 Research Article
Research Article
Predicting Behavioral Dysfunction in Alzheimer’s Disease
Paul S Foster1*, Cara Clark1, Dana Fuller1 and David W Harrison2
1Psychology Department Middle Tennessee State University, USA
2Virginia Polytechnic Institute, USA
Paul S Foster, Psychology Department, Middle Tennessee State University, 1500 Greenland Drive, Murfreesboro, TN 37132, USA
Received Date:February 19, 2024; Published Date:March 13, 2025
Abstract
Many patients with Alzheimer’s disease (AD) experience significant behavioral dysfunction that ultimately results in placement in managed care facilities. Right frontal lobe dysfunction is associated with a range of behavioral problems. Most patients with AD experience greater degeneration of the left hemisphere. However, some patients experience relative right hemisphere dysfunction and the possibility exists that these patients are more likely to exhibit significant behavioral deficits. The purpose of this study was to examine whether neuropsychological tests of right and left frontal lobe functioning may predict behavioral dysfunction in patients with AD. A total of 20 patients with AD were administered neuropsychological tests of left and right frontal lobe functioning and given the Neuropsychiatric Inventory Questionnaire as a measure of behavioral dysfunction. The result of a multiple regression indicated that the measure of right frontal lobe functioning was the best predictor of behavioral dysfunction. These findings have important implications in predicting patients who might experience significant behavioral dysfunction and hence greater likelihood of placement in a managed care facility.
Keywords:Alzheimer’s Disease; Behavioral Dysfunction; Prediction; Frontal Lobes
Introduction
Many patients with Alzheimer’s disease (AD) will eventually be placed in managed care and these admissions are often the result of significant behavioral problems as opposed to the memory disturbances that accompany the disease [1] reported an increased likelihood of disruptive behaviors in older adults who were at high risk of being institutionalized [2] conducted a one-year longitudinal study to investigate behavioral and psychological symptoms in patients with AD who were institutionalized versus those who were not institutionalized. Patients who were institutionalized exhibited higher agitation and disinhibition scores from the Neuropsychiatric Inventory, as well as lower Mini Mental Status Examination scores and increased caregiver burden. Agitation and aggression have been correlated with caregiver burden [3] and studies have found caregiver emotional distress and burden is significantly related to nursing home admission [4-6]. Hence, the expression of agitation, aggression, and disinhibition appear to be key factors in whether patients with dementia are eventually admitted to nursing homes or other managed care facilities.
Agitation, aggression, and other problematic social behaviors have been consistently related to right frontal lobe dysfunction and reduced capacity for regulatory control. Capacity limitations in these right frontal regions may leave negative emotional analyzers unregulated and even unbridled under duress or more extreme stress [7-10] proposed a neuropsychological model of hostility that related hostility and violence-prone behavior to problems with self-awareness from right cerebral dysfunction, with specific implications for the right frontal lobe [11] reported the case of an individual who exhibited aggression and callous disregard for others following trauma to the right frontal region. Ictal episodes of aggression related to a right frontoparietal epileptiform focus have also been reported [12]. Relatively increased left frontal and decreased right frontal activity has been associated with anger and aggression [13,14]. Research has also indicated that agitation in patients with AD is associated with reduced cerebral blood flow in the right inferior frontal gyrus [15]. Finally, [16] found right frontotemporal involvement in frontotemporal dementia patients with acquired sociopathy.
Many studies have related disinhibition to right frontal lobe functioning, particularly the right inferior frontal cortex [17]. Behavioral disinhibition tasks administered to healthy individuals activate the right middle frontal gyrus and right dorsolateral prefrontal cortex [18]. Injuries and lesions to the right orbitofrontal and basal temporal area have been associated with inappropriate social behaviors and other disinhibition behaviors [19]. Patients with degenerative diseases may exhibit this relationship between right frontal functioning and disinhibition. For instance, patients with frontotemporal dementia exhibit socially inappropriate behaviors and problems with disinhibition [20]. Others have reported a relationship between disinhibition and activation within regions of the right frontal lobe [21-23].
The neuropathological processes of AD follow a typical pattern, with initial involvement of the entorhinal cortex followed by the hippocampus, then other regions of the temporal lobe and association cortex, and finally involvement of the frontal lobes or orbitofrontal region [24-26]. However, there are lateral cerebral asymmetries to these neuropathological processes. Specifically, whereas both hemispheres are associated with a loss of gray matter, increased gray matter loss in the left hemisphere has been recorded in many patients with AD [27-29]. Asymmetry in cerebral glucose metabolism has also been reported [30].
The asymmetries in gray matter loss and cerebral glucose metabolism may be associated with different patterns of memory, cognitive, and behavioral deficits. For instance, greater left hemisphere atrophy has been associated with lower verbal abilities in patients with AD [31,32] reported four cases of patients with AD, three of whom had relative left hemisphere atrophy and presented with language deficits initially and one patient with relative right hemisphere atrophy who presented with predominant visuospatial problems as the first complaint. Other researchers have found relatively diminished metabolism in the left hemisphere for AD patients with predominant language dysfunction and relatively diminished metabolism in the right hemisphere for patients with predominant visuoconstructive dysfunction [33,34] conducted a factor analysis based on neuropsychological test performance and found two factors, including a verbal and a visuospatial factor. Their results also indicated that over one quarter of the patients exhibited asymmetry on their profile of deficits from neuropsychological testing. Further, these asymmetries persist and remain stable throughout the disease process [35].
Given the right frontal lobe involvement in aggression, disinhibition, and socially disruptive behaviors the possibility exists that patients with AD who have relatively greater right hemisphere involvement are at increased risk for developing significant behavioral problems. These emotional deficits, with increased sympathetic drive, poor insight to deficits, and negative emotional displays may be found in patients with right hemi-aging [29]. Hence, based on neuropsychological testing, it may be possible to predict patients who will later exhibit the aggression and disinhibition that ultimately results in placement in managed care facilities. Predicting patients who may develop significant problems with aggression and disinhibition would enable the families of these patient’s time to plan for appropriate treatments and interventions and also possibly begin addressing these problems earlier in the course of the disease. However, whereas many studies have examined the behavioral variables that predict nursing home placement, there has been a veritable paucity of research examining the prediction of the behavioral problems that lead to placement. An investigation by Galynker et al. (2000) [36] is among the few and they found that decreased perfusion in the frontal cortex was associated with negative symptom severity, but not with measures of cognitive functioning. However, neuropsychological assessment in this project was restricted to the use of the Mini Mental Status Examination (MMSE) as the measure of cognitive functioning. The purpose of the present study was to determine if neuropsychological test performance could predict scores on a measure of behavioral dysfunction in patients with AD. Given the aforementioned role of the right frontal lobe in aggression and disinhibition, we predicted that a measure of right frontal lobe functioning would constitute the best predictor. Research has found that the Ruff Figural Fluency Test (RFFT) is sensitive to right frontal lobe functioning [37]. Additionally, we have previously reported that heightened delta EEG amplitude over the right frontal lobe is predictive of reduced performance on the RFFT [38]. Performance on the Controlled Oral Word Association Test (COWAT), in contrast, is associated with left frontal lobe functioning [39-43]. Our specific prediction was that the RFFT would be the better predictor of behavioral dysfunction as measured by the Neuropsychiatric Inventory (NPI), followed by a measure of general cognitive functioning (MMSE), whereas performance on the COWAT was expected to make a nonsignificant contribution to the model.
Methods
Participants
The sample consisted of 20 patients diagnosed with probable AD (8 men and 12 women) with an age range of 66 to 87 years (M = 73.70, SD = 6.67) and an average education level of 11.45 years (SD = 3.09). The diagnosis of probable AD met NINDS-ADRDA and DSM-V criteria. Scores on the Mini Mental Status Exam ranged from 18 to 28 (M = 23.40, SD = 3.00). The patient sample was drawn from patients who were referred to the Memory Disorder Clinic at the Murfreesboro Medical Clinic to complete a neuropsychological evaluation for memory problems.
Apparatus
Mini Mental Status Exam: The Mini Mental State Exam (MMSE) is a screening test used to assess general cognitive functioning. Areas of functioning assessed include orientation, registration, attention, recall, working memory, language, and construction or drawing ability. The range of scores possible is from 0 to 30 and the raw score was used for this study.
Neuropsychiatric Inventory Questionnaire: The Neuropsychiatric Inventory Questionnaire [44] is a brief form of the Neuropsychiatric Inventory [45] consisting of 12 questions assessing behavioral changes and dysfunction in patients with neurological illnesses. Behaviors are rated in terms of severity on a scale of 1 to 3 and in terms of the degree of caregiver distress on a scale of 0 to 5. The specific behaviors rated include delusions, hallucinations, agitation or aggression, depression, anxiety, elation, apathy, disinhibition, irritability, motor disturbances, nighttime behaviors, and appetite and eating behaviors. The total severity rating across all behaviors included was used for the purpose of this study.
Controlled Oral Word Association Test: The Controlled Oral Word Association Test (COWAT) requires the subject to name as many words as possible that begin with a specified letter (F, A, and S) within 60 seconds. However, they cannot use proper nouns, they cannot count, and they cannot use a stem word and then simply provide different endings. The variable of interest for this study was the total number of words produced across the three different letters used in the test.
Ruff Figural Fluency Test: The Ruff Figural Fluency Test [46] is a measure of nonverbal fluency consisting of five individual parts, with each part consisting of a unique stimulus pattern. More specifically, each of the five parts contains a 5 x 7 array of 35 unique stimulus items, with each stimulus item being comprised of a 5-dot matrix. The test involves drawing as many unique designs as possible by connecting two or more of the dots within each of the matrices within a time limit of one-minute. The first three trials contain the same stimulus pattern but with different distracters placed in the background. The fourth and fifth trials each contain a different 5-dot matrix. The total number of unique designs produced across the five trials was used in this study.
Procedure
This study was approved by the Middle Tennessee State University Institutional Review Board and all participants were treated in accordance with the ethical principles of the American Psychological Association. Participants were evaluated at Murfreesboro Medical Clinic, a large clinic located in middle Tennessee. The COWAT, MMSE, NPI-Q, and RFFT were administered as part of a larger neuropsychological test battery, the purpose of which was to assess for and diagnose dementia. Each test was administered according to the standardized procedures or administration protocols.
Results
The data were initially analyzed by examining the correlations between the NPI-Q and the RFFT, MMSE, and COWAT. A Bonferroni correction was used to control for conducting multiple correlations (p < .017). The results indicated a significant correlation between the NPI-Q and the RFFT (r = -.64, p = .002, r2 = .41) and also between the NPI-Q and the MMSE (r = -.52, p = .013, r2 = .27). The correlation between the NPI-Q and the COWAT was not significant (r = -.42, p = .04). The variables were then entered into a multiple regression, using the Enter method. The NPI-Q was the dependent variable and the predictors included the RFFT, MMSE, and COWAT, entered in that order. The RFFT was entered first, given that it is a measure of right frontal lobe functioning. Therefore, it should have the strongest relationship with behavioral dysfunction (NPI-Q) and be the best predictor of NPI-Q outcome scores. The MMSE was entered second, since it is a measure of general cognitive functioning. Finally, the COWAT was entered last since it is a measure of left frontal lobe functioning and should therefore be the weakest predictor of NPI-Q outcome scores.
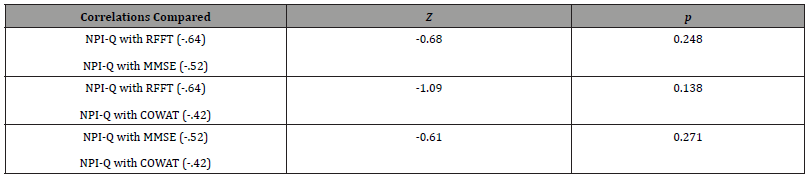
The result indicated that the RFFT was a significant predictor of the NPI-Q scores. Moreover, the addition of the MMSE and the COWAT did not significantly improve the basis for predicting the NPI-Q scores (see Table 1). Finally, we sought to determine if the aforementioned correlations between the variables of interest were significantly different [47]. The results indicated that no significant differences existed between any of the correlations (see Table 2).
Table 1:Hierarchical linear regression results.
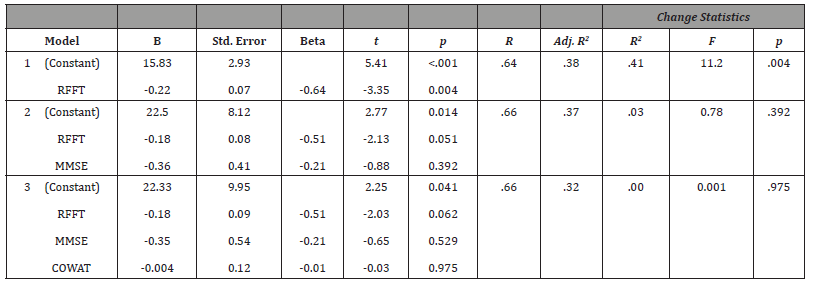
Table 2: Test results for differences between correlations.
Discussion
The results of this study supported the hypothesis investigated. Specifically, a significant negative correlation was found between the NPI-Q and the RFFT, indicating that as scores on the NPI-Q increase scores on the RFFT decrease. Hence, increasing behavioral dysfunction as measured by the NPI-Q is associated with increasing right frontal lobe dysfunction as measured by the RFFT. Further, the results indicated that performance on the RFFT is a significant predictor of NPI-Q scores and that the MMSE and COWAT measures did not improve the prediction of NPI-Q scores. Hence, as predicted, right frontal lobe dysfunction was associated with and predicted behavioral dysfunction in patients with Alzheimer’s disease. There have been a few reported studies examining the relationship between cognitive functioning and behavioral dysfunction in patients with Alzheimer’s disease. However, the findings of these studies have been somewhat mixed. Some researchers have failed to find a significant relationship between cognitive functioning or decline and behavioral disturbances [48, 49] or psychosis [50]. However, Cooper and colleagues reported that a number of behavioral variables were related to declining cognitive functioning [51]. Further, an association between cognitive decline and physical aggression in patients with AD has been reported [52]. The equivocal findings regarding the relationship between cognitive functioning and behavioral disturbance may be a result of the fact that these studies have all only used indices of general cognitive functioning, such as the MMSE. The present study is the first to use more specific neuropsychological measures to examine this relationship and the first to support that behavioral dysfunction may be specifically related to right frontal lobe dysfunction in patients with AD.
As mentioned previously, many patients with AD will be placed in managed care facilities. The presence of significant behavioral dysfunction leads to increased caregiver distress and increases the likelihood that patients with AD will be admitted to a nursing home. The ability to predict patients who may experience behavioral dysfunction would enable the family and caregivers valuable time for planning and provide important educational information regarding the potential for behavioral dysfunction and the types of behaviors that may be experienced. Further, treatments may be initiated earlier for those patients who are identified as being at increased risk. As a result, admission to managed care facilities may be delayed, which would have a significant monetary benefit for the patients and their families.
Although the present study found that performance on a measure of right frontal lobe functioning predicted behavioral dysfunction in patients with AD, the study was retrospective in nature. A more prospective, longitudinal study using the same neuropsychological tests would help to substantiate the present findings. Future research may also benefit from including additional measures of right versus left frontal lobe functioning to determine if the prediction of behavioral dysfunction may be increased. Finally, the present study inferred right frontal lobe functioning based on performance on a test that is purported to be sensitive to this region. Additional research may seek to include anatomical measures to corroborate the presence of relative right frontal dysfunction, such as by assessing gray matter loss or using functional imaging.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Strain LA, Blandford AA, Mitchell LA, Hawranik PG (2003) Cognitively impaired older adults: Risk profiles for institutionalization. International Psychogeriatrics 15(4): 351-366.
- Benoit M, Robert PH, Staccini P, Brocker P, Guerin O, et al. (2005) One-year longitudinal evaluation of neuropsychiatric symptoms in Alzheimer’s disease. The REAL.FR study. Journal of Nutrition, Health & Aging 9(2): 95-99.
- Dennehy EB, Kahle-Wrobleski K, Sarsour K, Milton DR (2013) Derivation of a brief measure of agitation and aggression in Alzheimer’s disease. International Journal of Geriatric Psychiatry 28(2): 182-189.
- Dauphinot V, Delphin-Combe F, Mouchoux C, Dorey A, Bathsavanis A, et al. (2015) Risk factors of caregiver burden among patients with Alzheimer’s disease or related disorders: A cross-sectional study. Journal of Alzheimer’s Disease 44(3): 907-916.
- Gaugler JE, Wall MM, Kane RL, Menk JS, Sarsour K, et al. (2011) Does caregiver burden mediate the effects of behavioral disturbances on nursing home admission? American Journal of Geriatric Psychiatry 19(6): 497-506.
- Gaugler JE, Yu F, Krichbaum K, Wyman JF (2009) Predictors of nursing home admission for persons with dementia. Medical Care 47(2): 191- 198.
- Comer CS, Harrison PK, Harrison DW (2015) The dynamic opponent relativity model: An integration and extension of capacity theory and existing theoretical perspectives on the neuropsychology of arousal and emotion. Springerplus 4: 345.
- Mitchell GA, Harrison DW (2010) Neuropsychological effects of hostility and pain on emotion perception. Journal of Clinical and Experimental Neuropsychology 32(2): 174-189.
- Williamson JB, Harrison DW (2003) Functional cerebral asymmetry in hostility: A dual task approach with fluency and cardiovascular regulation. Brain and Cognition 52(2): 167-174.
- Demaree HA, Harrison DW (1997) A neuropsychological model relating self-awareness to hostility. Neuropsychological Review 7(4): 171-185.
- Blair RJ, Cipolotti L (2000) Impaired social response reversal: A case of ‘acquired sociopathy’. Brain 123(Pt 6): 1122-1141.
- Sumer MM, Atik L, Unal A, Emre U, Atasoy HT (2007) Frontal lobe epilepsy presented at ictal aggression. Neurological Sciences 28(1): 48-51.
- Harmon-Jones E, Sigelman J (2001) State anger and prefrontal brain activity: Evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. Journal of Personality and Social Psychology 80(5): 797-803.
- Keune PM, van der Heiden L, Varkuti B, Konicar L, Veit R, et al. (2012) Prefrontal brain asymmetry and aggression in imprisoned violent offenders. Neuroscience Letters 515(2): 191-195.
- Banno K, Nakaaki S, Sato J, Torii K, Narumoto J, et al. (2014) Neural basis of three dimensions of agitated behaviors in patients with Alzheimer disease. Neuropsychiatric Disease and Treatment 10: 339-348.
- Mendez MF, Chen AK, Miller BL (2005) Acquired sociopathy and frontotemporal dementia. Dementia and Geriatric Cognitive Disorders 20(2-3): 99-104.
- Aron AR, Robbins TW, Poldrack RA (2004) Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences 8(4): 170-177.
- Asahi S, Okamoto Y, Okada G, Yamawaki S, Tokota N (2004) Negative correlation between right prefrontal activity during response inhibition and impulsiveness: A fMRI study. European Archives of Psychiatry and Clinical Neuroscience 254(4): 245-251.
- Starkstein SE, Robinson RG (1997) Mechanism of disinhibition after brain lesions. Journal of Nervous and Mental Disease 185(2): 108-114.
- Kirshner HS (2014) Frontotemporal dementia and primary progressive aphasia, a review. Neuropsychiatric Disease and Treatment 10: 1045-1055.
- Miller BL, Chang L, Mena I, Boone K, Lesser IM (1993) Progressive right frontotemporal degeneration: Clinical, neuropsychological and SPECT characteristics. Dementia 4(3-4): 204-213.
- O’Callaghan C, Naismith SL, Hodges JR, Lewis SJ, Hornberger M (2013) Fronto-striatal atrophy correlates of inhibitory dysfunction in Parkinson’s disease versus behavioural variant frontotemporal dementia. Cortex 49(7): 1833-1843.
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, et al. (2005) Neuroanatomical correlates of behavioural disorders in dementia. Brain 128(Pt 11): 2612-2625.
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica 82(4): 239-259.
- Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiology of Aging 16(3): 271-284.
- Delacourte A, David JP, Sergeant N, Buee L, Wattez A, et al. (1999) The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 52(6): 1158-1165.
- Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC (2002) Mapping the evolution of regional atrophy in Alzheimer’s disease: Unbiased analysis of fluid-registered serial MRI. Proceedings of the National Academy of Science 99(7): 4703-4707.
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, et al. (2003) Dynamics of gray matter loss in Alzheimer’s disease. Journal of Neuroscience 23(3): 994-1005.
- Harrison DW (2015) Right Hemi-aging Theory. In Brain Asymmetry and Neural Systems. Springer International Publishing pp: 469-474.
- Jagust WJ, Friedland RP, Budinger TF, Koss E, Ober B (1988) Longitudinal studies of regional cerebral metabolism in Alzheimer’s disease. Neurology 38(6): 909-912.
- Raz N, Raz S, Yeo RA, Turkheimer E, Bigler ED, et al. (1987) Relationship between cognitive and morphological asymmetry in dementia of the Alzheimer type: A CT scan study. International Journal of Neuroscience 35(3-4): 225-232.
- Bugiani O, Constantinidis J, Ghetti B, Bouras C, Tagliavini F (1991) Asymmetrical cerebral atrophy in Alzheimer’s disease. Clinical Neuropathology 10(2): 55-60.
- Foster NL, Chase TN, Fedio P, Patronas NJ, Brooks RA, et al. (1983) Alzheimer’s disease: Focal cortical changes shown by positron emission tomography. Neurology 33(8): 961-965.
- Strite D, Massman PJ, Cooke N, Doody RS (1997) Neuropsychological asymmetry in Alzheimer’s disease: Verbal versus visuoconstructional deficits across stages of dementia. Journal of the International Neuropsychological Society 3(5): 420-427.
- Grady CL, Haxby JV, Schlageter NL, Berg G, Rapoport SI (1986) Stability of metabolic and neuropsychological asymmetries in dementia of the Alzheimer type. Neurology 36(10): 1390-1392.
- Galynker II, Dutta E, Vilkas N, Ongseng F, Finestone H, et al. (2000) Hypofrontality and negative symptoms in patients with dementia of Alzheimer type. Neuropsychiatry, Neuropsychology and Behvavioral Neurology 13(1): 53-59.
- Ruff RM, Allen CC, Farrow CE, Niemann H, Wylie T (1994) Figural fluency: Differential impairment in patients with left versus right frontal lobe lesions. Archives of Clinical Neuropsychology 9(1): 41-55.
- Foster PS, Williamson JB, Harrison DW (2005) The Ruff Figural Fluency Test: Heightened right frontal lobe delta activity as a function of performance. Archives of Clinical Neuropsychology 20(4): 427-434.
- Baldo JV, Shimamura AP, Delis DC, Kramer J, Kaplan E (2001) Verbal and design fluency in patients with frontal lobe lesions. Journal of the International Neuropsychological Society 7(5): 586-596.
- Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, et al. (2000) A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology 14(3): 353-360.
- Herrmann MJ, Ehlis AC, Fallgatter AJ (2003) Frontal activation during a verbal-fluency task as measured by near-infrared spectroscopy. Brain Research Bulletin 61(1): 51-56.
- Ravnkilde B, Videbech P, Rosenberg R, Gjedde A, Gade A (2002). Putative tests of frontal lobe function: A PET-study of brain activation during Stroop’s test and verbal fluency. Journal of Clinical and Experimental Neuropsychology 24(4): 534-547.
- Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, et al. (1998) The effects of focal anterior and posterior brain lesions on verbal fluency. Journal of the International Neuropsychological Society 4(3): 265-278.
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, et al. (2000) Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. Journal of Neuropsychiatry and Clinical Neuroscience 12(2): 233-239.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, et al. (1994) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44(12s): 2308-2314.
- Ruff RM (1996) Ruff Figural Fluency Test. Odessa, FL: Psychological Assessment Resources.
- Steiger JH (1980) Tests for comparing elements of a correlation matrix. Psychological Bulletin 87(2): 245-251.
- Marin DB, Green CR, Schmeidler J, Harvey PD, Lawlor BA, et al. (1997) Noncognitive disturbance in Alzheimer’s disease: Frequency, longitudinal course, and relationship to cognitive symptoms. Journal of the American Geriatric Society 45(11): 1331-1338.
- Teri L, Borson S, Kiyak HA, Yamagishi M (1989) Behavioral disturbance, cognitive dysfunction, and functional skill. Prevalence and relationship in Alzheimer’s disease. Journal of the American Geriatric Society 37(2): 109-116.
- Kotrla KJ, Chacko RC, Harper RG, Doody R (1995) Clinical variables associated with psychosis in Alzheimer’s disease. American Journal of Psychiatry 152(9): 1377-1379.
- Cooper JK, Mungas D, Weiler PG (1990) Relation of cognitive status and abnormal behaviors in Alzheimer’s disease. Journal of the American Geriatric Society 38(8): 867-870.
- Holtzer R, Tang MX, Devanand DP, Albert SM, Wegesin DJ, et al. (2003) Psychopathological features in Alzheimer’s disease: Course and relationship with cognitive status. Journal of the American Geriatric Society 51(7): 953-960.
-
Paul S Foster*, Cara Clark, Dana Fuller and David W Harrison. Predicting Behavioral Dysfunction in Alzheimer’s Disease. Glob J Aging Geriatr Res. 3(5): 2025. GJAGR. MS.ID.000571.
-
Alzheimer’s Disease, Behavioral Dysfunction, Neuropsychiatric, Frontal Lobes, Prediction, Agitation, Aggression
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






