 Review Article
Review Article
Intranasal Emergency Drug Delivery: A Review
Kevin Eells1* and Inder Sehgal2
1Rocky Vista University College of Medicine, Parker, Colorado, USA
2Rocky Vista University College of Medicine, Ivins, Utah, USA
Kevin Eells at Rocky Vista University, 8401 S Chambers Road, Parker, CO, 80134, USA
Received Date: December 1, 2023; Published Date: January 26, 2024
Abstract
Development of systemic intranasal medications is of growing interest in pharmaceutical research. Intranasal administration offers a less invasive and more rapid delivery of medications compared to intramuscular or subcutaneous routes. Intranasal delivery can also allow some medications with low oral bioavailability to reach the brain. This delivery method offers an efficient and easily accessible route in both pediatric and adult patients allowing for rapid and acute treatment in outpatient settings. This review will discuss current and likely future intranasal medications for acute applications. A focused literature review was conducted and used to describe applicable nasal anatomy, mechanism of intranasal drug absorption, current clinical intranasal medications, advantages and obstacles of intranasal drug delivery systems, obstacles and future directions. Several characteristics make a drug suitable for intranasal delivery. These include small mass (<1 kDa), simple structure, and lipophilic character although absorption enhancers allow for even peptides to be administered. An appropriately small volume of administration is necessary to avoid runoff into the throat or out of the nostril. The most significant obstacles to intranasal drug delivery are presented by the chemistry of the drug molecules that can be formulated for delivery. Intranasal delivery is a non-invasive, rapid, and relatively simple method to systemically administer a growing variety of drug types.
Keywords:Intranasal; Drug delivery; Pharmaceutics; Adrenal insufficiency
Introduction
Intranasal or transnasal drug delivery relies on drug absorption through the nasal capillaries to reach systemic circulation. This alternative form of parenteral delivery can offer a less invasive and more rapid delivery of medication than intramuscular or subcutaneous routes. Intranasal delivery has also gained popularity since it can allow medications with low oral bioavailability to reach the brain [1] as the olfactory and trigeminal nerve pathways from the nares may deliver some drug structures bypassing the blood brain barrier [2]. Intranasal administration may also affect the pulmonary system, the lymphatic system and have some local direct effects. To reach systemic circulation rather than local tissues, drug physiochemistry and/or excipients must favor permeation across tissues and into capillaries. This review describes nasal anatomy, current clinical applications, and advantages and disadvantages of current intranasal drug delivery systems as they relate to mechanisms of action, costs, and future directions.
Anatomy of the Nasal Cavity
Anatomically, the nose can be divided into three areas: the vestibular region, respiratory region and olfactory region. The vestibular region is at the nostril opening and is composed of hair cells called vibrissae, squamous epithelial cells and spans 0.6 cm2 in the human nose [3]. Vibrissae filters out particles that are larger than 10 μm such as dust to prevent them from reaching the lungs. The respiratory region is the largest area of the nose and comprises 80-90% of the nasal cavity [3]. This region contains three turbinates: the superior, middle, and inferior. These serve to humidify, clean, warm and eliminate any potential debris in the air. While the vestibular region is a stratified squamous epithelium, the respiratory region is a pseudo stratified and columnar epithelium. It contains four main cell types: goblet cells, ciliated and nonciliated cells and basal cells. Goblet cells are mucus secreting cells that have barrier and antibacterial properties. Ciliated cells provide a propelling force that moves mucus along the airways and towards the larynx. Nonciliated cells function to increase the surface area which becomes a main factor in drug absorption. Basal cells have the unique ability to develop into any cell that may be needed [4].
The respiratory region is also the most vascular region of the three allowing for drug molecules reaching this region to be absorbed systemically. The entire nasal cavity is perfused by three arteries: the facial artery, the ophthalmic artery, and the maxillary artery. The maxillary artery branches sphenopalatine and descending palatine arteries perfusing the bulk of the nasal cavity. The veins that drain the nose and nasal cavity follow the arteries [5]. The venous drainage is vital as it is the venous return that takes drug molecules systemically. The maxillary capillary network combined with the respiratory region’s large surface area make an ideal environment for systemic drug delivery. The final anatomic region, the olfactory region, comprises roughly 10% of the nasal cavity [3]. This region is made up of ciliated pseudostratified columnar epithelium. The cilia in this region are sensory compared to the ciliated cells of the respiratory region which are motile. Another major distinction between the olfactory and respiratory epithelium is that both the capillary density and permeability are much lower than in the respiratory region [6]. This difference generally favors absorption of hydrophilic molecules in nasal respiratory regions than in olfactory regions. However, through selective targeting to the olfactory region, the use of lipophilic molecules or vasoconstrictive additives, the olfactory region can be used to transport drug molecules to the central nervous system [6].
Mechanisms for Drug Delivery
The first step in the delivery of an intranasal drug is the absorptive passage through the mucosal cells to reach the underlying capillaries. Systemic delivery occurs through capillary absorption facilitated by the vast number of vessels in the nasal cavity. This absorptive mechanism allows for the fastest onset of action and avoids first pass hepatic exposure increasing bioavailability [7]. Drug permeation to the capillaries can be accomplished through two different mechanisms: paracellular and transcellular [2]. Paracellular transport refers to passage between cells, whereas transcellular transport is through cells. Typically, lipophilic molecules with ability to cross the plasma membrane cross mucosal cells transcellularly, while more polar molecules utilize the paracellular route. Large molecular weight compounds (those >1 kDa) such as peptides and proteins often have a limited capability to cross the nasal mucosa. [3]. However, even peptides are delivered intranasally by using permeation enhancers such as surfactants [8].
Ideally, drugs for systemic intranasal delivery possess several characteristics. These include small mass (<1 kDa), simple structure, and amphipathic character (Table 1). Additionally, drugs should have pKa’s near that of mucosal pH. The normal human nasal mucosa has a pH of 6.3. Most nasal drug solutions, therefore, are formulated to lie within a range of 4.0-6.3 in order to maintain function of ciliary clearance and avoid nasal irritancies [9]. Another important consideration with intranasal drug formulation is the active ingredient’s concentration which determines the administration volume needed for effect. An appropriately small volume is needed to avoid runoff into the throat or out of the nostril. By finding the minimal necessary volume, intranasal administration can achieve both efficient absorption and significant bioavailability. Large molecules such as glucagon also are delivered by the intranasal route. For these drugs, formulation modifications are necessary to allow sufficient permeation. Detergents that separate intercellular junctions and/or liposomal delivery are methods commonly used to boost drugs through the nasal mucosa to the underlying capillaries [8]. Intranasal drugs that are successfully transferred into the CNS and across the blood brain barrier do so via reaching either the olfactory and/or respiratory anatomic regions [4]. Drug molecules reaching the dorsal nasal cavity can reach the olfactory sensory neurons, embedded in the support cells of the nasal cavity after passing through the mucosa. From here, the drug molecule may either be taken up extracellularly or intracellularly. In extracellular uptake, molecules do not pass through the olfactory sensory neuron itself; instead, they pass between support cells via tight junctions or paracellular clefts, then traverse the lamina propria and perineural space and ultimately reach the subarachnoid space where it can then be distributed to various sites in the CNS through cerebrospinal fluid [4]. For intracellular uptake, drug molecules enter the olfactory sensory neurons through endocytosis, then are sent to the Golgi apparatus where they undergo Golgi stacking and axonal transport to the olfactory bulb where they may reach the CNS [4].
Drug molecules can also gain CNS access through the respiratory anatomic region by maxillary and ophthalmic divisions (V1 and V2) of the trigeminal nerve innervating this region’s nasal mucosa [10]. Drug molecules follow the nerve pathways into the brainstem through the pons [1]. systemic drug delivery. Emulsifying agents can be added to enhance the tissue permeability (penetration across different tissue layers) of intranasal drugs such as with the glucagon peptide. [3,9].
Current Clinical Applications
While there are a wide range of medications delivered intranasally for chronic therapies such COPD, asthma, rhinitis and osteoporosis, intranasal delivery for acute care is an expanding delivery method because of its non-invasive nature, rapid action, and simplicity. This acute application of intranasal delivery has been adapted to administer glucagon, naloxone, and midazolam (Table 2).
Table 1:Characteristics needed for IN Drug Delivery.

Table 2:Current Successful Nasal Administered Drugs.
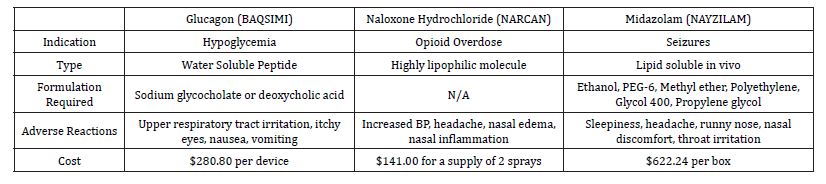
Table 2. Comparisons of three currently marketed intranasal drugs. The indications, solubility, formulation, adverse reactions and cost are shown [14,15,16,17,18].
BAQSIMI® is the formulation for intranasal administration of the peptide hormone glucagon. It is an emergency medication that can be administered by non-technical personnel to type 1 diabetics experiencing hypoglycemia [11]. The BAQSIMI® formulation is indicated in individuals who are 4 years of age or older. Glucagon is a single-chain polypeptide containing 29 amino acid residues with a molecular weight of 3483 g/mol and therefore appears to be a difficult molecule for intranasal delivery. However, glucagon is a relatively small peptide and when formulated with an emulsifying agent to enhance solubility (such as sodium glycocholate), it is now an accepted and useful drug product for intranasal administration [12]. Before the addition of emulsifying agents such as sodium glycocholate and Deoxycholic acid, delivery of peptides was limited to the parenteral route (IV, IM, and subcutaneous injections). The creation of these emulsifying agents offers renewed energy into developing intranasal medications that had previously been out of reach.
BAQSIM® is supplied in a one-time intranasal device delivering 3 mg of glucagon, as a preservative free, white powder that has the emulsifying agent dodecyl phosphocholine. This is a surfactant and absorption enhancer that has been shown to improve paracellular permeability of hydrophilic compounds by modulating the tight junctions. The medication can be stored in temperatures up to 86 °F and the device is discarded after use. After intranasal administration, plasma glucose levels reach ≥ 70 mg/dL or a ≥20 mg/dL from nadir within 30 minutes [13]. Glucagon is different from other peptide drugs in that it does not require a precise doseresponse that is critical to both safety and effectiveness. In studies conducted by [13], it was demonstrated that it is not necessary to achieve the very high blood glucagon levels obtained with SC or IM injection to achieve a clinically equivalent pharmacodynamic response [12]. Although the bioavailability of IN glucagon is less than that of injected glucagon, resulting in lower peak plasma glucagon concentrations, IN dosing results in a glycemic excursion that is not significantly different from injected glucagon in terms of return to normal glucose levels [12].
The list price of BAQSIMI is $280.80 per device; Similarly, the list price of a 1mg glucagon subcutaneous injection kit is approximately $280.80 [14]. The amount a patient actually pays will depend on any third party plans they may have. According to the BAQSIMI website, individuals on Medicaid will pay anywhere from $4-$9 on the device [14]. For individuals with private insurance, the average will be $25 out of pocket. Finally, individuals who have Medicare Part D coverage about 71% of BAQSIMI prescriptions cost between $0 and $100 per device, and the remaining prescriptions cost an average of $260 per device [14]. Some adverse reactions for injected glucagon differ from those associated with intranasal administration. Injection site reactions include erythema and swelling occur. In addition generalized allergic reactions including anaphylactic shock with breathing difficulties and hypotension are reported in trials. For intranasal glucagon, unique adverse reactions are upper respiratory tract irritation (i.e., rhinorrhea, nasal discomfort, nasal congestion, cough, and epistaxis), watery eyes, redness of eyes, and itchy nose, throat, and eyes. Both routes may lead to nausea, vomiting, and headaches. Naloxone hydrochloride (branded as NARCAN) was first granted US approval in 1971. It has become a mainstream medication to reverse opioid overdose. Naloxone is an opioid antagonist that is indicated for an individual experiencing effects of opioid overdose and subsequent respiratory and CNS depression. The mechanism of action of the drug involves competing for the same receptor sites that the opioid binds to. The nasal spray Narcan consists of 4 mg of naloxone hydrochloride in 0.1 mL [15]. The time to peak drug concentration for an individual receiving 1 spray of 4 mg is 0.50 hours. The highest concentration of the drug after an hour in the blood is 7.87 ng/mL. The half-life for one spray is 2.08 hours. For an individual receiving two sprays, 1 in each nostril for a total of 8 mg, the time to peak concentration is 0.33 hours [15]. Naloxone has a log P of 1.48 making it a highly lipophilic molecule. Due to this there is no need to add an agent to enhance solubility, as it is readily absorbed via the paracellular route.
Adverse reactions of NARCAN include increased blood pressure, musculoskeletal pain, headache, nasal dryness, nasal edema, nasal congestion, and nasal inflammation [15]. It is important to note that individuals who are given either intranasal or injected naloxone may also experience opioid withdrawal symptoms. Whenever this medication is administered, it requires immediate medical attention regardless of how well the individual responds. In February of 2023, the FDA approved intranasal naloxone to be sold over the counter. The cost for naloxone injectable solution (0.4 mg/mL) is around $61 for a supply of 10 milliliters, and is similar to the intranasal formulation [16]. This drug is often paid for by cash since insurance coverage is limited [16]
Midazolam nasal spray (NAYZILAM®) is a rescue medication indicated for individuals experiencing intermittent, stereotypic episodes of frequent seizure activity, such as seizure clusters, that are distinct from a patient’s usual seizure pattern. A single dose of NAYZILAM® consists of 5 mg midazolam per 0.1 mL solution. If a patient does not respond to the initial 5 mg dose, a second 5 mg can be administered to the opposite nostril after 10 minutes. The pH range of solution is approximately 5.0 to 9.0 [17]. Midazolam has a log P of 4.33, but the formulation consists of organic solvents including ethanol, PEG-6 methyl ether, polyethylene glycol 400, propylene glycol to increase the solubility and absorption of the medication [18]. Midazolam is believed to involve potentiation of GABAergic neurotransmission resulting from binding at the benzodiazepine site of the GABA receptor. This results in inhibition of excitatory neurons, causing a soothing effect on the CNS. Following nasal administration of a single 5 mg midazolam dose to healthy adults, midazolam was absorbed with median time to achieve maximal concentration (range) of 17.3 minutes. The highest concentration of the drug in the bloodstream or other part of the body after drug administration was 54.70 ng/mL [17]. The most common side effects of NAYZILAM® include sleepiness, headache, runny nose, nasal discomfort, and throat irritation [17]. The list price of NAYZILAM® is $622.242 per box. Each box contains two doses. For private insurance, 85% of NAYZILAM® prescriptions cost $0-$100 per box; for the remaining 15%, the most common cost for each box is $140 and for individuals with Part D coverage and the Extra Help coverage, the anticipated cost for each box is $10.35 or less [19].
In the United States, the FDA has approved three medications by two routes to be used as rescue medications in a non-hospital setting for the treatment of seizures. These are Diastat®, a diazepam rectal gel, Nayzilam® and Valtoco®, both of which are intranasal medications. Diastat® is a non-sterile gel provided in a prefilled, unit-dose, rectal delivery system [20]. The drug has a similar mechanism of action to Nayzilam as well as similar side effects, and precautions. Diazepam nasal spray (Valtoco®) can be used in individuals 6 years of age and older to treat seizures that are distinct from a patient’s usual seizure pattern. It is available in 5 mg, 7.5 mg, and 10 mg strengths all of which contain 0.1 mL of solution. The dosage depends on the age and weight of the individual receiving the drug. The highest plasma concentration after nasal administration of Valtoco® was reached in 1.5 hours [21]. The mean elimination half-life following administration of a 10 mg dose of Valtaco® was found to be about 49.2 hours [21]. The formulation includes dodecyl maltoside (Intravail A3; 0.25% weight/volume concentration), an alkylsaccharide, and vitamin E to increase absorption and solubility [18]. The cost for diazepam nasal spray (Valtoco), 5 mg/dose, is around $780 without insurance for a supply of 2 sprays [22]. The medication can be found on discount for a price of $685.11, making it the most expensive of the three [22]. Sleepiness, headache, runny nose, nasal discomfort, and throat irritation are also associated with this medication.
Advantages
Intranasal medications provide a unique set of advantages not found in other administration routes. One of the main advantages is that intranasal administration is a non-invasive, easily administered route that poses a minimal risk of infection. A study conducted by [23] assessed perceptions about glucagon delivery and potential effects of two glucagon delivery devices for severe hypoglycemia through qualitative interviews. The group interviewed a group of 45 individuals and asked them what an ideal device would look like to treat an emergent hypoglycemic event. The most frequently identified aspirational features for a new glucagon device were ease of use ([64%]), including being uncomplicated, premixed/ready to use, and ability to use quickly; small/easy to carry ([20%]); needlefree/ no long needles ([18%]); and easy instructions ([9%]). In general, the participants indicated they would prefer an intranasal glucagon due to the ease of carrying and use, the lack of a needle, and that non-patients would be more comfortable using it on a patient [23]. These authors later conducted a study investigating the attitudes of individuals with diabetes as well as their caregivers / acquaintances towards the use of intranasal glucagon vs. an autoinjector route using the Glucagon Device Attitudes Questionnaire [23]. Acquaintances and caretakers reported more anxiety using the autoinjector than with intranasal delivery and a higher likelihood of making an error while administering [24]. This data indicates the intranasal route provides an easy, non-invasive method that allows bystanders to have confidence in administering during an emergency.
Other advantages of the intranasal route for systemic administration include rapid absorption and no hepatic first pass metabolism of the drugs. In an emergency like an opioid overdose, the intranasal route allows for a rapid reversal allowing time for emergency responders to further patient treatments. Additionally, the intranasal route may allow for direct drug delivery to the brain by bypassing the blood-brain barrier through the trigeminal and olfactory pathways. This is an advantage lacking in other administration routes and allows for the intranasal treatment of CNS events such as seizures in non-hospital public settings where the intravenous or rectal routes are unavailable.
Disadvantages
Perhaps the most significant disadvantage to intranasal drug delivery is the types of drug molecules that can be formulated for intranasal delivery prior to the addition of emulsifying agents. The active ingredient in intranasal drugs must have a molecular weight <1 kDa to be efficiently absorbed [3]. Additionally, nasal drug administration is limited to very small volumes (25–200 μL), and thus only applicable to potent drugs with high water solubility. Absorption of polar drugs and some macromolecules are insufficient due to poor membrane permeability, rapid clearance, and enzymatic degradation into the nasal cavity [7]. While the drug characteristics certainly limit the number of drugs intranasal therapy can be applied to, the continued research of emulsifying agents is working to close that gap. Another disadvantage is drugs cannot irritate or injure the nasal mucosa [4]. Furthermore, the development of intranasal medications requires flexible doseresponse effects. Intranasal administration is not as precise as oral, IM or IV routes, so the drug being created must have a higher therapeutic index. Each of these factors limits the types of drugs that can effectively be formulated for intranasal delivery and poses unique challenges that must be overcome when developing new medications.
Table 3:Advantages & Obstacles to IN Systemic Drug Delivery.
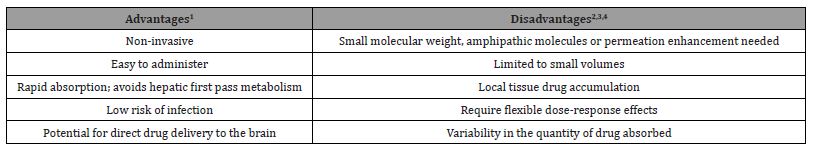
Table 3. Key advantages of IN systemic drug delivery are the non-invasive nature and ease. Key obstacles are small volume of the nasal cavity and variability of drug reaching systemic circulation; IN delivery is not suitable for drugs with precise dosing requirements [3,4,7,24].
Future Directions
Intranasal systemic drug delivery has become an increasingly popular area of investigation over the past decade. The numbers of interventional intranasal delivery studies, including patients of all age groups, sex and overall clinical phases increased around threefold compared with the time period from 2000 to 2010 [3]. While barriers must be overcome for successful formulation of specific intranasal drugs, the delivery advantages have led to expansion of this technique. Intranasal delivery research continues to expand to cover various disease states. Three potential future applications in this field are the administration of hydrocortisone to treat acute adrenal insufficiency in emergent situations, olanzapine for acute agitation and epinephrine for treatment of anaphylaxis [25].
Primary adrenal insufficiency is a rare disease with a reported prevalence of about 100 to 140 cases per million and an incidence of 4:1,000,000 per year [26]. Examples of primary adrenal insufficiency include Addison’s disease and Congenital Adrenal Hyperplasia. In both instances, individuals may experience an Addisonian, or adrenal crisis symptomized by fatigue, weakness, nausea, vomiting, abdominal pain, back pain, diarrhea, dizziness, hypotension, syncope, and if not treated, progression to obtundation, coma, and shock [27]. The current pharmacologic treatment for this crisis is administration of 100 mg intramuscular hydrocortisone injection. Hydrocortisone is administered as the sodium succinate salt, which is unstable in solution, necessitating two-chamber vials to separate hydrocortisone powder and diluent. Although hydrocortisone has a favorable molecular weight of 362.5 g/mol (National Center for Biotechnology Information, 2023), glucagon powder is also delivered intranasal. It is relatively insoluble in water, and not very lipophilic making it a difficult candidate for an intranasal medication. An area of intranasal delivery that is under investigation employs prednisolone or dexamethasone. An early-stage formulation under development at the University Medical Center Groningen utilizes a nebulized form of prednisolone powder to treat an adrenal crisis [28]. Prednisolone is 6 to 8 times more potent than hydrocortisone and has a longer duration of action; the time to onset is similar with peak concentrations being 1-2 hours. Acute agitation is often seen in individuals with schizophrenia, autism, and bipolar disorder. Agitation episodes related to neuropsychiatric disorders account for approximately 1.7 million visits to the ER each year, placing a significant economic and resource burden on the healthcare system (Impel NeuroPharma, 2019). Generic Olanzapine is an atypical second-generation antipsychotic that works by inhibiting dopamine receptors. Currently, the treatment of acute agitation has been limited to oral pills and intramuscular injections which are typically reserved for in-hospital settings. The intranasal route offers an at-home therapy for the first time. It allows for a noninvasive rapid onset medication that reduces the burden on the ER and minimizes the risk for needle sticks and assaults on healthcare staff. In the Phase 1 trial of INP-15, a drug-device combination which delivers an optimized formulation of olanzapine, results demonstrated that INP105 reached peak plasma levels twice as fast as intramuscular olanzapine (Zyprexa®), and ten-times faster than orally disintegrating tablets (ODT, Zyprexa Zydis®) (Impel NeuroPharma, 2019). Maximum and total plasma levels (Cmax and AUC) were similar to intramuscular delivery and exceeded the total plasma levels for ODT (Impel NeuroPharma, 2019). Approximately 25 to 40 million people in the United States have experienced severe Type 1 allergic reactions that may lead to anaphylaxis, but only about 3.3 million of them filled a prescription in 2021 for an epinephrine intramuscular injectable device [29]. ARS Pharma is currently in the process of developing an intranasal form of epinephrine named Neffy®. Data from the clinical trials demonstrated that Neffy® delivered consistent epinephrine levels to attain a pharmacokinetic (PK) and pharmacodynamic (PD) profile within the range of approved intramuscular (IM) injection products with a dose proportional exposure between once and twice dosing [29-32]. There are currently other companies developing nasal spray and sublingual routes as researchers continue to develop non-invasive rapid onset options.
Conclusions
Intranasal delivery is challenged by the drug solubility, yet it holds the promise to fill areas of therapy where existing products are severely limited by expertise required for easy administration. The cost of systemic intranasal drugs is similar to other routes of administration. The cost of each individual medication varies based on the insurance plan of the patient, but the similar list price allows for patients to pay the same amount for a potentially easier to administer device. Side effects of intranasal drugs are mostly systemic, and in most cases mirror those of the injection route. Although there are some nasal related adverse reactions, it has the benefit of avoiding injection-specific side effects seen in other routes. Further research into improvement of absorption enhancers will further spectrum of possible drugs that could be formulated for intranasal use.
Abbreviations: Central Nervous System, CNS; Intranasal, IN; Intravenous, IV; Intramuscular, IM
Contributions of Authors: KE: conceptualization, investigation and writing- original draft preparation. IS: supervision, writingreviewing and editing. All authors read and approved the final manuscript.
Funding
This research was funded by Rocky Vista University Department of Research.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Xu J, Tao J, Wang J (2020) Design and Application in Delivery System of Intranasal
- Kim D, Kim YH, Kwon S (2018) Enhanced nasal drug delivery efficiency by increasing
- Keller LA, Merkel O, Popp A (2022) Intranasal drug delivery: opportunities and toxicologic
- Franciska Erdő, Luca Anna Bors, Dániel Farkas, Ágnes Bajza, Sveinbjörn Gizurarson,
- Sobiesk JL, Munakomi S (2022) Anatomy, Head and Neck, Nasal Cavity. [Updated 2022 Jul 25]. In:
- Kumar N, Gautam M, Lochhead J, et al. (2016) Relative vascular permeability and
- Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC (2007) Intranasal delivery: physicochemical
and therapeutic aspects. Int J Pharm 337(1-2): 1-24.
- Upadhyay RK (2014) Drug delivery systems, CNS protection, and the blood brain barrier. BioMed
research international, (2014): 869269.
- Behl C, Pimplaskar H, Sileno A, deMeireles J, Romeo V (1998) Effects of physicochemical properties
and other factors on systemic nasal drug delivery. Adv Drug Deliv Rev 29: 89-116.
- TP Crowe, MHW Greenlee, AG Kanthasamy, WH Hsu, (2018) Mechanism of intranasal drug
delivery directly to the brain Life Sci 195: 44-52.
- Inzucchi S (2023) Hypoglycemia in Patients with Diabetes. ClinicalKey.
- Pontiroli AE (2015) Intranasal glucagon: a promising approach for treatment of severe hypoglycemia. Journal of diabetes science and technology 9(1): 38-43.
- BAQSIMI [package insert]. Indianapolis, IN: Eli Lilly and Company; 2020.
- Lilly USA. Baqsimi cost: With or without insurance: BAQSIMI® (glucagon) nasal powder. (2023).
- Lilly USA. Glucagon cost: With or without insurance: Glucagon (glucagon forinjection). (2023).
- NARCAN (naloxone hydrochloride) nasal spray [package insert]. Radnor, PA: Adapt Pharma;
(2015) National Center for Biotechnology Information. PubChem Compound Summary for CID
- Naloxone prices, coupons, Copay & Patient Assistance. Drugs.com. (2023).
- NAYZILAM® (midazolam) nasal spray [package insert]. Plymouth, MN: Proximagen, LLC;
(2019).
- Cloyd J, Haut S, Carrazana E, Rabinowicz AL (2021) Overcoming the challenges of
developing an intranasal diazepam rescue therapy for the treatment of seizure clusters.
Epilepsia, (2021) 62: 846-856.
- UCB Inc. How much should I expect to pay for Nayzilam® (midazolam) nasal spray,
- Diastat® (diazepam rectal gel) [package insert]. San Antonio, Texas: DPT Laboratories, Ltd;
(2023).
- VALTOCO® (diazepam) nasal spray [package insert]. San Diego, California: Neurelis, Inc;
(2020).
- GoodRx Inc. (2023). Valtoco. GoodRx.
- Bajpai SK, Cambron-Mellott MJ, Will O, Poon JL, Wang Q, et al. (2022) Mitchell, B. D., Peck, E.Y., Babrowicz, J., Raibulet, N. K., Child, C. J., & Beusterien, K. Development of a Measure to Assess Attitudes Towards Nasal versus Autoinjector Glucagon Delivery Devices for Treatment of Severe Hypoglycemia. Diabetes, metabolic syndrome and obesity: targets and therapy. 15: 3601-3615.
- Suman JD (2020) Nasal drug delivery: Past, present and future perspectives. Inhalation (2020) Nasal Drug Delivery_Past Present Future.
- Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, (2016) Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism 101(2): 364-389.
- Rathbun KM, Nguyen M, Singhal M (2023) Addisonian Crisis. StatPearls. Treasure Island (FL): StatPearls Publishing.
- S. National Library of Medicine. The treatment of adrenal crisis with inhaled
prednisolone - full text view. ClinicalTrials.gov 2022.
- ARS Pharmaceuticals Inc. Ars Pharmaceuticals announces presentation Of data supporting Neffy (epinephrine nasal spray) and real-world burden of needle injectors. ARS Pharmaceuticals 2023 February 23).
- Glucagon for injection (rDNA origin) [package insert]. Indianapolis, IN: Eli Lilly and Company;
1999.
- Babrowicz J, Child CJ, Raibulet NK, Beusterien K (2019) Perceptions About Glucagon Delivery Devices for Severe Hypoglycemia: Qualitative Research with Patients, Caregivers, and Acquaintances. Clinical therapeutics 41(10): 2073-2089.e6.
Antidepressants. Frontiers in bioengineering and biotechnology 8: 626882.
mechanical loading using hyper gravity. Scientific reports 8(1): 168.
challenges during drug development. Drug Deliv. and Transl 12(4): 735-757.
(2018) Evaluation of intranasal delivery route of drug administration for brain targeting. Brain
Research Bulletin. 143: 155-170.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
-
Kevin Eells* and Inder Sehgal. Intranasal Emergency Drug Delivery: A Review. Endo & Diab Opn Acc J. 1(2): 2024. EDOAJ. MS.ID.000508.
-
Intranasal; Drug delivery; Pharmaceutics; Adrenal insufficiency
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






