 Research Article
Research Article
Narrative Review of Shoulder Osteoarthritis Rotator Cuff Fatty Infiltration and Atrophy Interactions and Impacts
Ray Marks, Osteoarthritis Research Center, Canada
Received Date:July 07, 2025; Published Date:July 11, 2025
Abstract
Osteoarthritis, a disabling disease that induces high degrees of disability and high health costs in all parts of the globe can be very disabling despite advanced surgical joint replacement and remedial procedures. Adversely impacted in the face of muscle atrophy, as well as the encroachment of excess fatty tissue into vulnerable or injured muscles or tendons this report focuses on observations from studies examining rotator cuff muscle dysfunction, lesions or damage and its potential impact on shoulder arthritis, but that may well apply to other joints. In this regard, we found that with few exceptions and regardless of article examined a possible bi-directional role for muscle fat mass infiltration alongside muscle atrophy may have a strong bearing on the shoulder osteoarthritis disability cycle. Muscle fat infiltration appears to be involved, quite common and of high clinical significance in influencing the disease progression and its observed reversibility challenges.
Keywords:Fat Mass Infiltration, Muscle, Muscle Atrophy, Osteoarthritis, Rotator Cuff, Shoulder
Introduction
The disabling joint condition termed osteoarthritis remains an intractable problem affecting many older adults in all parts of the world despite decades of study and investigations of the many possible causes thereof. Related research is however alluding to a highly important role for muscle as a disease precursor, modifier, or mediator. This includes a specific condition termed sarcopenia denoting muscle wasting, as well as alterations in the desired muscle fat ratio. Their unique and interactive role as consistent features in many osteoarthritis cases and the knowledge that their presence may expose the associated joint tissues and cartilage lining to undue injurious impacts and forces is hence a key focal point of multiple current pathogenic oriented studies. In addition, associat ed muscle volume declines may have enormous functional implications for joint stability, joint mobility, joint proprioception, and pain that may all clearly exacerbate osteoarthritis disability significantly and progressively in their own right [1-7].
At the same time, this growing body of research points to muscle as a therapeutic target in the realm of osteoarthritis, including efforts to avert its severity and disabling impacts that are especially challenging for the older adult. Indeed, osteoarthritis, a prevalent chronic disease affecting one or more freely moving joints and characterized by progressive bone remodelling, articular cartilage degeneration and soft tissue capsular and ligament alterations is increasingly deemed to be associated with a complex interaction of local and systemic health negating factors. These possible muscle alterations may include various degrees of muscle spasm, muscle contractures, muscle fibrosis, ligament alterations, inflammation, and pathology [8]. In addition, all these conditions can be exacerbated or mediated to some degree by the presiding degree and extent of abnormal muscle fat infiltration or fraction relative to muscle mass, which is often noted to be declining in volume [9-12].
To this end, this brief specifically examines whether, a) a subnormal muscle fat ratio is a feature or predictor of osteoarthritis in general, b) possible insights from rotator cuff shoulder related studies as applied to shoulder osteoarthritis, c) the proposed mechanisms for fatty infiltration other than age and obesity at the shoulder joint. It was felt this information would be of interest to orthopaedic clinicians and therapists as well as those who contemplate surgical solutions for shoulder osteoarthritis, as well as in other shoulder surgical needs. It was anticipated the literature review would reveal a variety of studies highlighting the potential impact of excess muscle fat mass encroachment on muscle fibrosis and atrophy in the face of joint injury, and possibly pathology and surgery. One or more shoulder rotator cuff muscle studies were selected for review rather than all shoulder dysfunction sources as these commonly present enormous degrees of disablement and treatment challenges due to muscle atrophy and degeneration, fatty infiltration, immense pain, and muscle fibrosis [13].
Drawn largely from the PUBMED, database, the largest medically oriented data base, the overview aimed to provide the interested reader a general view of past work as well as current trends and gaps plus opportunities in this regard that might be worthy of further consideration and study, as well as serving as viable applications in the orthopaedic and aging health care field. The focus was on osteoarthritis, now assuming epidemic proportions, and which may occur independently as a separate health condition, or in conjunction with one or more chronic health conditions, and may well be an enormous activity obstacle and deterrent to successful aging goals due to its many adverse impacts on life quality and functioning, including immense self-care challenges and socioeconomic losses. While the world awaits a possible antidote in this regard, mounting evidence points to a possible role for the presence of muscle fat and its expansion post injury as well as aging impacts as a causative factor.
Although clinical research in this realm is however limited with the exception of a widespread focus on shoulder repair surgery, not all older adults at high risk for shoulder dysfunction can withstand surgery. Those who exhibit high muscle fat volumes also pose multiple post operative challenges in a significant proportion of cases, regardless of age [14-16]. As such, if found to be influential in any way, intervention in this regard may provide one avenue that is reasonably practical for purposes of securing the well-being of the aging person, especially in the case of the chronically ill older adult that lives in the community and must be self-reliant. This work is significant because the ability to minimize osteoarthritis severity is currently of the highest import, especially among those older adults who wish to reside in the community.
Aims
This purely narrative and descriptive introductory overview aimed to specifically examine the value of joint protection for minimizing the risk of acquiring and suffering from progressive bouts of osteoarthritis pain that are potentially compounded by declines in muscle volume and increases in muscle fat mass, for example at the shoulder, a non-weightbearing joint, but one essential for most life functions. Secondly, it aimed to offer insight into the processes underpinning the presence of muscle fat on a joint, an emergent topic of increasing interest. Third it aimed to provide recommendations for future consideration by clinicians and researchers in the field based on these findings.
Materials and Methods
To examine this issue, we elected to employ the PUBMED, PubMed Central and Google Scholar data bases largely using the ‘best match’ and ‘most current’ prompts and covering data published largely between January 2010-June 30 2025, using the key words: osteoarthritis, shoulder, muscle, muscle fat mass, atrophy and infiltration. Only articles focusing on osteoarthritis and how some form of shoulder muscle fat presence in various shoulder cuff lesions were deemed acceptable. Described in narrative form, are some general results of studies of all types that have chosen to examine aspects of the muscle fat fraction relative to lean muscle mass ratios in osteoarthritis or shoulder injury contexts using those data that pertain to one or more rotator cuff muscles which are four upper shoulder muscles (supraspinatus, infraspinatus, teres minor, subscapularis) and their tendons designed to permit shoulder movements and stability via their attachments to the upper aspect of the main shoulder bone or humerus. No systematic review was conducted, however, and points made are those that have emerged over time and comport with the author’s 25 years of research showing high degrees of obesity in cases with disabling hip and knee osteoarthritis, many young adults as well as older adults with shoulder dysfunction, and the fact that muscle fat mass volumes that are deemed ‘unhealthy’ are commonly seen in many young adults who are otherwise healthy as demonstrated by body impedance measures. The article is based on careful data extraction and author selection and no AI technology was employed at any point. The content chosen focuses largely on intramuscular fat mass, as opposed to subcutaneous fat and muscle atrophy or mass as this realm of exploration is quite new and might prove insightful in understanding arthritis manifestations in the frail underweight person, as well as in the importance of primary prevention efforts to counter progressive disabling in the overweight adult.
Accordingly, this narrative review first highlights some general
current 2025 reports and some reported recently that allude to an
array of observed muscle influences or responses in the osteoarthritis
disease cycle, rather than all those that have been published
to date. Second, studies that examine muscle fat attributes as this
pertains to the traumatized shoulder joint are discussed. Third,
some emerging evidence of a key role for preventive health behaviors
and injury prevention approaches as a mediating or moderating
pathogenic factor in this respect is discussed. The items chosen
were those thought to have a strong bearing on pain and the related
hallmark of osteoarthritis, namely cartilage tissue degeneration
and destruction and ensuing functional declines as experiences in
the case of shoulder arthritis. Reported on are largely publications
listed as of June 30, 2025 that best matched or were potentially relevant
to the current topic only selected after a careful examination
of their contents and if they addressed one of the author’s beliefs as
outlined below.
• Excess muscle fat mass may adversely impact articular cartilage
structural features as well as muscle composition,
strength, and joint stability.
• Obesity, aging and other chronic inflammatory diseases are associated
with increased fat mass and decreased muscle mass
and function that may invoke joint damage.
• A failure to prevent repetitive or acute injuries may cause a reactive
form of degrading arthritis in its own right.
• Lean muscle mass declines and increases in muscle fat ratios
may provoke and perpetuate an array of unwanted joint functional
outcomes and pain provoking adaptations both before
as well as after surgical joint replacement.
Results
In terms of the topic specifically sought, however, most publications that initially appeared potentially relevant most did not meet the desired criteria for this review that excluded invasive intervention discussions, nor any overall synthesis due to limited data, analyses extracted from animal models, secondary data rather than actual current research. Moreover, many were not based on prospective or targeted studies of the older adult with forms of osteoarthritis other than the knee and hip, and even then, were observational studies with dubious measurement tools, methods, and unknown properties were the rule not the exception. These were also generally non uniform in multiple design respects, exploratory or atheoretical, or only in the proposal stage. Three categories of publication emerged as per below.
General observations
As of 2025 increasing reports are highlighting the aging population and their common declines in wellbeing and challenges including those due to osteoarthritis. In this regard, past research shows that many aging adults who have a high risk of incurring sarcopenia and related muscle mass and strength declines often go on to manifest severe osteoarthritis. A disease with enormous disabling features in its own right, such as sarcopenic obesity, where lean muscle mass is replaced by fat, many with this painful disabling condition are also potentially obese and often reluctant to exercise even this is advocated with a resultant increased risk of muscle weakness and fatigue following or during long duration activities, and liable to possible falls and other injuries, as well as an emergent low life quality experiences.
Muscle fat mass observations
While a role for muscle in the osteoarthritis pathogenic cycle is a relatively recent perspective, increasing numbers of studies confirm its association with symptomatic osteoarthritis, including the notion that muscle fat deposition or infiltration may be a potent disease muscle mediator [eg., 21-24]. Indeed, while not widely documented, nor universally applied to a degree commensurate with its possible diverse pathogenic disease ramifications, attributes and associations, muscle abnormalities potentially explain and may predict diverse aspects of the progressive osteoarthritis progression phases. A fair number of past and present studies focus too on obesity and its implications for both joint loading, and as such highlight an independent role for muscle adipose tissue composition alterations including muscle fat deposition and possible changes in muscle mass or sarcopenia [a progressive muscle mass declining state] as this impacts mobility and sets the stage for inactivity and muscle fat fraction increases [25-27]. Others show a role for deforming contractures, varying degrees of muscle spasm and subnormal vector influences, plus functional changes in muscle biochemistry and muscle size [28] that have a unique or collective bearing on cartilage viability, and that may implicate or lead to muscle fat excesses and infiltration as well as deficits in lean muscle mass and muscle fibrosis [29-30].
Other data imply that there may be a progressively harmful adverse degree of joint loading that subsequently manifests as fully fledged osteoarthritis, as various degrees of muscle pathology and disordered biochemical expression and/or possible fears of moving due to pain. Collectively these factors have the potential to foster subnormal muscle forces and joint loading or attenuation responses, joint stability and the ability of an injured muscle to regenerate. As outlined by Chen et al. [31] osteoarthritis is a disease of joint degeneration and impaired function where muscle atrophy, fatty infiltration, and fibrosis are degenerative features of muscle injury and predict poor outcomes in some instances. Unsurprisingly, patients with glenohumeral joint osteoarthritis usually exhibit rotator cuff muscle degeneration, even though the rotator cuff may remain intact.
Rotator cuff fat mass attributes
Fatty infiltration of the rotator cuff evaluated with computerized tomography has been associated with asymmetric glenoid or shoulder joint cavity wear as well as humeral head subluxation in those patients with confirmed glenohumeral arthritis. This relationship arguably plays an important role in determining the optimal surgical management of advanced glenohumeral osteoarthritis, as well as its management in general. Compared with concentric forms of wear, posteriorly worn glenoids appear to have an imbalance in axial-plane rotator cuff fatty infiltration and an increased amount of fatty infiltration of the infraspinatus and teres minor compared with the subscapularis. The authors suggest these imbalances may contribute to the higher rates of failure after anatomic total shoulder arthroplasty in patients with posterior wear compared with those with concentric wear, but may also cause initial damage to a vulnerable joint [31].
Other data show rotator cuff muscle degeneration, bone morphology, and humeral head subluxation are known risk factors for failure of anatomic total shoulder arthroplasty in patients with B-glenoid shoulder osteoarthritis [32]. Others reveal observations of prevailing or emergent muscle fat associated metabolic muscle alterations, alterations in muscle quality, volume, mitochondrial muscle energetic alterations and/or myopathy in the face of any perpetual state of undue soft tissue damage, persistent joint stresses, pain, and signs of muscle weakness. There may also be emergent indications of a gradually diminishing joint range of motion and stiffness, plus an abnormal degree of muscle related joint biomechanics, an ongoing cycle of disordered joint destruction, and inflammation [33-36].
In cases where rotator cuff tears prevail in particular, these are commonly accompanied by fatty infiltration of the associated muscles to varying degrees depending on the tear pattern, and that can substantially limit daily activities and affect quality of life. Ample research also shows that after a tendon tear, the rotator cuff muscle will commonly undergo fatty infiltration within and around the muscles as well as declines in muscle volume often associated with poorer functional and surgical outcomes after reconstruction surgery [37, 38]. In this regard, the etiology of rotator cuff disease is likely to be a multi-factorial one due to age-related degeneration, genetics, high choledterol levels, smoking, micro- and macro trauma Those classified as having full-thickness rotator cuff tears tend to show a progressive fat encroachment that enlarges over time, as dies pain, or worsening pain [39] [see Figure 1].
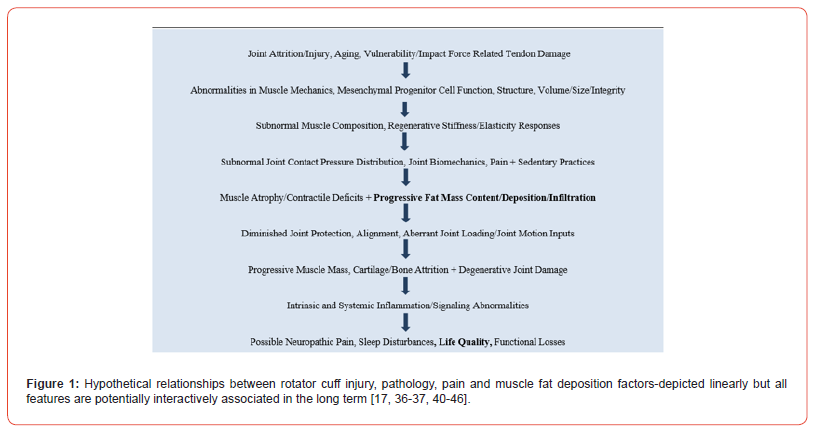
In essence, this envisioned series of adverse interactions including heightened muscle force capacity and contractile abnormalities and others that may induce or be mediated by muscle fat encroachment and is increasingly shown to be associated with progressive subnormal joint biodynamical, structural and osteoarthritis joint destruction processes that are not readily reversible [42]. As well, this idea is consistent with known age associated degrees of the gradual onset of subnormal muscle metabolic physiological states, losses of lean muscle mass, increasingly sedentary behaviors, the loads needed for muscle regeneration that are increasingly excessive for the associated muscle volume declines [43]. In addition, there may be a reactive or causative array of intrinsic muscle cell mesenchymal alterations [44] that prevent, inhibit, or impair muscle repair.
Underlying local factors may also include possible painful muscle reactive adaptations due to persistent abnormal sensory inputs from one or more of the surrounding tissues in the presence of an increased muscle fat mass and that may impact the ability to attenuate joint loads significantly and effectively. Also known as my steatosis, its presence in muscle exhibits a negative correlation with muscle mass, strength, mobility, and a decrement in muscle quality, while serving as a biomarker for sarcopenia, cachexia, and metabolic syndromes. It also induces pro-inflammatory changes that clearly contribute to declines in muscle function, compromise mitochondrial function, and increase muscle inflammatory responses [35] and possibly postural stability [46]. Worse effects occur in cases of higher muscle fat content and area presence [47].
Others show the presence of muscle fat derived inflammation that may not only evoke local muscle pain signals, but may elicit more widespread pain, bone attrition, and sensory sympathetic inputs that contribute to the arthritis disease and disability cycle [48]. It also appears both externally or extrinsic as well as internally or intrinsic induced muscle fat infiltration and subsequent fibrosis may have a separate or combined cumulative and progressive effect on osteoarthritis structural pathology of the shoulder joint and others [49], and regardless of whether this situation is causative or reactive. Moreover, unless identified early on, it may be highly challenging to heighten the subject’s physical function or relieve longstanding pain, especially if there is progressive muscle atrophy [49], plus a possible unwillingness or fear on the part of the affected adult to move their arm to counter increasing pain and stiffness, alongside muscle mass and sensory declines, and joint stability deficits that may influence them to adopt abnormal and awkward movements to avoid pain and injury but that may place strain on the joints, further pain and possible diverse muscle associated adverse structural alterations that may vary depending on the degree of muscular fat infiltration.
Other muscle-based determinants that may be related to the degree of muscle fat encroachment and presence involved in osteoarthritis include declines in physical performance and quality of life. As well, there is the possible development of a muscle nerve entrapment process by osteoarthritis bone spurs [50], muscle fibrosis, muscle inflammation, joint instability, and an altered cartilage structure with the added risk of incurring more extensive joint pathology and pain than those generated in the presence of sufficient fat free lean muscle mass [56]. Adding to this subnormal cycle is a possible role for osteoarthritis associated muscle fat infiltration impacts on muscle metabolic alterations [57] alongside widespread pain, atrophic muscle weaknesses, muscle volume deficits, inflammation, and muscle architecture alterations [58]. At the shoulder joint, it appears muscle resident fibromatogenic cells do serve as a key a contributor to rotator cuff tear pathological changes that cause immense pain and disability [13].
While mechanical injury is the most likely cause of osteoarthritis in most instances, emerging evidence showing functional ability declines in the presence of excess muscle fat could be causative or reactive or both. As well, those cases displaying atrophic muscle weakness are likely to also display disruptions in muscle genetics, and muscular alterations such as declines in muscle force capacity and responsiveness that may further amplify the pain and dysfunction attributable to the local condition even after surgery where muscle fatty tissue infiltration and deposition may emerge or persist [59, 60].
Discussion
As per a number of diverse current and past studies, it can be concluded shoulder osteoarthritis and impaired function can be significantly influenced by muscle atrophy, fatty muscle infiltration, and fibrosis usually accompanying rotator cuff muscle degeneration [31]. In addition, MRI type scans and analyses, further reveal increased fatty infiltration in the infraspinatus muscle with age [41]. Moreover, in full-thickness rotator cuff tears in human samples it tears of all sizes show significantly greater lipid content and smaller myofiber cross-sectional areas compared with partial-thickness tears and control muscles [61]. Moreover, a preclinical study of rabbit supraspinatus tenotomy outcomes showed this artificial injury to recapitulate key features of the pathophysiology of human rotator cuff tears, including muscle atrophy and degeneration, a lack of regenerative ability, fat accumulation, and fibrosis [13]. Indeed, despite years of study and insights, what remains is a high re-tear rate after a successful repair of the rotator cuff that is a major clinical challenge attributed to muscle atrophy and fat accumulation of rotator cuff muscles over time [62]. Moreover, after successful arthroscopic repair, even if tendon tear-induced fatty infiltration can be almost negated, and muscle atrophy slightly reversed in the case of a failed repair, these changes are further pronounced during the first 3 postoperative months [63].
Gerber et al. [45] note that it appears that muscle changes that occur after tendon release, may be exacerbated in the presence of denervation of the muscle due to an ensuing decrease in the pennation angle of lengthened muscle fibers, with a reduced mean cross-sectional area of pooled muscle fibers, a slow- to fast-type transformation, and an increase in the area percentage of hybrid fibers, leading to overall significantly greater atrophy of the corresponding muscle. The pattern of fat infiltration within the supraspinatus muscle of the shoulder joint changes from a laterally based location around the muscle-tendon junction to a more diffuse, global infiltration pattern when the whole muscle fat content exceeds 10% according to Wallenberg et al. [30]. Hence, more translational research on diverse osteoarthritis samples of higher ages may prove promising in stressing a role for fat mass correlates that can foster extensive alterations in muscle force capacity and immense irreparable disabling outcomes if overlooked.
In addition, even though the source of muscle fat infiltration and why this may enlarge and become pathogenic is not well clarified [17], and nutrition and physical activity behaviors may clearly be of high import, basic preclinical studies speak to a series of adaptive responses in the face of joint damage including those induced by neurological nerve damage [45] as one factor, even if this does not apply in all cases. As well, even if fatty tissue can be removed artificially the underlying muscle abnormalities, for example fibrosis [17] may prevail. The rotator cuff tendons can degenerate and/or tear from the greater tuberosity of the humerus, which is associated with several anatomical, physiological, biochemical, and molecular changes in tendon and muscle. immunobiological responses following the rotator cuff lesion and the inherent repair mechanisms elicited by the body. The greatest difficulty in treating this pathology is that the muscle can undergo irreversible fatty infiltration in the setting of chronic tears that is associated with poor surgical outcomes. The article also investigates the key molecular pathways of the muscle homeostasis and energy metabolism to propose a possible mechanism for fatty infiltration [64]. The radiologic grading of muscle fat content was associated with the expression of various genes, including adipogenic, fibrotic, inflammatory, and atrophy-related genes, and these genes were closely correlated with each other in terms of expression [65].
Siso et al. [14] who studied patients with primary glenohumeral osteoarthritis awaiting total shoulder arthroplasty showed B3 glenoids had the greatest degree of fat presence of all rotator cuff muscles, implying a possible disabling correlation. Although the cause effect association is unclear, high-grade rotator cuff muscle fatty infiltration is associated with B3 glenoids-subtype of arthritis, increased pathologic glenoid retroversion, and increased joint-line medialization [25]. An additional thought is that posterior humeral head subluxation and glenoid retroversion, which are pathognomonic of the Walch type B shoulder, may lead to a disturbance in the length-tension relationship of the posterior rotator cuff, causing fatty infiltration [66]. What can be gleaned from the presently retrieved sources of peer reviewed information on osteoarthritis of the shoulder as well as osteoarthritis of other joints is the notion of how injury alone can induce joint attrition and pain, as well as why it is not easy to reverse. Moreover, if this involves one or more of the shoulder rotator cuff muscles the use of empirically supported preventive approaches may be more helpful and insightful than relying on surgery alone.
Data also imply that our assumptions listed earlier cannot be ignored. Indeed, it can be assumed that a high proportion of muscle fat infiltration and its progression will ensue if ignored, as well as by failing to appreciate the complexity of what we do know about the triadic fatty tissue, sarcopenia, and their osteoarthritis associations. In particular, strategies directed across adulthood, towards enhancing muscle mass, strength and endurance, plus efforts towards avoidance of repetitive or acute injurious joint loading activities appear indicated. Muscle stress protection seems especially crucial as recent analyses reveal that in the presence an injury, a hostile microenvironment characterized by heightened inflammation, fibrosis, and muscle weakness may be forthcoming and is one shown to stimulate intramuscular adipose tissue expansion and impair its regeneration capacity and contractile functions especially in the face of a sedentary lifestyle [66]. In patients with rotator cuff tears, and shoulder osteoarthritis, and instability, clinicians can track or look out for these features and work with the client to mitigate their impacts. Finally, rehabilitation programs targeting shoulder muscle function and pain should not neglect to examine shoulder proprioception that is showing promising initial results in restoring function and returning injured athletes to play.
At present, it appears safe to say that even though more solid uniform information on this topic is needed in multiple realms [25], most clinical researchers currently assume muscle problems of some sort underlie and impact osteoarthritis pain and if minimized will prove to have a bearing on osteoarthritis outcomes. However, distinctive efforts to study and address muscle fat deposition origins and impacts such as fears of movement, muscle deconditioning [68], muscle strength declines, pain, and pain behaviours are desirable due to their possible cross talk effect between fat and body tissues and pain [68-74]. Developing effective osteoarthritis preventive as well as treatment strategies using more advanced technologies and analyses can also help to greatly advance the field as cited by most current researchers, including those who conduct joint replacement surgery, especially at the shoulder joint [25].
Although publication bias may prevail, these aforementioned observations are largely in agreement that one can expect a negative role for the presence of fatty tissue in muscles around an osteoarthritis damaged joint as well as how its enlargement in the face of poor management and non-protective behaviors. At the shoulder, avoidance of an acute injury is essential as this is a noteworthy determinant of muscle fat infiltration and its persistence and adverse signalling influences [75]. Hence although osteoarthritis is currently deemed a chronic progressively disabling condition with no known cure, research over the past 15 years or more has indicated that there is strong possibility that an array of muscle related factors that can contribute to the osteoarthritis pain and disease cycle. However, while a diverse array of intervention approaches that focus on maximizing muscle structure and function appear advocated to potentially reduce the degree of disability in knee and hip osteoarthritis, that at the shoulder is rarely alluded to except for surgical research purposes. Muscle fat mass, a predictor of adverse joint events [68] alongside pain, regardless of its precise muscle site and disease severity is also not well articulated in the realm of shoulder exercise therapies.
This lack of attention may inadvertently impact outcomes of surgery to replace a diseased joint, as well as rehabilitation in general especially in the cases where muscle fat content is not assessed and sensory motor and strength training, and joint protection are not carefully construed. Additionally, symptomatic cases need to be carefully educated as to the considerable care they must take, to avoid overexertion, muscle fatigue, and repetitive movements, which can traumatise muscle, heighten muscle pain inputs and accelerate cartilage destruction, or encourage excess sedentary practices. Additional care and careful monitoring to avoid overstretching the joint, and helping those with severe overweight to lose weight is advocated as well. As well, avoiding high frequency loading activities after periods of immobilization found to hasten cartilage destruction is clearly of additional import [75].
In short, although limited, current findings strongly highlight the degree to which muscle fat infiltration and/or enlargement as well as muscle atrophy may play a disabling role in the osteoarthritis disease cycle, as well fostering multiple levels of focal and systemic dysfunction, severe pain, and biomechanical and metabolic challenges. Moreover, at the shoulder it can be observed that although the temporal and causal relationships of these associations remain ambiguous, muscle atrophy and fat infiltration should be considered two discrete processes in the natural history of gleno humeral osteoarthritis [69]. Indeed, it appears increasingly difficult to ignore a role for muscle fat mass deposition and/or its expansion as a potential pathogenic factor or co-factor in the cycle of progressive shoulder pathology and requiring attention even if surgery is contemplated [4]. Additionally, it appears that rotator cuff muscle volume is significantly decreased in those adults with definitive osteoarthritis and can thereby impact outcomes including muscle force capacity and joint protection [60].
However, physical therapies that address this pathogenic disease feature may greatly help to mitigate this disabling condition especially if they encourage efforts to safely increase lean muscle mass and balanced well-modulated movements and stability, while reducing pain and the risk of further joint destruction [50]. In particular, to avert rapid or excess disease progression and disability, and its association with immense social and mobility-related loss es, there appears to be an increasing body of research that supports the view that efforts to reduce muscle fat encroachment is a highly salient osteoarthritis disease correlate and possible efficacious mitigation strategy.
Unfortunately, since this idea is by no means a universally accepted or practice based one, thus more studies that tease out the possible relationship between muscle factors and osteoarthritic pain along with central factors that affect pain and muscle fat encroachment may enable progress especially if conducted over extensive time periods utilizing a variety of possible patient specific interventions. As shown in Figure 1 efforts to untangle these interactive reactionary states may prove especially revealing and help in the design of optimal osteoarthritis rehabilitation plans as well as reducing the need for surgery and its oftentimes less than optimal outcomes. Similarly conservative treatments of the shoulder and their scope and sequence may be enhanced such that: 1) older adults, and those at risk for excess reactive or age associated muscle atrophy declines or both will suffer less in multiple respects; 2). It may be possible to elucidate how adverse joint impact generated internally or externally or both can induce of exacerbate osteoarthritis joint damage; 3). while not definitive, prevention appears highly promising as an adjunctive approach to avert and ameliorate the magnitude of muscle mass declines and attrition generated by injury both macro and micro and osteoarthritis disease severity even in cases that undergo joint replacement surgery; 4) securing an ample or desired level of motion as regards muscle health in a controlled progressive manner and avoiding excessive joint stretches may yet help to protect against excess movement generation, age related joint damage and injury, while speeding up recovery.
In this regard, concerted efforts to educate as to how important it is for vulnerable adults, such as those who are overweight, to protect against cartilage damage and injury, including repetitive joint loading impacts may be of the highest import in allaying the onset or progression of excess muscle fat infiltration at the shoulder joint and others. Another point is that the presence of any subnormal muscle composition has previously been linked not only to poor function, but comorbidity, and increased hospitalization and all-cause mortality [76]. However, to advance this field successfully, well-powered and carefully conceived long term clinical, as well as epidemiological and basically oriented studies that employ advanced technologies, careful subgroup allocation, and healthy as well as impaired subjects are sorely needed and strongly encouraged.
Conclusion
Although modern medicine has been quite successful in managing
many acute health conditions and reducing infection and injury
risk and magnitude in the older adult population, no similar simple
solution prevails against osteoarthritis especially among those older
adults who are possibly either over- or underweight. There may
also be associated emergent signs of pain and need for surgery if
nothing is done to avert preventable disease correlates. Meantime
it appears:
• High degrees of shoulder osteoarthritis impairment and suffering
will persist among older adults if a role for muscle fat
mass as both a precursor and progenitor remain fragmentary
or unaccounted for.
• Dedicated efforts to assess and unravel the origins and profound
impact of muscle fat mass deposition on shoulder joint
health and how exercise may exacerbate this, rather than
thwart this if poorly devised and implemented are indicated.
• Injuries involving macro or micro tears of the shoulder rotator
cuff along with subsequent or pre-existing deconditioning and
poor health and are poorly treated are likely to prove key contributors
to shoulder osteoarthritis rather than age alone and
warrant upstream and downstream prevention efforts.
• Research that examines the link between intramuscular fat
and joint sensory functions and pain will prove insightful.
• A study of disease-free older adults and how muscle fat affects
joint loading and muscle regeneration and how this knowledge
can be applied pre-emptively would also greatly advance the
field.
Until more is known, we further assert shoulder osteoarthritis determinants that affect many older adults adversely are possibly compounded by muscle fat mass invasion and/or reactive infiltration and enlargement associated with a variety of subnormal bone-muscle interactions. Moreover, neglecting or overlooking the relevance of possible associated increases in disordered muscle composition, especially that attributable to injury risk and related fatty infiltration processes may hasten the onset of progressive muscle weakness, dysfunction, neuropathic pain, cartilage failure and its disabling life quality and functional implications.
By contrast, early detection, careful follow ups and record keeping, and primary preventive, rather than late end stage interventions, will likely avert much suffering, and pain among numerous at risk older adult populations as regards shoulder arthritis disability and where possible surgery, often fraught with varying benefits, persistent pain and muscle strength decreases and long recovery periods [75]. It may affect the need for reparative surgery and spread of muscle fat to unaffected shoulder cuff muscles in the case of further muscle attrition [76, 77]. It may help avoid or reduce the negative impact of excess body weight as well as frailty and poor proprioception on muscle fat mass, especially among those deemed at risk [78]. In essence, although seemingly an obscure topic in the realm of osteoarthritis mitigation - it is apparent much osteoarthritis suffering can be avoided or undone. In particular, a concerted desire to identify, track, and examine muscle factors such as muscle fat mass in general in the realm of both osteoarthritis research and the design of optimal osteoarthritis rehabilitation plans and their scope and sequence may well be far reaching at low cost and generate much success.
Funding
None
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Andrew Nguyen, Philip Lee, Edward K Rodriguez, Karen Chahal, Benjamin R Freedman, et al. (2025) Addressing the growing burden of musculoskeletal diseases in the ageing US population: challenges and innovations. Lancet Healthy Longev. Published online 6(5): 100707.
- Weiwei Ma, Honggu Chen, Qipeng Yuan, Xiaoling Chen, Huanan Li (2025) Global, regional, and national epidemiology of osteoarthritis in working-age individuals: insights from the global burden of disease study 1990-2021. Sci Rep 15(1): 7907.
- Fei Wang, Yu Cao, Hao Lu, Yuehan Pan, Youping Tao, et al. (2025) Osteoarthritis incidence trends globally, regionally, and nationally, 1990-2019: an age-period-cohort analysis. Musculoskeletal Care 23(1): e70045.
- Qiming Wu, Zhuyan Xu, Xiaomin Ma, Juan Li, Jun Du, et al. (2024) Association of low muscle mass index and sarcopenic obesity with knee osteoarthritis: a systematic review and meta-analysis. J Int Soc Sports Nutr 21(1): 2352393.
- Kelsey H Collins, Kristin L Lenz, Eleanor N Pollitt, Daniel Ferguson, Irina Hutson, et al. (2021) Adipose tissue is a critical regulator of osteoarthritis. Proc Natl Acad Sci U S A 118(1): e2021096118.
- Shogo Okada, Masashi Taniguchi, Masahide Yagi, Yoshihiro Fukumoto, Tetsuya Hirono, et al. (2025) Degeneration of the cartilage quality is correlated with a higher intramuscular fat infiltration of the vastus medialis in older adults with pre-to-mild knee osteoarthritis. Eur J Radiol 183: 111930.
- Grigorios Svarnas, Vlad Popa, Theofania-Sotiria Patsiou, Joseph Michael Schwab, Moritz Tannast (2025) Postoperative muscle atrophy and fatty degeneration with respect to surgical approaches in total hip arthroplasty. Arch Orthop Trauma Surg 145(1): 177.
- Li Zhang, Peng Xu, Hui Li, Chao Lu, Weikun Hou, et al. (2025) Contributions of external, muscle, and ligament forces to tibiofemoral contact loads in patients with knee osteoarthritis and healthy individuals. Bioengineering 12(6): 600.
- Gakuto Kitamura, Manabu Nankaku, Takumi Kawano, Shinichi Kuriyama, Shinichiro Nakamura, et al. (2025) Characteristics of skeletal muscles in lower limb from the perspective of muscle quality in patients with knee osteoarthritis. J Orthop Res.
- Merve Karapınar, Veysel Atilla Ayyıldız, Meriç Unal, Tüzün Fırat (2024) Effect of intramuscular fat in the thigh muscles on muscle architecture and physical performance in the middle-aged women with knee osteoarthritis. J Orthop Sci 29(1): 194-199.
- Giovanna Distefano, Stephanie Harrison, John Lynch, Thomas M Link, Philip A Kramer, et al. (2024) Skeletal muscle composition, power, and mitochondrial energetics in older men and women with knee osteoarthritis. Arthritis Rheumatol 76(12): 1764-1774.
- Shorter E, Sannicandro AJ, Poulet B, Goljanek-Whysall K (2019) Skeletal muscle wasting and its relationship with osteoarthritis: a mini-review of mechanisms and current interventions. Current Rheumatol Rep 21(8): 40.
- Mario A Vargas-Vila, Michael C Gibbons, Isabella T Wu, Mary C Esparza, Kenji Kato, et al. (2022) Progression of muscle loss and fat accumulation in a rabbit model of rotator cuff tear. J Orthop Res 40(5): 1016-1025.
- Deniz Siso, Hwabok Wee, Padmavathi Ponnuru, Gregory S Lewis, Jing Du, et al. (2024) The association of rotator cuff muscle morphology and glenoid morphology in primary glenohumeral osteoarthritis. Shoulder Elbow:17585732241269193.
- Wu HT, Liu Q, Lin JH (20250 Fatty infiltration predicts retear and functional impairment following rotator cuff repair: systematic review and meta-analysis. J Orthop Surg Res 20(1): 303.
- Qianlin Weng, Ting Jiang, Tuo Yang, Yuqing Zhang, Weiya Zhang, et al. (2025) Association of thigh intramuscular fat infiltration with incident knee and hip osteoarthritis: a longitudinal cohort study. Arthritis Rheumatol.
- Alessandra M Norris, Kiara E Fierman, Jillian Campbell, Rhea Pitale, Muhammad Shahraj, et al. (2024) Studying intramuscular fat deposition and muscle regeneration: insights from a comparative analysis of mouse strains, injury models, and sex differences. Skelet Muscle 14(1): 12.
- Gabby B Joseph, Zehra Akkaya, Wynton M Sims, Charles E McCulloch, Michael C Nevitt, et al. (2025) MRI-based analysis of thigh intramuscular fat and its associations with age, sex, and BMI using data from the osteoarthritis initiative data. Sci Rep 15(1): 6188.
- Xini Zhang, Xiaoyu Pan, Liqin Deng, Weijie Fu (2020) Relationship between knee muscle strength and fat/muscle mass in elderly women with knee osteoarthritis based on dual-energy X-Ray absorptiometry. Int J Environ Res Publ Hlth 17(2): 573.
- Jean-Pierre Raynauld, Jean-Pierre Pelletier, Camille Roubille, Marc Dorais, François Abram, et al. (2015) Magnetic resonance imaging-assessed vastus medialis muscle fat content and risk for knee osteoarthritis progression: relevance from a clinical trial. Arthritis Care Res 67(10): 1406-1415.
- Stephanie Inhuber, Nico Sollmann, Sarah Schlaeger, Michael Dieckmeyer, Egon Burian, et al. (2019) Associations of thigh muscle fat infiltration with isometric strength measurements based on chemical shift encoding-based water-fat magnetic resonance imaging. Eur Radiol Exp 3(1): 45.
- Marc A Merriman, James H Chapman, Taraje Whitfield, Fatemeh Hosseini, Debolina Ghosh, et al. (2025) Fat expansion not fat infiltration of muscle post rotator cuff tendon tears of the shoulder: regenerative engineering implications. Regen Eng Transl Med 11(1): 1-14.
- Masashi Taniguchi, Yoshihiro Fukumoto, Masahide Yagi, Tetsuya Hirono, Momoko Yamagata, et al. (2023) A higher intramuscular fat in vastus medialis is associated with functional disabilities and symptoms in early stage of knee osteoarthritis: a case-control study. Arthritis Res Ther 25(1): 61.
- Jessica B Aily, Marcos de Noronha, Ricardo J Ferrari, Stela M Mattiello (2025) Differences in fatty infiltration in thigh muscles and physical function between people with and without knee osteoarthritis and similar body mass index: a cross-sectional study. BMC Musculoskelet Disord 26(1): 109.
- Kenneth W Donohue, Eric T Ricchetti, Jason C Ho, Joseph P Iannotti (2018) The association between rotator cuff muscle fatty infiltration and glenoid morphology in glenohumeral osteoarthritis. J Bone Joint Surg Am 100(5): 381-387.
- Eddo Wesselink, Edwin de Raaij, Philip Pevenage, Nick van der Kaay, Jan Pool (2019) Fear-avoidance beliefs are associated with a high fat content in the erector spinae: a 1.5 Tesla magnetic resonance imaging study. Chiropr Man Therap 27: 14.
- Jufeng Luo, Qiao Xiang, Taiping Lin, Rui Liang, Yuzhao Dai, et al. (2025) Associations between total and regional fat-to-muscle mass ratio and osteoarthritis incidence: a prospective cohort study. Osteoarthritis Cartilage: S1063-4584(25)01005-2.
- Alexander W Aleem, Peter N Chalmers, Daniel Bechtold, Adam Z Khan, Robert Z Tashjian, et al. (2019) Association between rotator cuff muscle size and glenoid deformity in primary glenohumeral osteoarthritis. J Bone Joint Surg Am 101(21): 1912-1920.
- Deepak Kumar, Thomas M Link, S Reza Jafarzadeh, Michael P LaValley, Sharmila Majumdar, et al. (2021) Association of quadriceps adiposity with an increase in knee cartilage, meniscus, or bone marrow lesions over three years. Arthritis Care Res 73(8): 1134-1139.
- Wang L, Valencak TG, Shan T (2024) Fat infiltration in skeletal muscle: Influential triggers and regulatory mechanism. iScience 27(3): 109221.
- Chuanshun Chen, Hecheng Zhou, Yuesong Yin, Hai Hu, Binbin Jiang, et al. (2023) Rotator cuff muscle degeneration in a mouse model of glenohumeral osteoarthritis induced by monoiodoacetic acid. J Shoulder Elbow Surg 32(3): 500-511.
- Matthew J Hartwell, Ryan E Harold, Patrick T Sweeney, Amee L Seitz, Guido Marra, et al. (2021) Imbalance in axial-plane rotator cuff fatty infiltration in posteriorly worn glenoids in primary glenohumeral osteoarthritis: an MRI-based Study. Clin Orthop Relat Res 479(11): 2471-2479.
- James Elliott, Gwendolen Jull, Jon Timothy Noteboom, Ross Darnell, Graham Galloway, et al. (2006) Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine 31(22): E847-E855.
- Nazanin Daneshvarhashjin, Philippe Debeer, Harold Matthews, Peter Claes, Filip Verhaegen, et al. (2025) Covariation between rotator cuff muscle quality and shoulder morphometric bony features in B-glenoids: a statistical modeling approach. Biomech Model Mechanobiol.
- Ana Isabel Garcia-Diez, Marta Porta-Vilaro, Jaime Isern-Kebschull, Natali Naude, Roman Guggenberger, et al. (2024) Myosteatosis: diagnostic significance and assessment by imaging approaches. Quant Imaging Med Surg 14(11): 7937-7957.
- Severin Ruoss, Christoph B Möhl, Mario C Benn, Brigitte von Rechenberg, Karl Wieser, et al. (2018) Costamere protein expression and tissue composition of rotator cuff muscle after tendon release in sheep. J Orthop Res 36(1): 272-281.
- Georg C Feuerriegel, Benjamin Fritz, Adrian A Marth, Stefan Sommer, Karl Wieser, et al. (2025) Assessment of the rotator cuff muscles: state-of-the-art MRI and clinical implications. Radiology 315(2): e242131.
- L Deraedt, C Diependaele, D Cardon, A Jalalijam, L DE Wilde, et al. (2024) 3D quantitative CT study to assess rotator cuff muscle fatty infiltration. Acta Orthop Belg 90(2): 221-227.
- Tashjian RZ (2012) Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med 31(4): 589-604.
- Tatiane Gorski, Nicola C Casartelli, Gillian Fitzgerald, Astrid M H Horstman, Evi Masschelein, et al. (2024) Intramuscular fatty infiltration and its correlation with muscle composition and function in hip osteoarthritis. Skelet Muscle 14(1): 32.
- Lara Riem, Silvia S. Blemker, Olivia DuCharme, Elizabeth B Leitch, Matthew Cousins, et al. (2023) Objective analysis of partial three-dimensional rotator cuff muscle volume and fat infiltration across ages and sex from clinical MRI scans. Sci Rep 13(1): 14345.
- Nikoo Saveh Shemshaki, Ho-Man Kan, Mohammed Barajaa, Takayoshi Otsuka, Amir Lebaschi, et al. (2022) Muscle degeneration in chronic massive rotator cuff tears of the shoulder: addressing the real problem using a graphene matrix. Proc Natl Acad Sci U S A 119(33): e2208106119.
- Ori Safran, Kathleen A Derwin, Kimerly Powell, Joseph P Iannotti (2005) Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J Bone Joint Surg Am 87(12): 2662-2670.
- Xuhui Liu, Anne Y Ning, Nai Chen Chang, Hubert Kim, Robert Nissenson, et al. (2016) Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J 6(1): 6-15.
- Christian Gerber, Dominik C Meyer, Martin Flück, Paola Valdivieso, Brigitte von Rechenberg, et al. (2017) Muscle degeneration associated with rotator cuff tendon release and/or denervation in sheep. Am J Sports Med 45(3): 651-658.
- Tsubasa Mitsutake, Maiko Sakamoto, Yuji Chyuda, Shinichiro Oka, Hirokatsu Hirata, et al. (2016) Greater cervical muscle fat infiltration evaluated by magnetic resonance imaging is associated with poor postural stability in patients with cervical spondylotic radiculopathy. Spine 41(1): E8-E14.
- Yimeng Yang, Longhua Qiu, Xueping Gu, Jun Chen, Shiyi Chen, et al. (2022) Monitoring rotator cuff muscle fatty infiltration progression by magnetic resonance imaging T1 mapping: correlation with direct evaluation findings in rats. Am J Sports Med 50(4): 1078-1087.
- Bahram Mohajer, Mahsa Dolatshahi, Kamyar Moradi, Nima Najafzadeh, John Eng, et al. (2022) Role of thigh muscle changes in knee osteoarthritis outcomes: osteoarthritis initiative data. Radiology 305(1): 169-178.
- McCrum E (2020) MR Imaging of the rotator cuff. Magn Reson Imaging Clin N Am 28(2): 165-179.
- Peter J Millett, Jean-Yves Schoenahl, Matthew J Allen, Tatiana Motta, Trevor R Gaskill (2013) An association between the inferior humeral head osteophyte and teres minor fatty infiltration: evidence for axillary nerve entrapment in glenohumeral osteoarthritis. J Shoulder Elbow Surg 22(2): 215-221.
- Walter Sepúlveda-Loyola, Yshoner Antonio Silva-Díaz, Mário Molari, Erikson Alexander Jiménez Torres, Cintya Odar-Rojas, et al. (2025) Association between the fat mass/fat-free mass ratio and muscle strength, static balance and exercise capacity in older adults: a cross-sectional study. Nutr Hosp 43(3): 464-469.
- Aderson Loureiro, Maria Constantinou, Belinda Beck, Rod S Barrett, Laura E Diamond (2019) A 12-month prospective exploratory study of muscle and fat characteristics in individuals with mild-to-moderate hip osteoarthritis. BMC Musculoskelet Disord 20(1): 283.
- Tahere Seyedhoseinpoor, Mohammad Taghipour, Mehdi Dadgoo, Mohammad Ali Sanjari, Ismail Ebrahimi Takamjani, et al. (2022) Alteration of lumbar muscle morphology and composition in relation to low back pain: a systematic review and meta-analysis. Spine J 22(4): 660-676.
- Suzanne J Snodgrass, Peter Stanwell, Kenneth A Weber, Samala Shepherd, Olivia Kennedy, et al. (2022) Greater muscle volume and muscle fat infiltrate in the deep cervical spine extensor muscles (multifidus with semispinalis cervicis) in individuals with chronic idiopathic neck pain compared to age and sex-matched asymptomatic controls: a cross-sectional study. BMC Musculoskelet Disord 23(1): 973.
- Devin J Drummer, Jeremy S McAdam, Regina Seay, Inmaculada Aban, Kaleen M Lavin, et al. (2022) Perioperative assessment of muscle inflammation susceptibility in patients with end-stage osteoarthritis. J Appl Physiol (1985) 132(4): 984-994.
- Jake A Fox, Lauren Luther, Eden Epner, Lance LeClere (2024) Shoulder proprioception: a review. J Clin Med 13(7): 2077.
- Iva Miljkovic, Allison L Kuipers, Jane A Cauley, Tanushree Prasad, Christine G Lee, et al. (2015) Greater skeletal muscle fat infiltration is associated with higher all-cause and cardiovascular mortality in older men. J Gerontol Series A: Biomedical Sci and Med Sci 70(9): 1133-1140.
- Ryan B Wallenberg, Mckenna L Belzer, Duncan C Ramsey, Dayton M Opel, Mark D Berkson, et al. (2022) MRI-based 3-dimensional volumetric assessment of fatty infiltration and muscle atrophy in rotator cuff tears. J Shoulder Elbow Surg 31(6): 1272-1281.
- Jean-David Werthel, François Boux de Casson, Valérie Burdin, George S Athwal, Luc Favard, et al. (2021) CT-based volumetric assessment of rotator cuff muscle in shoulder arthroplasty preoperative planning. Bone Jt Open 2(7): 552-561.
- Lindsey Ruderman, Abigail Leinroth, Helen Rueckert, Troy Tabarestani, Rafeal Baker, et al. (2022) Histologic differences in human rotator cuff muscle based on tear characteristics. J Bone Joint Surg Am 104(13): 1148-1156.
- Nikoo Saveh Shemshaki, Ho-Man Kan, Mohammed A Barajaa, Amir Lebaschi, Takayoshi Otsuka, et al. (2023) Efficacy of a novel electroconductive matrix to treat muscle atrophy and fat accumulation in chronic massive rotator cuff tears of the shoulder. ACS Biomater Sci Eng 9(10): 5782-5792.
- Karl Wieser, Jethin Joshy, Lukas Filli, Philipp Kriechling, Reto Sutter, et al. (2019) Changes of supraspinatus muscle volume and fat fraction after successful or failed arthroscopic rotator cuff repair. Am J Sports Med 47(13): 3080-3088.
- Thankam FG, Dilisio MF, Agrawal DK (2016) Immunobiological factors aggravating the fatty infiltration on tendons and muscles in rotator cuff lesions. Mol Cell Biochem 417(1-2): 17-33.
- Se-Young Ki, Yong-Soo Lee, Ja-Yeon Kim, Taewoo Lho, Seok Won Chung (2021) Relationship between fatty infiltration and gene expression in patients with medium rotator cuff tear. J Shoulder Elbow Surg 30(2): 387-395.
- Antonio Arenas-Miquelez, Victor K Liu, Joseph Cavanagh, Petra L Graham, Louis M Ferreira, et al. (2021) Does the Walch type B shoulder have a transverse force couple imbalance? A volumetric analysis of segmented rotator cuff muscles in osteoarthritic shoulders. J Shoulder Elbow Surg 30(10): 2344-2354.
- Thomas Brioche, Allan F Pagano, Guillaume Py, Angèle Chopard (2016) Muscle wasting and aging: experimental models, fatty infiltrations, and prevention. Molecular Aspects Med 50: 56-87.
- Barbara Melis, Michael J DeFranco, Christopher Chuinard, Gilles Walch (2010) Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clin Orthop Rel Res 468(6): 1498-1505.
- Michael A Moverman, Richard N Puzzitiello, Mariano E Menendez, Nicholas R Pagani, Paul-Anthony J Hart, et al. (2022) Rotator cuff fatty infiltration and muscle atrophy: relation to glenoid deformity in primary glenohumeral osteoarthritis. J Shoulder Elbow Surg 31(2): 286-293.
- Ziqi Jiang, Kexin Wang, Hongda Zhang, Yuanzhi Weng, Deming Guo, et al. (2025) Correlation between paraspinal muscle fat infiltration and thoracic vertebral degeneration based on phantom-less QCT: a novel insight into thoracic vertebral degeneration. Eur Spine J 34(3): 837-852.
- Al Saedi A, Duque G, Stupka N (2021) Targeting intramuscular adipose tissue expansion to preserve contractile function in volumetric muscle loss: a potentially novel therapy? Curr Opin Pharmacol 58: 21-26.
- Yilong Huang, Ling Wang, Baofa Luo, Kaiwen Yang, Xiaomin Zeng, et al. (2022) Associations of lumber disc degeneration with paraspinal muscles myosteatosis in discogenic low back pain. Front Endocrinol (Lausanne) 13: 891088.
- Yihua Zhu, Yue Hu, Yalan Pan, Muzhe Li, Yuanyuan Niu, et al. (2024) Fatty infiltration in the musculoskeletal system: pathological mechanisms and clinical implications. Front Endocrinol (Lausanne) 15: 1406046.
- Theret M, Rossi FMV, Contreras O (2021) Evolving roles of muscle-resident fibro-adipogenic progenitors in health, regeneration, neuromuscular disorders, and aging. Front Physiol 12: 673404.
- Edward Bowen, Aboubacar Waque, Favian Su, Michael Davies, Gabriella Ode, et al. (2025) Muscle health & fatty infiltration with advanced rotator cuff pathology. Curr Rev Musculoskeletal Med 18(4): 160-172.
- Yaiza Lopiz, Raul Herzog, Camilla Arvinius, Carlos Garcia, Esperanza Anhui, et al. (2025) Functional outcomes and complications of classic Grammont-style reverse shoulder arthroplasty in patients with Os Acromiale: a retrospective case-control study. Int Orthop.
- Jennifer Linge, Mikael Petersson, Mikael F Forsgren, Arun J Sanyal, Olof Dahlqvist Leinhard (2021) Adverse muscle composition predicts all-cause mortality in the UK Biobank imaging study. J Cachexia Sarcopenia Muscle 12(6): 1513-1526.
- Anthony Herve, Hervé Thomazeau, Luc Favard, Michel Colmar, Pierre Mansat, et al. (2019) Clinical and radiological outcomes of osteoarthritis twenty years after rotator cuff repair. Orthop Traumatol Surg Res 105(5): 813-818.
- Amanda L Ager, Fabio Carlos Lucas de Oliveira, Jean-Sébastien Roy, Dorien Borms, Michiel Deraedt, et al. (2023) Effects of elastic kinesiology taping on shoulder proprioception: a systematic review. Braz J Phys Ther 27(3): 100514.






