therapy, occupational therapy, speech therapy, deep brain stimulation, and lifestyle modifications. They complement drug therapy to improve mobility, daily function, and overall well-being [16, 20, 21].
Table 2: Non-Pharmacological treatment for Parkinson’s Disease management.
Complementary and Alternative Medicine in Parkinson’s Disease
Complementary and alternative medicine (CAM) therapies are gaining attention for their role in managing Parkinson’s disease (PD) symptoms. Recent meta-analyses provide statistically significant evidence supporting various CAM interventions, including acupuncture, traditional Chinese exercises, dance, Tai Chi, Mediterranean diet adherence, and probiotic supplementation. Acupuncture has emerged as an effective intervention, significantly improving neuropsychiatric symptoms such as depression (SMD = -0.70, 95% CI [-0.98, -0.42], p < 0.00001), anxiety (SMD = -0.78, 95% CI [-1.43, -0.14], p = 0.02), cognitive function (MoCA scores; WMD = 2.74, 95% CI [2.43, 3.05], p < 0.00001), and overall quality of life among PD patients [22]. The therapeutic efficacy of acupuncture, combined with its favorable safety profile, positions it as a viable adjunctive therapy for managing PD-related neuropsychiatric manifestations [22]. Additionally, recent evidence supports acupuncture as an effective complementary treatment for constipation in Parkinson’s disease (PD). A meta-analysis involving 960 PD patients found that acupuncture significantly increased complete spontaneous bowel movements (WMD = 1.49, 95% CI [0.86, 2.11], p < .00001), enhanced patients’ constipation-related quality of life (PAC-QOL: WMD = -11.83, 95% CI [-15.67, -7.99], p < .00001), improved chronic constipation severity (CCS: SMD = -0.99, 95% CI [-1.40, -0.58], p < .01), and effectively increased rectal resting pressure (RRP: WMD = 2.13, 95% CI [0.44, 3.82], p < .05) [23].
Probiotic supplementation also effectively manages constipation in PD patients. A systematic review and meta-analysis of 11 randomized controlled trials involving 756 participants demonstrated significant improvements in weekly complete bowel movements (SMD = 0.73, 95% CI [0.54, 0.92], p < .00001) and improving constipation-related quality of life scores (PAC-QOL: SMD = -0.79, 95% CI [-1.19, -0.39], p < .001), although probiotics did not significantly alter fecal characteristics, defecation effort, or general motor symptoms (MDS-UPDRS III scores) [24]. Traditional Chinese exercises (TCEs), encompassing Tai Chi, Qigong, and related practices, have also demonstrated substantial therapeutic benefits. TCEs significantly alleviate depression (SMD = -1.30, 95% CI [-2.10, -0.49], p = 0.002), anxiety (SMD = -1.11, 95% CI [-2.14, -0.08], p = 0.03), sleep disorders, and enhance cognitive functions (SMD = 0.91, 95% CI [0.44, 1.38], p = 0.0001), thereby substantially improving patients’ quality of life [25]. Tai Chi specifically has shown notable improvements in balance (MD = 2.06, 95% CI [1.35, 2.78], p < 0.00001), mobility (MD = -1.59, 95% CI [-2.28, -0.91], p < 0.00001), and gait speed (SMD = 0.59, 95% CI [0.28, 0.91], p = 0.0002), though its effects on gait amplitude and endurance remain uncertain [26].
Dietary interventions, notably the Mediterranean diet (MD), also present protective effects against PD development and progression. Adherence to the MD significantly reduces PD risk, with an overall odds ratio (OR) of 0.75 (95% CI [0.66, 0.84]) compared to low adherence groups. The protective effect is even stronger during prodromal stages (OR = 0.67, 95% CI [0.59, 0.76]), emphasizing the preventive potential of this diet.27 The MD’s anti-inflammatory and antioxidant-rich components may counteract neurodegeneration, positioning it as a recommended lifestyle modification for PD management [27].
Future Scope and Challenges
Symptomatic treatments are the sole therapies for Parkinson’s disease (PD), which is still an incurable disease despite tremendous progress in the disease biology. A few of the newer topics of research covered in this section are the role of neuroinflammation and gut microbiota, early diagnosis, artificial intelligence (AI)-aided diagnostics, the possibility of disease-modifying treatments (DMTs), and geriatric-focused clinical trials.
Advances in Early Detection and Targeted Treatment
Since Parkinson’s symptoms usually present after significant neuronal loss, the earlier diagnosis continues to be an issue. Recent biomarker discoveries such as dopamine transporter (DaT) imaging, neurofilament light chain (NfL), and cerebrospinal fluid (CSF) α-synuclein have made it possible to detect earlier and more accurately. Further, subgroups with monogenic Parkinson’s disease (PD) caused by mutations of the LRRK2, GBA, and SNCA genes have also been identified because of improvements in genetic profiling [28]. There are various patient subgroups that possess various underlying biology for Parkinson’s disease (PD), and there are customized treatment options in the pipeline that act on such pathways. A case in point is glucocerebrosidase (GCase) enhancers in GBA-associated PD and LRRK2 inhibitors in those who are genetically predisposed. The hope is that the trend towards personalization in the treatment pipeline would improve therapy outcomes and reduce toxicity [29].
Potential for AI-Driven Diagnostics and Disease Monitoring
The detection of Parkinson’s disease (PD) and the tracking of disease progression are being transformed by artificial intelligence (AI) and machine learning (ML). AI platforms can read high-dimensional data, including neuroimaging scans, gait signals, and voice recordings, to identify faint preclinical signals of PD. AI and wearable sensor technology provide continuous and non-invasive monitoring of motor symptoms with objective quantification of disease outside of clinic visits [30]. Moreover, the identification of new PD biomarkers may be aided by AI-driven examination of multi-omics data, such as transcriptomics, metabolomics, and genomes. AI-based predictive modelling can be able to classify patients according to the rates at which their diseases progress, which can be useful in designing enhanced clinical trials and optimizing individualized treatment regimens [30].
Role of Neuroinflammation and Gut Microbiome in PD Pathogenesis
It is increasingly well recognized that neuroinflammation plays a pivotal role in the pathophysiology of PD. Elevated levels of pro-inflammatory cytokines, including TNF-α and IL-1β, and microglial activation have been linked to dopaminergic neuron degeneration. Inhibition of neuroinflammatory pathways with immunomodulatory drugs, including NLRP3 inflammasome inhibitors and colony-stimulating factor 1 receptor inhibitors, is one of the promising treatment options. Concurrently, mounting evidence indicates that dysbiosis of gut microbiota is implicated in Parkinson’s disease [31]. Patients with Parkinson’s disease have been found to show a shift in gut microbiota composition, which is defined by a decrease in SCFA-producing species and an increase in pro-inflammatory bacteria. Neurodegeneration is believed to be controlled by the gut-brain axis, which is controlled by vagal nerve circuits, immunological signals, and microbial metabolites. Possible disease-altering approaches, such as fecal microbiota transplantation, prebiotic treatment, and probiotic therapy, are being studied [31].
Potential for Disease-Altering Therapies
Levodopa and the dopamine agonists are examples of the pharmacologic treatments of Parkinson’s disease that now have beneficial effects on symptoms but do not stop the disease’s course. Various experimental therapies attempt to alter the disease course by acting upon the basic pathophysiologic mechanisms. α-Synuclein aggregation inhibitors: There are ongoing clinical trials for small molecules and monoclonal antibodies (e.g., prasinezumab) that block α-synuclein from misfolding and transmission [32]. Gene therapy: Gene-based therapies are being researched, including CRISPR-mediated gene-editing approaches to target LRRK2 mutations and AAV delivery of neurotrophic factors (e.g., GDNF and neurturin) [33]. Mitochondrial and Autophagy Enhancers: To recover mitochondrial function and cellular homeostasis, drugs like ursodeoxycholic acid (UDCA), coenzyme Q10 derivatives, and mitophagy inducers are being explored [34]. Assuming these strategies are promising, there are still many issues to be resolved, including blood-brain barrier penetration, long-term safety, and efficacy measurement in human trials.
Geriatric-Focused Clinical Trials and Pipeline Study Status Needed
The lack of older persons in clinical studies is a principal barrier in PD clinical studies. Although patients over 60 years account for most PD cases, frailty and comorbidities related to advanced age tend to exclude them from study participation. The external validity of trial results to actual PD populations is constrained by exclusion. Designing clinical trials with a geriatric emphasis is critical to ensuring that experimental therapies are effective and safe for aging citizens. Elderly patients may be incorporated using patient-centred outcomes, remote monitoring equipment, and adaptive trial design.
Several disease-modifying drugs are currently under development for clinical use, targeting various pathological mechanisms. LRRK2 inhibitors, such as DNL151 and BIIB122, have advanced to Phase II/III trials, aiming to address genetic mutations linked to Parkinson’s disease. Additionally, glucocerebrosidase enhancers like Ambroxol and Venglustat are being evaluated in Phase II trials to improve lysosomal function. Promising late-phase trials are also underway for α-Synuclein-targeting immunotherapies, including Prasinezumab and Cinpanemab, which aim to mitigate protein aggregation [32]. Furthermore, early-phase trials are exploring neurotrophic factor-based gene therapies, such as AAV-GDNF and AAV-Neurturin, to promote neuronal survival and regeneration. Careful validation through large, well-controlled studies in a variety of patient populations will be necessary for the successful introduction of these candidates to clinical application [28].
Conclusion
Parkinson’s disease (PD) is a complex neurodegenerative disorder with rising prevalence, necessitating constant advances in diagnosis, treatment, and management. This review emphasized on recent advancements in diagnostic biomarkers, imaging modalities, and AI-driven assessment that support early diagnosis and differentiation of the disease. Levodopa-based therapies remain the gold standard for symptom management despite the emergence of new disease-modifying therapies like α-synuclein aggregation inhibitors, gene therapy, and mitochondrial enhancers that hold the potential for regulating disease course. Alternative medicine, including acupuncture, traditional Chinese exercises, Mediterranean diet, and probiotics has proven beneficial in relieving the symptoms, enhancing quality of life. However, there are some concerns such as under-enrolment of older subjects in clinical trials, the requirement of more treatment regimens individualized according to patient needs, and the heterogeneity of PD pathophysiology. Future studies should focus on precision medicine, AI-aided surveillance, and gut microbiota-directed interventions to optimize therapeutic approaches. These advances will be crucial in the progression of PD management, enhancing patient outcomes, and moving towards successful disease-modifying therapies, with the goal of slowing down disease progression.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- (2025) Parkinson’s Disease | National Institute of Neurological Disorders and Stroke.
- (2025) Parkinson’s Disease: Causes, Symptoms, and Treatments | National Institute on Aging.
- Jinqiao Zhu, Yusha Cui, Junjiao Zhang, Rui Yan, Dongning Su, et al. (2024) Temporal trends in the prevalence of Parkinson’s disease from 1980 to 2023: a systematic review and meta-analysis. Lancet Healthy Longev 5(7): e464-e479.
- (2025) Launch of WHO’s Parkinson disease technical brief.
- (2025) Parkinson’s Disease: Challenges, Progress, and Promise | National Institute of Neurological Disorders and Stroke
- Arshad U, Rahman F, Hanan N, Chen C (2023) Longitudinal Meta-Analysis of Historical Parkinson’s Disease Trials to Inform Future Trial Design. Movement Disorders 38(9): 1716-1727.
- Kunvariya AD, Dave SA, Modi ZJ, Patel PK, Sagar SR (2023) Exploration of multifaceted molecular mechanism of angiotensin-converting enzyme 2 (ACE2) in pathogenesis of various diseases. Heliyon 9(5): e15644.
- Coleman C, Martin I (2022) Unraveling Parkinson’s Disease Neurodegeneration: Does Aging Hold the Clues? J Parkinsons Dis 12(8): 2321-2338.
- Ben-Shlomo Y, Darweesh S, Llibre-Guerra J, Marras C, San Luciano M, et al. (2024) The epidemiology of Parkinson’s disease. The Lancet 403(10423): 283-292.
- Zarkali A, Thomas GEC, Zetterberg H, Weil RS (2024) Neuroimaging and fluid biomarkers in Parkinson’s disease in an era of targeted interventions. Nat Commun 15(1): 5661.
- Adrina Habibzadeh, Vahid Reza Ostovan, Omid Keshavarzian, Sina Kardeh, Seyed Sasan Mahmoudi, et al. (2023) Volatile organic compounds analysis as promising biomarkers for Parkinson’s disease diagnosis: A systematic review and meta-analysis. Clin Neurol Neurosurg 235: 108022.
- Costa MFBNA da, Reisdorfer E, Kempfer SS, Fernandes GCM, Porporatti AL, et al. (2018) Diagnostic validity of biomarkers in Parkinson’s Disease: systematic review and meta-analysis. Rev Bras Enferm 71(6): 3074-3083.
- Yi Qu, Jiangting Li, Qixiong Qin, Danlei Wang, Jingwei Zhao, et al. (2023) A systematic review and meta-analysis of inflammatory biomarkers in Parkinson’s disease. NPJ Parkinsons Dis 9(1): 18.
- Li W, Li JY (2024) Overlaps and divergences between tauopathies and synucleinopathies: a duet of neurodegeneration. Transl Neurodegener 13(1): 16.
- Ella Burchill, Cameron James Watson, Jack B Fanshawe, James Brunton Badenoch, Emma Rengasamy, et al. (2024) The impact of psychiatric comorbidity on Parkinson’s disease outcomes: a systematic review and meta-analysis. The Lancet Regional Health - Europe 39: 100870.
- Chopade P, Chopade N, Zhao Z, Mitragotri S, Liao R, et al. (2023) Alzheimer’s and Parkinson’s disease therapies in the clinic. Bioeng Transl Med 8(1): e10367.
- Power D, Crotty GF (2025) Advances in the Pharmacological Management of Parkinson’s Disease. Curr Treat Options Neurol 27(1): 22.
- Halli-Tierney AD, Luker J, Carroll DG (2020) Parkinson Disease. Am Fam Physician 102(11): 679-691.
- Cummins L, Cates ME (2022) Istradefylline: A novel agent in the treatment of ‘“off”’ episodes associated with levodopa/carbidopa use in Parkinson disease. Mental Health Clinician 12(1): 32-36.
- Lee TK, Yankee EL (2022) A review on Parkinson’s disease treatment. Neuroimmunol Neuroinflamm 8: 222.
- Foltynie T, Bruno V, Fox S, Kühn AA, Lindop F, et al. (2024) Medical, surgical, and physical treatments for Parkinson’s disease. The Lancet 403(10423): 305-324.
- Tan W, Xie F, Zhou J, Pan Z, Liao M, et al. (2024) Efficacy and safety of acupuncture therapy for neuropsychiatric symptoms among patients with Parkinson’s disease: A systematic review and meta-analysis. Clin Rehabil 38(8): 1044-1062.
- Li Z, Niu Q, Yang K, Zhao K, Yin S, et al. (2024) Acupuncture for constipation in Parkinson’s disease: A systematic review and meta-analysis of randomized controlled trials. Medicine (United States) 103(29): e38937.
- Jin X, Dong W, Chang K, Yan Y, Liu X (2024) Efficacy of probiotic supplements on Parkinson’s disease: A systematic review and meta-analysis. Complement Ther Med 82: 103045.
- Weiqiang Tan, Zhaoquan Pan, Jiawei He, Tiexiong Wu, Feng Wu, et al. (2025) Traditional Chinese exercises for the treatment of neuropsychiatric symptoms in Parkinson’s disease: A systematic review and meta-analysis. Complement Ther Med 89: 103134.
- Lou L, Xiang C, Hu Y, Yang J (2025) Tai Chi improves balance, mobility and gait function of the lower limbs in patients with Parkinson’s disease: a systematic review and meta-analysis. Eur J Med Res 30(1): 107.
- Zhao J, Peng Y, Lin Z, Gong Y (2025) Association between Mediterranean diet adherence and Parkinson’s disease: a systematic review and meta-analysis. Journal of Nutrition, Health and Aging 29(2): 100451.
- Francesco Cavallieri, Rubens G Cury, Thiago Guimarães, Valentina Fioravanti, Sara Grisanti, et al. (2023) Recent Advances in the Treatment of Genetic Forms of Parkinson’s Disease: Hype or Hope? Cells 12(5): 764.
- Menozzi E, Toffoli M, Schapira AHV (2023) Targeting the GBA1 pathway to slow Parkinson disease: Insights into clinical aspects, pathogenic mechanisms and new therapeutic avenues. Pharmacol Ther 246: 108419.
- Yuzhe Yang, Yuan Yuan, Guo Zhang, Hao Wang, Ying-Cong Chen, et al. (2022) Artificial intelligence-enabled detection and assessment of Parkinson’s disease using nocturnal breathing signals. Nat Med 28(10): 2207-2215.
- Kalyanaraman B, Cheng G, Hardy M (2024) Gut microbiome, short-chain fatty acids, alpha-synuclein, neuroinflammation, and ROS/RNS: Relevance to Parkinson’s disease and therapeutic implications. Redox Biol 71: 103092.
- E Srinivasan, G Chandrasekhar, P Chandrasekar, K Anbarasu, A S Vickram, et al. (2021) Alpha-Synuclein Aggregation in Parkinson’s Disease. Front Med (Lausanne) 8: 736978.
- Poojitha Pinjala, Kamatham Pushpa Tryphena, Renuka Prasad, Dharmendra Kumar Khatri, Woong Sun, et al. (2023) CRISPR/Cas9 assisted stem cell therapy in Parkinson’s disease. Biomater Res 27(1): 46.
- Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ (2012) The role of autophagy in Parkinson’s disease. Cold Spring Harb Perspect Med 2(4): a009357.
-
Shivani A Dave*, Dhruv J Shah,a, Khushi V Pandya and Kunal Vora. Recent Advances and Emerging Challenges in Parkinson’s Disease Management: Diagnostic Innovations, Therapies, and Complementary Approaches. Glob J Aging Geriatr Res. 3(5): 2025. GJAGR. MS.ID.000572.
-
Parkinson’s Disease, Diagnostic Innovations, Therapies, Neuroimaging, Pharmacological Treatment, Artificial Intelligence
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






