 Review Article
Review Article
Testosterone Therapy and Association with All- Cause Mortality Decrease in Men with Adult-Onset Testosterone Deficiency
Mruga M Dhebar1, Richard C Strange2 and Sudarshan Ramachandran1,2,3*
1Department of Clinical Biochemistry, University Hospitals Birmingham NHS Foundation Trust, West Midlands, United Kingdom
2School of Pharmacy and Bioengineering, Keele University, Staffordshire, United Kingdom
3Department of Mechanical and Aerospace Engineering, Brunel University London, United Kingdom
Sudarshan Ramachandran, Department of Clinical Biochemistry, University Hospitals Birmingham NHS Foundation Trust, Good Hope Hospital, Sutton Coldfield, West Midlands B75 7RR, United Kingdom
Received Date: May 30, 2025; Published Date: June 03, 2025
Abstract
Adult-onset testosterone deficiency (TD) is diagnosed in men with low serum testosterone levels and associated symptoms, after excluding primary and secondary hypogonadism and Klinefelter syndrome. In this review we initially describe the exponential relationship between age and mortality, exploring the Gompertz/Makeham model. We examine work by Vaupel determining the age category that mortality decrease leads to greatest increase in population longevity. This is followed by examining the evidence regarding mortality changes in men with adult-onset TD; there appears accumulating evidence that these patients when untreated are at greater mortality risk and importantly testosterone therapy decreases the risk. The mediators of the change in mortality in the untreated and treated men is not clear. We finally show using a cohort of men with adultonset TD and type 2 diabetes, that mortality is in accordance with the Gompertz/Makeham model. However, although the exponential pattern was maintained in men treated/untreated with TTh, the graphs varied demonstrating benefit which appeared greater in the older age-group. Thus, it is possible that based on the work of Vaupel, TTh in adult-onset TD and T2DM could have a major bearing on male longevity.
Keywords: Age; Adult-Onset Hypogonadism; Type 2 Diabetes; Testosterone Therapy; All-Cause Mortality; Haematocrit; Phosphodiesterase Type 5 Inhibitors; Sex Hormone Binding Globulin
Introduction
Benjamin Gompertz in 1825 suggested that mortality is an exponential function of age; the proposed model being μ(x) = α * eβx, (μ(x) = mortality rate, age = x, α and β = constants representing the ageing rate) [1]. The Gompertz law of mortality underwent a modification by Makeham with the inclusion of extrinsic mortality (y); μ(x) = α * eβx + y [2]. Prior to the work of Gompertz, Joseph Addison and Kay Pearson focused on external factors of mortality, describing life as a walkthrough over a bridge [3]. With the absence or decrease of significant external factors associated with mortality such as conflict, mortality due to intrinsic factors increases exponentially with age as suggested by Gompertz. Interestingly the Gompertz/Makeham model takes into account population heterogeneity with each subgroup perhaps demonstrating varying coefficients and constants found in the equations [3,4]. It is perhaps reasonable to speculate that treatment of chronic pathologies in adulthood could add to the heterogeneity (presentation phenotypes and clinical outcomes) with different coefficients and constants (in the Gompertz/Makeham model) being evident in the untreated and treated cohorts. Postponing individual death can be considered to increase population longevity. An interesting conundrum was posed by Vaupel in 1986 as to the age decade of mortality aversion that would be associated with the greatest increase in population life expectancy [5]. Swedish life tables, in contrast to common perception, suggested that life expectancy would be maximally increased if mortality was decreased in men and women aged 67-77 and 74-84 years, respectively [5]. Adult-onset testosterone deficiency (TD) is prevalent in 0.6-12% of men over 50 years of age and 40-70% in men with type 2 diabetes (T2DM), and has been associated with increased mortality [6-8]. The prevalence is seen to increase with age as demonstrated by the European Male Ageing Study (EMAS); 0.1%, 0.6%, 3.2%, and 5.1% in men aged between 40-49, 50-59, 60-69, and 70-79 years respectively [9]. Hence, in view of potential increase in life expectancy in this age group, based on the work by Vaupel [5], and the Gompertz law of mortality [1,2], we review mortality patterns observed in this highly prevalent condition, and changes observed following testosterone therapy (TTh). This review focuses principally on work carried out by our research network and describes initially the association between mortality and adult-onset TD, then changes in the association observed with TTh. We then speculate on factors that could be mediators of these associations. Finally, we show that the relationship between age and mortality in untreated men with adult-onset TD was exponential and adhered to the Gompertz/ Makeham model. The relationship following TTh although changed in character remained exponential. This review is based on data from basic science, trials and reviews familiar to our research group or selected from PubMed (US National Library of Medicine) in the separate sections described.
Mortality in men with adult-onset TD
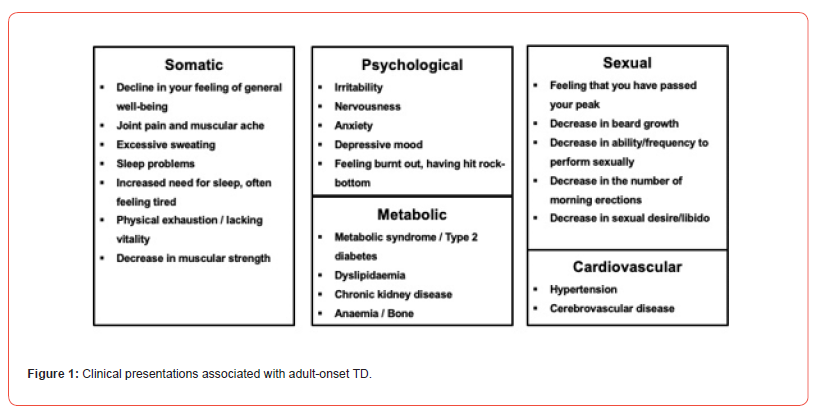
Adult-onset TD is considered in men with low serum total testosterone (TT) <12.1nmol/L and/or free testosterone (FT) < 0.225nmol/L together with clinical phenotypes associated with low testosterone levels (Figure 1) following exclusion of primary hypogonadism, hypothalamic-pituitary-gonadal axis pathology and Klinefelter syndrome - British Society for Sexual Medicine guidelines [6]. Prior to describing mortality changes associated with treatment(s), it is useful to study mortality rates in untreated men..
Analysis of EMAS in 2014 data by Pye et al. of 2599 men aged between 40-79 years followed-up for 4.3 years (median) indicated that those with three or more hypogonadal symptoms and serum TT levels <8nmol/l were associated with both cardiovascular disease (CVD) mortality (hazard ratio (HR): 3.8, 95% confidence interval (CI): 1.3-10.8) and all-cause mortality (HR: 3.1, 95% CI: 1.7-5.7), the models were adjusted for age, recruitment site, smoking status and general health [10]. A further analysis of the updated EMAS data of 1788 men by Antonio et al. in 2022 demonstrated various phenotypes defining sexual health were associated with mortality (independent of serum TT and FT), over a mean follow-up of 12.6 years [7]. Similarly changes in serum TT and FT were associated with mortality in the Danish Monitoring Trends and Determinants of Cardiovascular Disease (MONICA10, a subgroup of the MONICA1 cohort with a 10-year examination during follow-up) [11]. Analysis of this registry in 2018 included 1167 men aged between 30-60 years over a mean 15.2 years of follow-up. All-cause mortality occurred in 36.1% (421 men) of the men and those with greater baseline serum TT and FT levels demonstrated lower mortality. Further, men with the highest age-related decrease in serum TT and FT had increased mortality, these associations appeared independent of baseline hormone values [11].
The association between adult-onset TD in men with T2DM and mortality was evident in 2 further studies that also included a treatment arm (hormone replacement) [12-15]. Muraleedaran et al in 2013 evaluated mortality in 581 men with T2DM over a mean follow-up of 5.8 years [12]. The cohort were split into two using a serum TT of 10.4nmol/L at baseline. Men in the lower serum TT (≤10.4nmol/L) demonstrated a near doubling of mortality (HR: 2.02, 95% CI: 1.2-3.4) than their counterparts with higher hormone values. Importantly the regression models were adjusted for body mass index, glycaemic control, prior CVD, smoking status, statin therapy and antihypertensive such as angiotensin-converting enzyme inhibitors/angiotensin receptor blocker use [12].
A similar study by Hackett et al analysed the men screened for the BLAST (Burntwood, Lichfield, Atherstone Sutton Coldfield and Tamworth) randomised controlled study (RCT) with allcause mortality as the outcome [13,14]. This BLAST screened cohort comprising 857 men with T2DM evaluated the association between serum TT and FT levels and all-cause mortality over a mean follow-up of 3.8 years [13,14]. The cohort was stratified into normal testosterone (320 men with serum TT >12nmol/L and FT >0.25nmol/L) and low (537 men with serum TT ≤12.0nmol/L or FT ≤0.25nmol/L) groups. The latter group of 537 men was further split into 362 men remaining untreated and the remaining 175 men on TTh (testosterone undecanoate (TU)) [13,14]. Mortality in men with low serum testosterone not on TTh had significantly (HR: 0.62, 95% CI: 0.41-0.94, the Cox regression model adjusted for baseline age) higher mortality (16.9%) compared with the subgroup with normal values (11.3%) [13].
Mortality in men with adult-onset TD on TTh
The above section clearly shows that mortality is increased in men with adult-onset TD. The following section investigates whether this increment is countered with hormone replacement. A few studies with design, methodology and analyses that have been criticised for fundamental flaws, hinted that TTh was associated with increased risk of CVD and mortality and led to safety concerns [16]. Vigen et al in 2013 analysed data of 8709 patients from the Veterans Administration healthcare system who had previously had coronary angiography with serum TT <10.4nmol/L and subsequently prescribed TTh [16]. They studied a composite of all-cause mortality, myocardial infarction, and stroke in the study cohort; the event rate over 3 years of follow-up appeared lower (10.1%) in individuals treated compared with their untreated counterparts (21.2%) [16]. However, statistical models including adjustment with over 50 variables led to a reversal of the event rate (TTh: 25.7%, untreated: 19.9%). Further, 100 women were acknowledged to have been included in this supposedly all male cohort. Whilst Vigen et al. used a composite of mortality and CVD as the study outcome, there were other studies that reported increased CVD rates associated with TTh [17,18]. Finkle et al. studied 55,593 insurance claims and compared myocardial infarction rates in the period before (12 months) and after (3 months) TTh [17]. Nonfatal myocardial infarctions appeared higher in men after TTh was commenced; especially in men aged 65 years or more [17]. It is essential to highlight that the reference group of men were men using phosphodiesterase type 5 (PDE5)-inhibitors, which have shown to independently decrease cardiovascular and allcause mortality [13,14,19-21]. Further, serum TT values were not available for analysis in the pre and post TTh phases. We would also wish to make the point that a 3-month post TTh period appeared insufficient to evaluate clinical outcomes post TTh. It is important to note that the authors accepted the points that we have raised [17]. The Testosterone in Older men with Mobility Limitations (TOM) Trial consisted of 209 men (included in the safety assessment) with limited mobility randomised to placebo or testosterone gel with maximal voluntary muscle strength during leg press exercise as the primary outcome (the target sample size was 252 men) [18]. Although the primary outcome showed significant improvement, the RCT was prematurely halted due to ‘adverse events cardiovascular in nature’, a heterogeneous composite of stenting, bypass procedures, peripheral oedema, hypertension, arrythmias, electrocardiographic changes, stroke and syncope, appearing higher in the TTh arm (23 men) compared to those on placebo (5 men) [18]. Adverse events more specific to CVD termed ‘atherosclerosis related events’ comprising myocardial infarction, sudden death, angioplasty, coronary artery bypass surgery and stroke was also seen to be higher in the TTh arm (7 men) than the placebo arm (1 man). The authors acknowledged limitations such as the small sample size, the nature of adverse events data collection, CVD not being either a primary or secondary end point with no structured evaluation of events performed [18].
Contrary to the above findings there is accumulating evidence from longitudinal studies of TTh being associated with a decrease in mortality in addition to improvement in hypogonadal symptoms of [22]. A study by Shores et al in 1031 men (>40 years of age) with serum TT ≤8.7 nmol/L showed that over a follow-up of 4 years allcause mortality was lower in the 398 men prescribed TTh (10.3%) compared to the remaining 633 untreated men (20.7%), the analysis was adjusted for confounding variables [23]. Subgroup analysis suggested that significant mortality decrease was restricted to men with T2DM and those free from coronary heart disease at baseline. This indicated that sample heterogeneity could influence findings in studies of similar cohort size and follow-up [23]. The significant observation of TTh associated decrease in mortality in men with T2DM was confirmed by Muraleedaran et al. [12] and Hackett et al. in the United Kingdom [13,14].
Muraleedaran et al. stratified 238 men with baseline serum TT ≤10.4nmol/L into 64 men commenced on TTh and compared mortality rate with the remaining untreated 174 men; the untreated men were at higher risk of mortality (HR: 2.3, 95% CI: 1.3-3.9) [12]. Hackett et al. further stratified the previously mentioned BLAST screened cohort of 537 men with T2DM with low serum testosterone levels (serum TT ≤12.0nmol/L or FT ≤0.25nmol/L) into 175 men on TTh and the remaining untreated 362 men [13,14]. The untreated men were at significantly higher risk of mortality (16.9%) over a mean follow-up of 3.8 years compared to those on TTh (HR: 0.38, CI: 0.16 - 0.90, the Cox regression analysis adjusted for age) [13]. Mann et al in 2024 analysed a registry of 737 men with adult-onset TD over a median follow-up period of 9.5 years [24]. Treatment with TTh was associated with significant lower mortality compared to the men who opted against TTh (HR: 0.23, CI: 0.14-0.40, and when adjusted for age at baseline HR: 0.41, CI: 0.23-0.72). The association remained independent of age, waist circumference, glycaemic control, dyslipidaemia, blood pressure, smoking status and T2DM [24]. Interestingly the decrease in mortality was seen to be statistically significant only in men with greater CVD risk factors (waist circumference, lipids, and blood pressure >median values, as well as men currently smoking, and diagnosed with T2DM at baseline) [24]. These results suggest once again that cohort heterogeneity could affect the association between TTh and mortality even after a near 10-year follow-up [24].
systematic review/meta-analysis of 35 RCTs with 17 RCTs providing individual level datasets in 5601 men aged >18 years and follow-up >3 months by Hudson et al in 2022 [25]. Th mortality data from 14 of the trials with mortality data showed lower mortality in 1621 men on TTh (0.4%) than their 1519 untreated counterparts (0.8%), although statistical significance was not reached (odds ratio (OR): 0.46, 95% CI: 0.17-1.24, p=0.13) [25]. The association between TTh and mortality could have been affected by the relatively short treatment exposure (mean follow-up: 9.5 months), low mortality rate and phenotypic heterogeneity of the individual trial cohorts [25].
Factors possibly mediating the associations between mortality in men with adult-onset TD and TTh
Although there appeared a few, highly criticised studies suggesting that TTh was associated with increased mortality, subsequent longitudinal studies have robustly refuted the findings. We now speculate as to possible factors that may lead to the abovementioned findings. Before delving through individual factors, we must try and provide an understanding of adult-onset TD. Serum TT and FT alone cannot provide the diagnostic criteria for this condition as the hypogonadal presentation phenotypes may be influenced by differential effects of free and bound hormone levels [26]. This in turn can be affected by binding proteins such as SHBG and receptor resistance mediated by factors such as androgen receptor CAG repeats that are not routinely measured in clinical practice. Including symptoms attributed to low testosterone is a neat interim solution (until more individual drivers of adult-onset TD are determined) to diagnosis of the condition as the symptoms may be considered a surrogate for testosterone action [26]. It is possible that severity of each symptom will differ between each patient, hence this could perhaps provide an explanation for heterogeneity of clinical outcomes following TTh.
SHBG levels are often seen to be low in adult-onset TD and it appears to correlate inversely with body mass index in men on or not on TTh [27]. Interestingly a positive correlation is evident between SHBG levels and the severity of the classifying symptoms of adult-onset TD, this appeared independent of serum TT [28]. We can speculate that an elevated SHBG could exert this effect by virtue of a negative association with FT [29]. Serum SHBG levels were seen to decrease in men on TTh in the BLAST RCT and we cannot provide a robust explanation for this phenomenon [30]. Tint et al. and Ramachandran et al. showed that increased SHBG was associated with higher mortality, independent of age (this is important as SHBG increases with age [31,32] Ramachandran et al. analysed the BLAST screened cohort and found that mortality was positively associated with age and SHBG (independent of calculated FT) and inversely associated with serum TT [32]. It was speculated that the wide distribution of SHBG made a causative relationship between the binding protein and mortality unlikely and the association was likely to be mediated by serum FT. It was suggested that serum FT calculated via algorithms may not be a complete reflection of testosterone activity [32].
Elevation of haematocrit (HCT) levels is the commonest adverse effect of TTh [6]. Gagnon et al., using the Framingham cohort of 5209 individuals over a follow-up of 34 years, showed the highest HCT quintile was associated with greater mortality and CVD [33]. Interestingly, the association between HCT and CVD appeared non-linear, perhaps J or U shaped [33] and this was confirmed by Boffetta et al investigating 49,983 adult Iranians [34]. A U-shaped relationship between categories of HCT and mortality was seen in both sexes; low and high HCT being associated with increased mortality. It was noted that the optimal HCT level associated with the lowest mortality varied between the sexes [34]. Strange et al. analysed a registry database of 737 men with adult-onset TD where 353 men were on TTh whilst the remaining 384 men opted against hormone replacement [35]. Mortality rates in the men on TTh with TU over a follow-up >105 months varied between the tertiles of HCT at final assessment; 7.4% (HCT of 46-48%), 8.9% (HCT of 49%) and 0.8% (HCT of 50-52%) [35]. Logistic regression analysis of the men on TTh demonstrated that mortality was inversely associated with baseline (OR: 0.49, 95% CI: 0.31-0.79) and increase in HCT (OR: 0.54, 95% CI: 0.34-0.86) up to a HCT level of 52% (the highest value at final assessment in the cohort) [35]. A decrease in HCT is evident with increasing age in men over 18 years [36]. Evidence that the point of inflexion in a U-shaped relationship between HCT and safety may be 52% was provided by Ory et al. who showed that a HCT >52% in men on TTh >12 months was associated with higher CVD and venous thromboembolism, importantly no change mortality was apparent [37].
Adult-onset TD has been associated with the metabolic syndrome (MetS), defined in 2009, as a cluster of phenotypes; central obesity (based on ethnicity specific waist circumference cut-off values), which is considered the principal driver, and a minimum of two of the following 4 risk factors (triglyceride ≥1.7mmol/L, high density lipoprotein cholesterol <1.03mmol/L (males) or <1.29mmol/L (females), blood pressure ≥135/85 mm Hg and fasting plasma glucose ≥5.6mmol/L) [38,39]. The MetS prevalence like that of adult-onset TD increases with age [40]. The MetS and the classifying phenotypes are seen to be associated with increased mortality [41,42]. Thus, it is tempting to consider that improvement in the classifying factors of the MetS that has been described following TTh, may mediate the association between TTh and decrease in mortality [24,43,44]. However, analysis of the BLAST screened cohort did not show that the association between TTh and decreased mortality was not mediated by CVD risk factors included in the MetS classification [45].
Apart from the TTh related HCT increase (up to 52%) possibly increasing oxygen delivery and leading to lower mortality, none of the other studies discussed demonstrate mechanisms that may facilitate the associations between increased mortality and adultonset TD and the decrease observed with TTh. We would state that adult-onset TD, a highly prevalent pathology with many longitudinal studies suggesting TTh related benefit requires a well-designed study with a long follow-up. We would also add that such a trial should include a mechanistic arm so that mechanisms of benefit could be elicited. The interaction between TTh and PDE5 inhibitors should also be evaluated as many men are co-prescribed these agents. PDE5 inhibitors have been associated with decrease in CVD and mortality in men with adult-onset TD [13,14,19-21]. Analysis of the BLAST screened cohort suggested that both therapies were independently associated with decreased mortality in men with T2DM and adult-onset TD [13].
How TTh affects age-related mortality
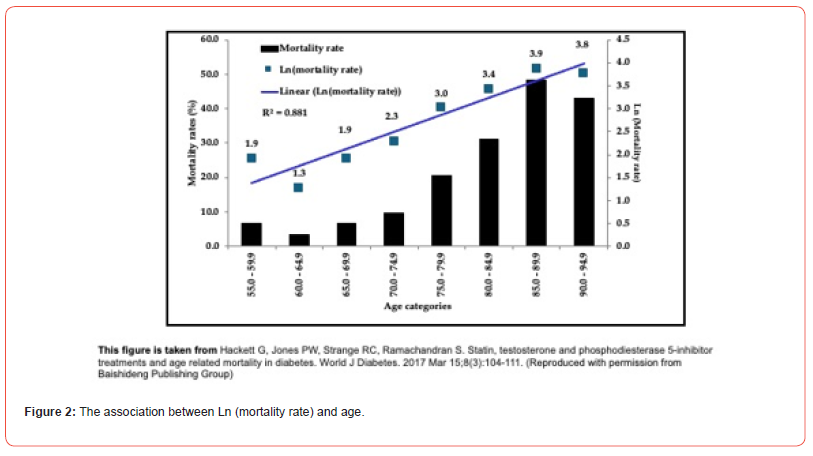
We have shown that adult-onset TD, a condition prevalent in men aged over 50 years has a higher mortality and TTh is associated with a reduction of the mortality. It would be interesting to see whether TTh alters the association between age and the probability of mortality, this association is expected to be exponential if the Gomperz/Makeham model is adhered to [1,2]. This analysis of the BLAST screened cohort showed the relationship between mortality rate and age categories to be exponential (demonstrated in Figure 2 by a linear association between Ln (mortality rate) and age with a R2 value of 0.881) [14]. Logistic (and logit) regression analyses were carried out on the study cohort to initially study the relationship between death (reference: survival) and age (in years) at death or final follow-up visit as the independent variable; age was significantly associated with mortality (OR: 1.08, 95% CI: 1.06-1.11) [14]. Following this, the analysis was repeated in men with adult-onset TD and T2DM, with age and testosterone status/therapy at final assessment as independent variables in a single model. Low T (362 men with serum TT ≤12.0nmol/L or FT ≤0.25nmol/L)/untreated with TTh was selected as reference and compared with the Low T (175 men with serum TT ≤12.0nmol/L or FT ≤0.25nmol/L)/treated with TTh subgroup. Mortality in the Low T/treated subgroup was significantly lower (OR:0.31, 95% CI: 0.13-0.75) than the Low T/untreated reference subgroup [14]. Age remained significantly associated with mortality (OR: 1.10, 95% CI: 1.07-1.12).
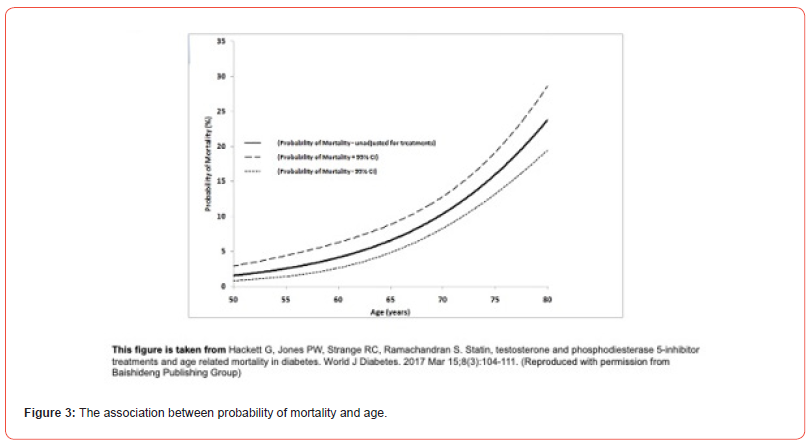
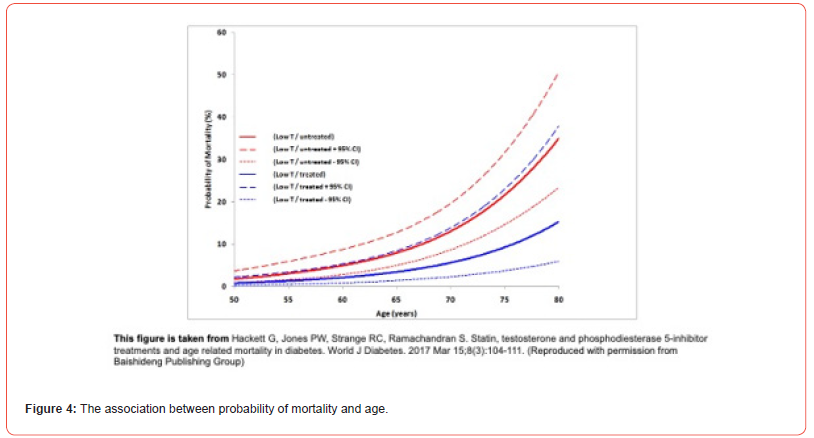
Probability of mortality and 95% CI for each man was estimated using the different logistic regression models and plotted in Figures 3 and 4 [14]. Figure 4 shows that the subgroups stratified by testosterone status and therapy did alter the relationship between age and mortality, however, the exponential pattern was preserved. Figure 4 suggests that the decrease in mortality is perhaps greater in the older men. The unadjusted mortality rates of men on/not on TTh with adult-onset TD (BLAST screened cohort) suggests this as well; mortality rates in the 50.0-59.9 years, 60.0-69.9 years, 70.0- 79.9 years age groups were 4.0%, 7.3%, and 17.5% respectively in the untreated men, whilst 2.1%, 1.7%, and 5.3%, respectively in the men on TTh [13]. Thus, it is possible that based on the work of Vaupel (mortality decrease in men aged 67-77 years was related to the greatest increase in population longevity) TTh in adult-onset TD and T2DM could have a major bearing on male longevity.
Author Contributions
SR, RCS and MMD planning of the paper, preparation of manuscript.
Data Availability
Not applicable.
Sources of Funding:
None.
Acknowledgement
None.
Conflict of Interest
SR has received research grants, travel grants and speakers’ honoraria from Basins Healthcare. MMD and RCS have no disclosures.
References
- Kirkwood TB (2015) Deciphering death: a commentary on Gompertz (1825) On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philos Trans R Soc Lond B Biol Sci 370(1666): 20140379.
- Hallen A (2009) Makeham’s addition to the Gompertz law re-evaluated. Biogerontology 10(4): 517- 522.
- Avraam D, Arnold-Gaille S, Jones D, Vasiev B (2014) Time-evolution of age-dependent mortality patterns in mathematical model of heterogeneous human population. Exp Gerontol 60: 18-30.
- Ledberg A (2020) Exponential increase in mortality with age is a generic property of a simple model system of damage accumulation and death. PLoS One 15(6): e0233384.
- Vaupel JW (1986) How change in age-specific mortality affects life expectancy. Population Studies 40(1): 147-157.
- Hackett G, Kirby M, Rees RW, Jones TH, Muneer A, et al. (2023) The British Society for Sexual Medicine Guidelines on Male Adult Testosterone Deficiency, with Statements for Practice. World J Mens Health 41(3): 508-537.
- Antonio L, Wu FCW, Moors H, Mathei C, Huhtaniemi IT, et al. (2022) EMAS Study Group. Erectile dysfunction predicts mortality in middle-aged and older men independent of their sex steroid status. Age Ageing 51(4): afac094.
- Kapoor D, Aldred H, Clark S, Channer KS, Hugh-Jones T (2007) Clinical and Biochemical Assessment of Hypogonadism in Men with Type 2 Diabetes: Correlations with Bioavailable Testosterone and Visceral Adiposity. Diabetes Care 30(4): 911-917.
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, et al. (2010) EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 363(2): 123-135.
- Pye SR, Huhtaniemi IT, Finn JD, Lee DM, O'Neill TW, et al. (2014) EMAS Study Group. Late-onset Hypogonadism and Mortality in Aging Men. J Clin Endocrinol Metab 99(4): 1357-1366.
- Holmboe SA, Skakkebæk NE, Juul A, Scheike T, Jensen TK, et al. (2018) Individual testosterone decline and future mortality risk in men. Eur J Endocrinol 178(1): 123-130.
- Muraleedaran V, Marsh H, Kapoor D, Channer KS, Jones TH (2013) Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol 169(6): 725-733.
- Hackett G, Heald AH, Sinclair A, Jones PW, Strange RC, et al. (2016) Serum testosterone, testosterone replacement therapy and all-cause mortality in men with type 2 diabetes: retrospective consideration of the impact of PDE5 inhibitors and statins. Int J Clin Pract 70(3): 244-253.
- Hackett G, Jones PW, Strange RC, Ramachandran S (2017) Statin, testosterone and phosphodiesterase 5-inhibitor treatments and age related mortality in diabetes. World J Diabetes 8(3): 104-111.
- Morgentaler A, Lunenfeld B (2014) Testosterone and cardiovascular risk: world's experts take unprecedented action to correct misinformation. Aging Male 17(2): 63-65.
- Vigen R, O'Donnell CI, Barón AE, Grunwald GK, Maddox TM, et al. (2013) Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 310(17): 1829-1836.
- Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, et al. (2014) Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One 9(1): e85805.
- Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. (2010) Adverse events associated with testosterone administration. N Engl J Med 363(2): 109-122.
- Andersson DP, Trolle Lagerros Y, Grotta A, Bellocco R, Lehtihet M, et al. (2017) Association between treatment for erectile dysfunction and death or cardiovascular outcomes after myocardial infarction. Heart 103(16): 1264-1270.
- Anderson SG, Hutchings DC, Woodward M, Rahimi K, Rutter MK, et al. (2016) Phosphodiesterase type-5 inhibitor use in type 2 diabetes is associated with a reduction in all-cause mortality. Heart 102(21): 1750-1756.
- Kloner RA, Stanek E, Desai K, Crowe CL, Paige Ball K, et al. (2024) The association of tadalafil exposure with lower rates of major adverse cardiovascular events and mortality in a general population of men with erectile dysfunction. Clin Cardiol 47(2): e24234.
- Haider KS, Zitzmann M, Ramachandran P, Konig CS, Hackett G, et al. (2024) Testosterone therapy over 60 months improves ageing male symptoms scores in all men with functional testosterone therapy. Aging Male 27(1): 2357548.
- Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM (2012) Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab 97(6): 2050-2058.
- Mann A, Strange RC, König CS, Hackett G, Haider A, et al. (2024) Testosterone replacement therapy: association with mortality in high-risk patient subgroups. Andrology 12(6): 1389-1397.
- Hudson J, Cruickshank M, Quinton R, Aucott L, Aceves-Martins M, et al. (2022) Adverse cardiovascular events and mortality in men during testosterone treatment: an individual patient and aggregate data meta-analysis. Lancet Healthy Longev 3(6): e381-e393.
- Strange RC, Lorde N, Maarouf A, Hackett G, Ramachandran S. Changes in SHBG and the role of calculated free testosterone. (Chapter 3) - “Testosterone treatment for men with hypogonadism and prediabetes/diabetes” edited by Prof. Geoff Hackett and Prof. Mike Kirby, published by Edizioni Minerva Medica (in press).
- Winters SJ (2020) SHBG and total testosterone levels in men with adult onset hypogonadism: what are we overlooking? Clin Diabetes Endocrinol 6: 17.
- Rastrelli G, Corona G, Cipriani S, Mannucci E, Maggi M (2018) Sex hormone-binding globulin is associated with androgen deficiency features independently of total testosterone. Clin Endocrinol (Oxf) 88(4): 556-564.
- Ramachandran S, Hackett GI, Strange RC (2019) Sex hormone binding globulin: A review of its interactions with testosterone and age, and its impact on mortality in men with type 2 diabetes. Sex Med Rev 7(4): 669-678.
- Ramachandran S, Hackett GI, Strange RC (2020) Testosterone replacement therapy: Pre-treatment sex hormone-binding globulin levels and age may identify clinical subgroups. Andrology 8(5): 1222-1232.
- Tint AN, Hoermann R, Wong H, Ekinci EI, MacIsaac RJ, et al. (2016) Association of sex hormone-binding globulin and free testosterone with mortality in men with type 2 diabetes mellitus. Eur J Endocrinol 174(1): 59-68.
- Ramachandran S, Strange RC, Fryer AA, Saad F, Hackett GI (2018) The association of sex hormone-binding globulin with mortality is mediated by age and testosterone in men with type 2 diabetes. Andrology 6(6): 846-853.
- Gagnon DR, Zhang TJ, Brand FN, Kannel WB (1994) Hematocrit and the risk of cardiovascular disease--the Framingham study: a 34-year follow-up. Am Heart J 127(3): 674-682.
- Boffetta P, Islami F, Vedanthan R, Pourshams A, Kamangar F, et al. (2013) A U-shaped relationship between haematocrit and mortality in a large prospective cohort study. Int J Epidemiol 42(2): 601-615.
- Strange RC, König CS, Ahmed A, Hackett G, Haider A, et al. (2021) Testosterone Therapy: Increase in Hematocrit is Associated with Decreased Mortality. Androgens 2(1): 150-159.
- Mahlknecht U, Kaiser S (2010) Age-related changes in peripheral blood counts in humans. Exp Ther Med 1(6): 1019-1025.
- Ory J, Nackeeran S, Balaji NC, Hare JM, Ramasamy AR (2022) Secondary Polycythemia in Men Receiving Testosterone Therapy Increases Risk of Major Adverse Cardiovascular Events and Venous Thromboembolism in the First Year of Therapy. J Urol 207(6): 1295-1301.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, et al. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16): 1640-1645.
- Shipman KE, Strange RC, Ramachandran S (2016) Use of fibrates in the metabolic syndrome: a review. World J Diabetes 7(5): 74-88.
- Bonomini F, Rodella LF, Rezzani R (2015) Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis 6(2): 109-120.
- Guize L, Thomas F, Pannier B, Bean K, Jego B, et al. (2007) All-cause mortality associated with specific combinations of the metabolic syndrome according to recent definitions. Diabetes Care 30(9): 2381-2387.
- Zambon S, Zanoni S, Romanato G, Corti MC, Noale M, et al. (2009) Metabolic syndrome and all-cause and cardiovascular mortality in an Italian elderly population: the Progetto Veneto Anziani (Pro.V.A.) Study. Diabetes Care 32(1): 153-159.
- Li SY, Zhao YL, Yang YF, Wang X, Nie M, et al. (2020) Metabolic Effects of Testosterone Replacement Therapy in Patients with Type 2 Diabetes Mellitus or Metabolic Syndrome: A Meta-Analysis. Int J Endocrinol 2020: 4732021.
- Tishova Y, Kalinchenko S, Mskhalaya G, Hackett G, Livingston M, et al. (2024) Testosterone therapy reduces insulin resistance in men with adult-onset testosterone deficiency and metabolic syndrome. Results from the Moscow Study, a randomized controlled trial with an open-label phase. Diabetes Obes Metab 26(6): 2147-2157.
- Hackett GI, Cole N, Mulay A, Strange RC, Ramachandran S (2019) Long-term Testosterone Therapy in Type 2 Diabetes is associated with reduced Mortality without improvement in conventional cardiovascular risk factors. BJU Int 123(3): 519-529.
-
Mruga M Dhebar, Richard C Strange and Sudarshan Ramachandran*. Testosterone Therapy and Association with All- Cause Mortality Decrease in Men with Adult-Onset Testosterone Deficiency. Glob J Aging Geriatr Res. 3(5): 2025. GJAGR. MS.ID.000574.
-
Parkinson’s Disease, Diagnostic Innovations, Therapies, Neuroimaging, Pharmacological Treatment, Artificial Intelligence
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






