 Research Article
Research Article
Melatonin’s Protective Role in Gestational Chronodisruption: Effects on Corticosterone Rhythms in Offspring
Salazar Petres E, Corvalan F, Mendez N, Vergara K, Torres-Farfan C*
Laboratorio de Cronobiología del Desarrollo, Instituto de Anatomía, Histología y Patología, Facultad de Medicina, Universidad Austral de Chile, Valdivia, Chile
Claudia Torres-Farfan, PhD. Casilla (PO box) 567 - Valdivia, Chile. Phone: 56 (63) 2293021. Fax: 56 (63) 2221604.
Received Date:January 10, 2024; Published Date:March 13, 2024
Abstract
Chronodisruption, a common issue in urbanized societies, has been linked to detrimental health effects from gestation to adulthood, including metabolic and cardiovascular dysfunctions. Aligned with the Developmental Origins of Health and Disease (DOHaD) hypothesis, our research focuses on maternal-fetal circadian interactions, with melatonin as a key mediator. This study investigates the therapeutic potential of supplementing maternal melatonin to alleviate the impact of gestational chronodisruption on offspring. corticosterone rhythms. By using Sprague-Dawley rats, pregnant females were divided into three groups: a control group (LD-Control) maintained in a standard photoperiod, a chronodisrupted group (CPS) subjected to chronicphotoperiod shifting, and a CPS melatonin-treated group (CPS+Mel) undergoing photoperiod shifting with melatonin supplementation. Plasma corticosterone levels were measured at 200 days postpartum. The CPS group displayed desynchronized corticosterone rhythms, while the LD-Control group showed normal synchronization. Remarkably, the CPS+Mel group exhibited significantly normalized rhythms, with corticosterone secretion patterns closely resembling the control group. This suggests that prenatal melatonin supplementation can counteract the adverse effects of gestational chronodisruption, potentially restoring circadian homeostasis in offspring. These findings highlight melatonin’s role in fetal programming and suggest its potential in managing environmental disruptions affecting circadian biology, reinforcing the importance of circadian factors in developmental health.
Introduction
In urbanized societies, the disruption of natural light/ dark cycles, known as chrono disruption, significantly impacts human health from gestation to adulthood [1-4]. Studies have stablished a connection between gestational chrono disruption and adverse pregnancy outcomes, as well as altered physiological development in offspring, highlighting its role in metabolic and cardiovascular dysfunctions [4-12]. This phenomenon aligns with the Developmental Origins of Health and Disease (DOHaD) models, emphasizing the importance of maternal-fetal circadian interactions [4,13-16]. Melatonin, a key regulator of circadian rhythms, emerges as a potential mediator in mitigating these effects. Alterations in melatonin are linked to the developmental programming of Non- Communicable Diseases and may play a pivotal role in addressing the consequences of gestational chrono disruption [17-21]. This study investigates the therapeutic role of maternal melatonin supplementation in normalizing offspring corticosterone rhythms disrupted by gestational chrono disruption, proposing it as a critical factor in fetal programming.
Materials and Methods
Animals and experimental procedures: Sprague-Dawley rats were obtained from Charles River (CRL International Inc., Kingston, NY) and were housed in our facility under standard conditions, including a 12:12h light-dark cycle photoperiod with light on at 07:00 AM; approximately ~400 lx at the head level;
controlled temperature (20 ± 2ºC), food and water ad libitum. Then, young females (90-110 days of age) were mated during the night, and the following morning at 09:00 h, vaginal smears were conducted to confirm sperm presence and stablish zero days of gestation or embryonic day (E0). At this stage pregnant females were randomly allocated into three groups and maintained under the following photoperiod protocols, as reported previously [22]. LD-Control: Pregnant rats maintained during gestation under a standard photoperiod 12:12h (light: dark cycle; lights on at 07:00 AM). CPS: Chronic photoperiod shifting, pregnant rats exposed to chronic photoperiod shifting (simulating rotating shift work) until 18 days of gestation. Subsequently, pregnant dams returned to normal LD photoperiod until delivery 8 CPS+Mel: Pregnant rats exposed to chronic photoperiod shifting and treated with melatonin in the drinking water (2μg/ml; about 60 μg total; doses 150-165 μg /kg of weight) during subjective night (19:00 - 07:00h) from day E0 until 18 days of gestation, as reported previously by us [23]. Animal handling and care was performed following the Guide for the Care and Use of Laboratory Animals of the Institute for Laboratory Animal Research of the National Research Council. The protocols were 2 approved by the Bioethics Commission from Universidad Austral de Chile (CBA#352/2019). After delivery mothers and newborns were maintained together until weaning (day 21) and under LD photoperiod cycle. At 200 days old, male offspring were subjected to corticosterone daily rhythms assessment. Daily plasma rhythms of corticosterone measurement: Males from each experimental group (LD, n=9; CPS, n=9; CPS+Mel, n=10) were deeply anaesthetized (isoflurane 2.0-3.0%) for about 10 minutes and blood samples (200 μl) were collected from the tail every 4 h around the clock. The animals returned to their room until the next sample. Blood samples were immediately centrifuged at 13,000xg for 5 minutes and serum was separated and stored at −20°C for subsequent measurements. Corticosterone levels were determined using MILLIPLEX MAP Rat Stress Hormone Millipore (cat. no. RSHMAG-69K) on the Luminex® xMAP® platform (EMD Millipore Corporation, Billerica, MA, USA), following the manufacturer’s instructions. Both intra- and inter-assay variabilities were lower than 10% and 15%, respectively. The results were expressed as ng/ ml.
Statistical analysis: To evaluate 24 h changes on daily corticosterone measurement in adult offspring, mean data for each clock time was fitted to a theoretical cosine function to calculate the hour of acrophases and correlation coefficients for the experimental group. To determine clock time changes within a group we used one-way ANOVA and Tukey test. To compare data between groups we used two-way ANOVA with Bonferroni multiple comparison tests. Analyzes were performed using Graph Pad Prism Software V10.0, USA. Differences with P < 0.05 were considered statistically significant.
Results
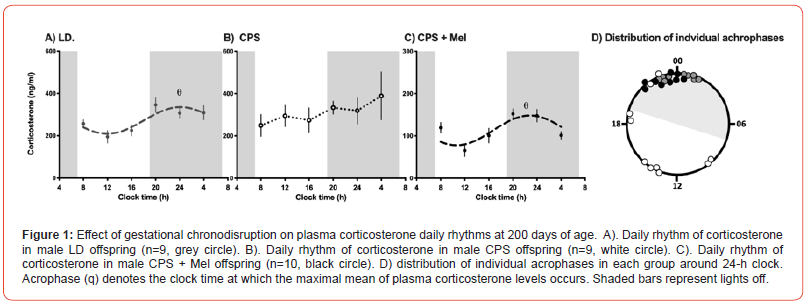
Control (gestated in a normal 12 h:12 h photoperiod) and CPS (gestated under chronodisruption) adult rats displayed significantly different daily rhythms of plasma corticosterone, as has been described in other studies at other postnatal ages. Control rats showed synchronized daily plasma corticosterone rhythms, with individual acrophases between 20.00 h and 01.00 h (Figure. 1A-D), while the daily rhythm of the mean data fitted a synchronized daily rhythm (acrophase q: 00.09h; Figure 1A). In contrast, the individual plasma corticosterone acrophases of CPS offspring were widely distributed around the clock (between02.00 and 23.00 h, Figure. 1D), thus the average data did not fit a synchronized daily rhythm (Figure. 1B).Notably, no differences were detected between these groups regarding the 24-hour mean of corticosterone, suggesting that the misalignment of the circadian rhythm of CPS offspring, at least at 200 days of age did not impact the total production of corticosterone. Conversely, CPS + Mel offspring at 200 days of age exhibited robust daily corticosterone rhythms, with individual acrophases during the first half of the dark phase (Figure 1CD, please note that Figure 1C is in other scales). The mean data reflected a synchronizeddaily rhythm (acrophase q: 22.56 h), indicating that prenatal melatonin treatment restores regulatory mechanisms in the adrenal gland, strongly associated with corticosterone 24-h rhythmicity. In contrast with those observed in LD and CPS offspring, CPS+Mel offspring display significant differences in 24-hour mean of corticosterone, displaying almost a half of corticosterone that the other groups (LD: 223.8 ±54 ng/ml vs CPS: 248.7 ± 31.1 ng/ml vs CPS + Mel 107.2 ± 30.6 ng/ml).
Conclusion
Our study highlights the potential of maternal melatonin supplementation as a therapeutic intervention for mitigating the impacts of gestational chronodisruption on offspring. The normalization of corticosterone rhythms in the CPS+Mel group suggests that melatonin may play a crucial role in maintaining circadian homeostasis, which is disrupted under conditions of altered light/dark cycles during gestation. These findings contribute to our understanding of the DOHaD model, emphasizing the importance of circadian factors in fetal programming and longterm health outcomes. Further research is imperative to explore the underlying mechanisms of melatonin’s protective effects and to evaluate its potential clinical applications in managing the impacts of environmental disruptions on circadian biology.
References
- Erren TC, Reiter RJ (2009) Defining chronodisruption J Pineal Res 46: 245-247.
- Bonmati Carrion M et al. (2014) Protecting the Melatonin Rhythm through Circadian Healthy Light Exposure. Int J Mol Sci 15: 23448-23500.
- Richter HG, Mendez N, Halabi D, Torres-Farfan C, Spichiger C (2022) New integrative approaches to discovery of pathophysiological mechanisms triggered by night shift work. Chronobiol Int 39: 269-284.
- Méndez N, Fernando Corvalan, Diego Halabi, Pamela Ehrenfeld, Rodrigo Maldonado, et al. (2023) From gestational chronodisruption to noncommunicable diseases: Pathophysiological mechanisms of programming of adult diseases, and the potential therapeutic role of melatonin. J Pineal Res 75: e12908.
- Cai C, Ben Vandermeer, Rshmi Khurana, Kara Nerenberg, Robin Featherstone, et al. (2019) The impact of occupational shift work and working hours during pregnancy on health outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol 221: 563-576.
- Abeysena C, Jayawardana P, De A Seneviratne R (2009) Maternal sleep deprivation is a risk factor for small for gestational age: A cohort study. Aust. NZJ Obstet Gynaecol 49: 382-387.
- Ferreira DS, Fernanda G Amaral, Caroline C Mesquita, Ana Paula L Barbosa, et al. (2012) Maternal Melatonin Programs the Daily Pattern of Energy Metabolism in Adult Offspring. PLoS ONE 7: e38795.
- Mendez N, Diego Halabi, Carlos Spichiger, Esteban R Salazar, Karina Vergara, et al. (2016) Gestational chronodisruption impairs circadian physiology in rat male offspring, increasing the risk of chronic disease. Endocrinology 157(12): 4654-4668.
- Salazar ER, H G Richter, C Spichiger, N Mendez, D Halabi, et al. (2018) Gestational chronodisruption leads to persistent changes in the rat fetal and adult adrenal clock and function: Fetal programming of adrenal dysfunction by photoperiod shifting. J Physiol 596(23): 5839-5857.
- Mendez, N, Claudia Torres Farfan, Esteban Salazar, Pía Bascur, et al. (2019) Fetal programming of renal dysfunction and high blood pressure by chronodisruption. Front Endocrinol 10: 362.
- Varcoe TJ, Wight N, Voultsios A, Salkeld MD, Kennaway DJ (2011) Chronic Phase Shifts of the Photoperiod throughout Pregnancy Programs Glucose Intolerance and Insulin Resistance in the Rat. PLoS ONE 6: e18504.
- Halabi D, Hans G Richter, Natalia Mendez, Thilo Kähne, Carlos Spichiger, et al. (2021) Maternal Chronodisruption Throughout Pregnancy Impairs Glucose Homeostasis and Adipose Tissue Physiology in the Male Rat Offspring. Front. Endocrinol 12: 678468.
- Astiz, M, Oster H (2018) Perinatal Programming of Circadian Clock-Stress Crosstalk. Neural Plast 1-12.
- Mark PJ, Crew RC, Wharfe MD, Waddell BJ (2017) Rhythmic Three-Part Harmony: The Complex Interaction of Maternal, Placental and Fetal Circadian Systems. J Biol Rhythms 32: 534-549.
- Suzuki K (2018) The developing world of DOHaD. J Dev Orig Health Dis 9: 266-269.
- Osmond C, Barker DJP (2000) Fetal, Infant, and Childhood Growth Are Predictors of Coronary
- Agil A, et al. (2013) Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J Pineal Res 54: 381-388.
- Cipolla-Neto J, Amaral FG do, (2018) Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr Rev 39: 990-1028.
- Serón-Ferré M, et al. (2002) Perinatal neuroendocrine regulation. Development of the circadian time-
- Torres-Farfan C, et al. (2011) A Circadian Clock Entrained by Melatonin Is Ticking in the Rat Fetal Adrenal. Endocrinology 152: 1891-1900.
- Mendez N, et al. (2016) Gestational Chronodisruption Impairs Circadian Physiology in Rat Male Offspring, Increasing the Risk of Chronic Disease. Endocrinology 157: 4654-4668.
- Vilches N et al. (2014) Gestational Chronodisruption Impairs Hippocampal Expression of NMDA
Heart Disease, Diabetes, and Hypertension in Adult Men and Women. Environ. Health Perspect 108: 9.
keeping system. Mol Cell Endocrinol 186: 169-173.
Receptor Subunits Grin1b/Grin3a and Spatial Memory in the Adult Offspring. PLoS ONE 9: e91313.
-
Salazar Petres E, Corvalan F*, Mendez N, Vergara K, Torres-Farfan C*. Melatonin’s Protective Role in Gestational Chronodisruption: Effects on Corticosterone Rhythms in Offspring. Endo & Diab Opn Acc J. 1(2): 2024. EDOAJ.MS.ID.000510.
-
Chronodisruption; Cardiovascular Dysfunctions; Corticosterone
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






