 Research Article
Research Article
Excessive Weigth in Survivors of Acute Lymphoblastic Leukemia: What’s Going on with Children and Adolescent in Northeast Brazil?
Caroline Kupsch Medrado*, Lucas Rodrigo Gomes de Carvalho and Ana Marice Teixeira Ladeia
Bahian Medical and Public Health School and Post-Graduate of Bahian Medical and Public Health School, Science Development Foundation of Bahia, Brazil
Caroline Kupsch Medrado, Bahian Medical and Public Health School and Post-Graduate of Bahian Medical and Public Health School, Science Development Foundation of Bahia, Brazil
Received Date:Febuary 24, 2024; Published Date:March 13, 2024
Abstract
Background: Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy. Therapeutic advances increased survival rates to
more than 90% at 5 years. However, this treatment may have unfavorable consequences. Obesity is a well-recognized late effect in these survivors,
which increases cardiovascular morbidity and mortality. This research aims to describe the prevalence of excessive weight in ALL survivors, to test
the association between clinical, laboratory and treatment characteristics and excessive weight.
Method: Cross-sectional study including ALL survivors from a tertiary pediatric oncology hospital in Bahia. Results were described with
measures of central tendency and dispersion for numeric variables and absolute and relative frequencies for categorical variables. To test the
association between ALL and excessive weight, Fisher’s exact test or chi-square test were performed, when appropriate. P values < 0.05 were
considered significant.
Results: We evaluated 128 patients with a mean age of 12.3 3.4 years, most of them male. Of the examined sample, 45 patients (35%) had
excessive weight, of which 26 (20.3%) overweight, 15 (11.7%) obesity and 4 (3.1%) severe obesity. There was an association between family history
of obesity and excessive weight (56.3% vs 14.1%; p <0.001), and between family history of obesity and increased waist circumference (24% vs 15%;
p = 0,002) with no association with gender, type of ALL, treatment protocol used and time after the end of treatment.
Conclusion: In this sample, there was a high prevalence of excessive weight in children and adolescents after ALL treatment and an association
of excessive weight and increased waist circumference with a family history of obesity.
Keywords:Acute Lymphoblastic Leukemia, Obesity, Childhood Cancer Survivors, excessive weigth.
Abbreviations:ALL: Acute Lymphoblastic Leukemia; GBTLI: Brazilian Group of Childhood Leukemia Treatment; BFM: Berlin-Frankfurt Münster backbone.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy, accounting for nearly a quarter of all childhood cancers [1]. While advances in treatment strategies have led to fiveyear survival rates approaching 90% [1], curative therapy for pediatric ALL is associated with an increased risk for numerous chronic health conditions [2]. The St. Jude Lifetime Cohort Study report states that at 45 years of age, 95.5% of childhood cancer survivors have at least one chronic disease, including obesity [2,3]. As obesity is known to contribute to an increased risk of hypertension, type 2 diabetes,cardiovascular disease,cancer,and premature death, it is imperative to develop and apply interventions in this at-risk population. However, effective intervention strategies for ALL survivors requires a clear understanding of those who are at greatest risk of becoming obese and the mechanisms of obesity in survivors [4]. Some etiologic mechanisms have been proposed, including patient characteristics and treatment received [5,6]. Nevertheless, the factors related to excess weight in patients treated even with the most modern protocols have not yet been fully elucidated6. Even so there are few studies reporting this risk in developing countries especially in Brazil in which the problem of dietary deficiency was rapidly shifting to one of dietary excess in last decades. The aim of this study was (1) to assess the frequency of excessive weight in children and adolescents’ survivors of ALL, (2) to identify the clinical and laboratory findings of these individuals and (3) to test their association with excess weight gain in an important public hospital that is reference in childhood cancer in Bahia-Brazil. We intend to contribute for evidence about the characteristics of ALL survivors in Brazilian children.
Material and Methods
This is a cross-sectional study based on a convenience sample of 128 patients with a previous diagnosis of ALL, in regular follow- up with pediatric endocrinology at Martagão Gesteira Hospital, in Salvador, Bahia, from October 2016 to December 2021. In this Hospital, all ALL cases are referred and regularly followed with endocrinological evaluation, regardless of nutritional status. The primary objective of a descriptive study was followed. Considering an estimated prevalence of 30% of excessive weight after ALL treatment, 127 patients were needed to estimate the prevalence of excessive weight in this sample, with 8% precision and an alpha of 5%. The sample calculation was performed using a specific calculator (WinPepi Version 11.65 of 2016). Patients, of both genders, were included if, at first endocrinological evaluation, aged between 2 and 18 years old, and had completed curative treatment for ALL at least 12 months before. Patients excluded from the study were those who undergone bone marrow transplantation, who had secondary obesity or any concomitant diseases that could serve as confounding factors, such as Cushing’s syndrome and Prader Willi syndrome. This study was approved by a local Research Ethics Committee under protocol 48166121.5.0000.5544, available at the “Plataforma Brasil”. Informed consent was obtained from all subjects and/or their families before enrollment in the study, after explaining the purpose of the study and the procedure.
Three treatment protocols for the underlying disease were identified using a medical record review: Brazilian Group of Childhood Leukemia Treatment-1999 (GBTLI 99) [7], Berlin-Frankfurt- Münster-backbone (BFM 2002 [8] and BFM 2009 [9] protocols). In these treatment protocols, prednisone and dexamethasone were equivalent in terms of glucocorticoid effect. It was not possible to access the complete anthropometric measurements of these patients before the ALL diagnosis or upon admission. Anthropometric and pubertal development data were obtained during the appointment with a pediatric endocrinologist. Anthropometric measurements were made with the children wearing light clothing and without shoes. Measurements of height were obtained in duplicate with an accuracy of 0.1 cm. Weight was measured using a digital scale with a capacity of 2 to 150 kg and an accuracy of 0.1 kg. The average of the two height measurements was used to calculate BMI [BMI = weight (kg)/height2 (cm)]. The boys and girls were classified according to BMI Z-score ranges (BMI curves established for each gender and age), using the parameters of the population curves defined by the World Health Organization (WHO) in 2007 [10]. The children’s nutritional status was categorized according to BMI z-score. A second categorization was performed: all overweight, obese, or severely obese individuals were considered as excessive weight. The waist circumference was measured using an inelastic tape measure graduated in centimeters. The reference point was the midpoint between the lower costal margin and the anterior superior iliac crest. Furthermore, the patient was upright, presenting a relaxed abdomen after a gentle exhalation. The reference value used was the North American standard described by Fernandez in 2004 [11]. Values over the 90 percentile were considered increased for both gender. Blood pressure was measured in the right arm using an aneroid sphygmomanometer, with appropriate cuffs for the patient’s size. As a reference, we used the values recommended by the Department of Nephrology of the Brazilian Society of Pediatrics [12]. The pubertal staging was also assessed using the Marshall and Tanner criteria to dichotomize into prepubertal (stage 1) and pubertal (from stage 2 on). The family history of obesity variable was defined if the father, mother and/or grandparents were obese by measuring their weight and height (BMI).
Blood samples were collected after overnight 12-hour fasting to measure total cholesterol, Low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG) and fasting glucose (mg/dL); glycated hemoglobin (A1c %) by the HPLC method (high performance liquid chromatography); insulin (μUI /mL), TSH (mUI/mL) and free tiroxin T4L (ng/dL) by electrochemiluminescence. The reference values from the Brazilian Guidelines on Dyslipidemia and Prevention of Atherosclerosis [13] were adopted for the lipid profile. The HOMA-IR (homeostatic model assessment) calculation was based on fasting insulin and glucose levels to assess insulin resistance; the HOMA-IR cut-off point was > 4.07 for pubertal adolescents and > 2.91 for non-pubertal adolescents [14]. Hand and wrist radiography to evaluate bone age was determined by the Greulich-Pyle method [15]. Statistical analysis: All statistical analyses were carried out using the Statistical Packages for Social Sciences (SPSS) software version 26.6 [16]. The results were described with measures of central tendency and dispersion for numeric variables and absolute and relative frequencies for categorical variables. Frequencies were compared using the Chisquare test or Fisher’s exact test, when appropriate. Statistical tests with a p-value < 0.05 were considered statistically significant.
Results
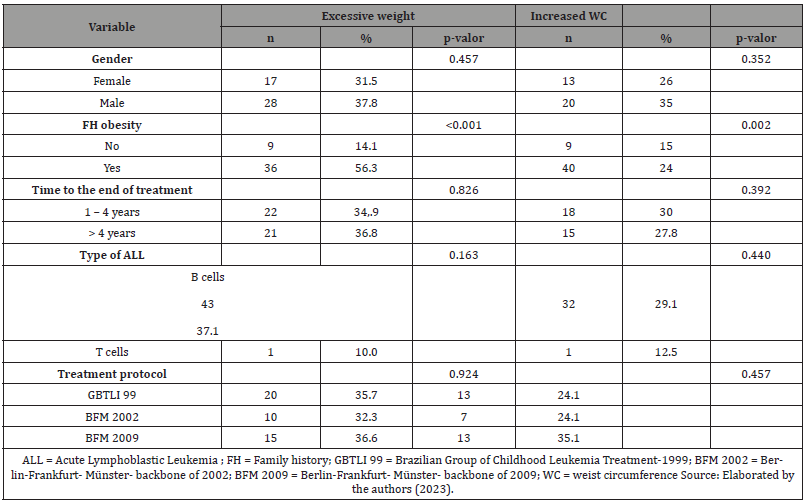
Among 128 ALL survivors patients, 74 (57.8%) were male, 43.8% were treated by GBTLI 99 protocol, 24.2% by BFM 2002, and 32% by BFM 2009. At diagnosis, the mean age was 5.2 ± 3.6 years, and 116 individuals (92.1%) presented an immunophenotypic classification of B-precursor ALL. In the sample, eight patients (6.3%) underwent cranial radiotherapy (Table 1). The duration of treatment ranged from 2.0 to 2.6 years, with a median of 2.4 years; the time between concluding this treatment and the first endocrinological evaluation had a median of 4 years, with 52.5% of patients between 1 and 4 years out of therapy and 47.5% over 4 years. At the endocrinological appointment, the mean age was 12.3 ± 3.4 years, and most patients were already in puberty (68.5%). Fifty percent had mother and/or father and/or grandparents with history of obesity. Forty-five (35.2%) patients presented with excessive weight. Of these, 26 (20.3%) were overweight, 15 (11.7%) obese, and 4 (3.1%) severely obese. Thirty-three (27.5%) patients had altered waist circumference (>P90) (Table 2). The mean systolic blood pressure (BP) was 106,3 ± 14,8 mmHg, and the mean diastolic BP was 68,6 ± 11,6 mmHg. When BP was analyzed according to gender, age, and height, no blood pressure abnormalities were found. Increased HOMA-IR was identified in 31.4% of the individuals. The lipid and glycemic profiles and thyroid function were not significantly altered. Furthermore, no significant difference was found between bone ages and chronological ages. Table 3 shows the laboratory characteristics of the sample studied. Individuals with a family history of obesity were observed to have a higher frequency of excessive weight than those without a family history (56.3% vs. 14.1%; p <0.001). Besides, no differences were observed between the presence of excessive weight and gender, type of ALL, treatment protocol used, and time after the end of treatment (Table 4) Furthermore, it was observed that individuals with a family history of obesity had a higher frequency of increased waist circumference than those without this family history (24% vs. 15%; p = 0.002). No differences were observed between the presence of increased waist circumference and gender, type of ALL, treatment protocol used, and time after the end of treatment (Table 4).
Table: 1Demographic characteristics of the study participants.
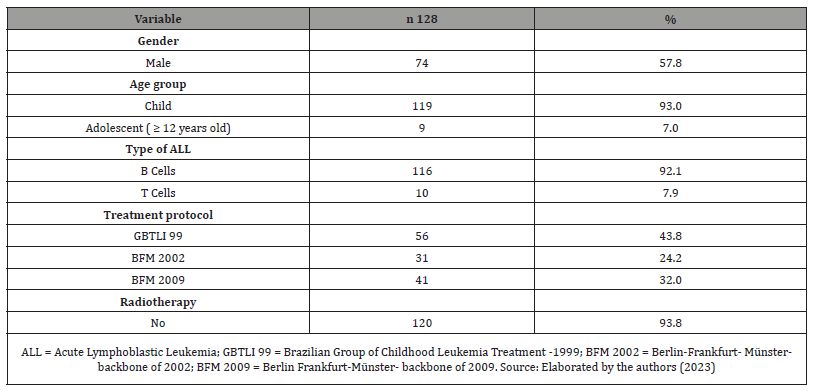
Table: 2Characteristics of the survivors at the endocrinology appointment.
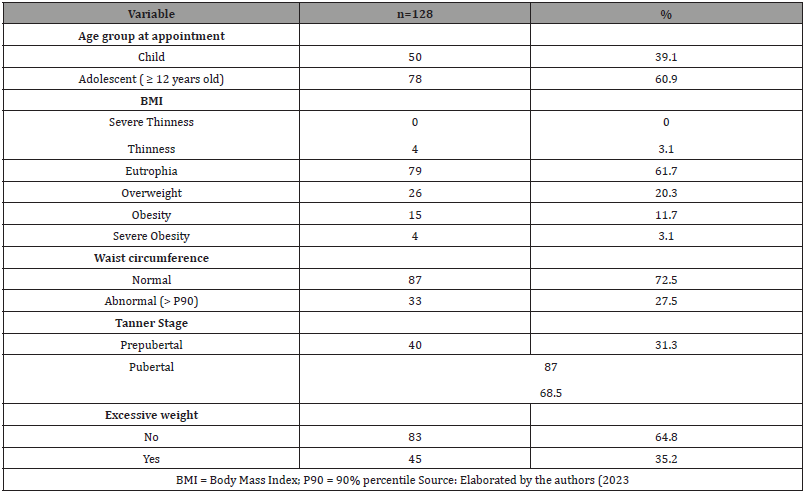
Table: 3Laboratory characteristics of ALL survivors.
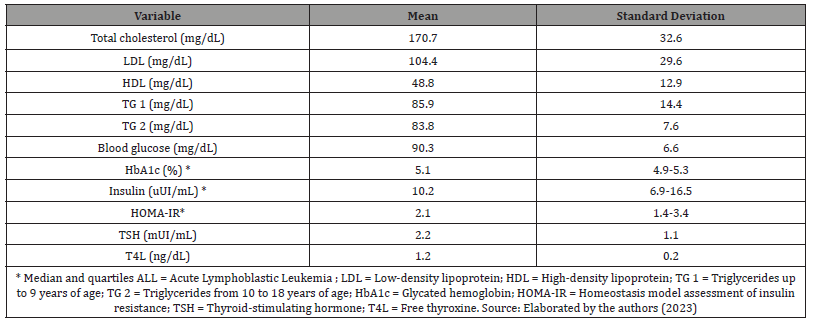
Table: 4Association between clinical variables with excessive weight and increased waist circumference in ALL survivors.
Discussion
The findings of this study indicated a high prevalence of excessive weight (35.2%) in children and adolescents who survived ALL. These findings are according with other Brazilian studies, as well as from other countries. Moreover, the numbers reflect the magnitude of the problem since excessive weight at the end of treatment was higher than in the Brazilian population aged 5-9 years (29.3%) [17] and 12-17 years (25.5%) [18] in general. At the endocrinological appointment, excessive weight had a frequency like that reported by Alves et al19 (38.3%) in a study with Brazilian children. In international literature, there are even higher prevalence, such as those reported by Asner et al. (48%) and Breene et al. (47.2%) [20,21]. However, it is relevant to highlight that differences can be attributed to the diversity of protocols, excess weight definitions, and small sample size in these studies [6,22]. There is a strong relation between the quantity of adipose tissue and their disfunction. Adipose tissue quantity, as measured with either BMI or waist circumference (WC) is related to plasma concentrations of adipokines, to morphologic characteristics of adipose tissue, and to the development of the metabolic syndrome23. Given the importance of insulin resistance in the pathophysiology of metabolic syndrome, it is relevant to emphasize that we found 27.5% of patients with increased abdominal circumference (>P90) – all of whom were part of the excessive weight group. The present study also observed a 31.4% frequency of HOMA-IR elevation, a method to assess insulin resistance that is closely related to childhood obesity. This percentage is like that found in other studies with ALL survivors [24,25]. There is well-documented evidence linking the risk of obesity after ALL treatment with older protocols. Former late effects studies in ALL survivors demonstrated that CRT was a major risk factor for overweight/obesity in the long-term. Discussed hypotheses and mechanisms suggested that radiation damage to hypothalamic neurons controlling eating behaviors and leptin resistance were mainly involved in the development of late excessive weight gain in this population20. In this sample, only eight patients underwent cranial radiotherapy, all children with B-cell ALL.
However, after the 1990s, there was progress in therapies, with the development of protocols that include replacing cranial radiotherapy by intrathecal chemotherapy, more intense systemic chemotherapy, and the use of high-dose corticoids. Despite this changes and the use of the most modern protocols, some studies still mention the risk of obesity [20,26]. The analysis of associated factors in the present sample does not show that excessive weight is associated with the type of leukemia or with the treatment received. However, a statistically significant association was observed between excessive weight and family history of obesity. Family history is important in determining obesity and the result of the present study is consistent with those in the literature. In a study with a sample of 699 children in the second largest city in Bahia, Oliveira AL, et al [27], identified parental obesity as a significant risk factor in the development of childhood overweight/obesity, especially when both parents had this condition. - Children of obese parents were 3.5 times more likely to have excessive weight. The genetic factor is sufficient cause to determine obesity, but it is not always necessary. This factor is strongly influenced by the environment in which children live, and it is known that the lifestyle adopted by parents is generally transferred to their children, which perpetuates the overweight phenotype27. It is important to notice that the association between a family history of obesity was also demonstrated with an increase in waist circumference, which reinforces the role of family history in the genesis of obesity, whether global or central, thus suggesting the etiopathogenesis of obesity after treatment for ALL is not different from that of children in the general population.
Research in healthy children shows that the parents’ eating behavior (i.e., the way and frequency they feed their children) is closely related to caloric intake and children’s BMI [28]. Thus, it is relevant to note that, to minimize weight loss during the disease, there is a tendency to provide more calories to children and adolescents affected by ALL, which often lasts for years after the end of treatment [29]. Other possible contributing mechanisms to the development of excessive weigth after ALL therapy are increased sedentary behavior and reduced physical activity [3]. The strength of this study includes that children and adolescents survivors of ALL of the main pediatric oncology center in Bahia were studied for the first time. Furthermore, the importance of this research is also the demonstration of the high frequency of excessive weight and increased abdominal circumference in this group. With this knowledge, plans and guidelines can be drawn up for an adequate approach to obesity, already at that time, to avoid future complications. Joint monitoring of all ALL survivors with an endocrinologist, nutritionist and psychologist is planned to be implemented, with periodic reassessments of excessive weight cases. We know that obesity is a chronic and multifactorial disease that causes damage to individual health and increases the costs of the public health system. In the case of childhood patients, guidance from caregivers regarding eating habits, physical activity and mental health care is essential. This investigation presented some limitations: there was no systematic record of the nutritional status of patients before and during treatment for ALL. When analyzing factors associated with obesity, the literature suggests that excess baseline weight could be one of the factors associated with excess weight after treatment [30]. Therefore, the treatment period would be critical for nutri tional intervention and preventing future complications. Although a control group with minimal pairing by sex and age group was desirable, this was not possible since the Pediatric Oncology at Martagão Gesteira Hospital is a tertiary service, however this limitation does not invalidate the results presented, considering that they were compared with those of the general population with the same sociodemographic profile and ALL survivors from other centers.
Conclusion
In the present study, a high prevalence of excessive weight was found in a sample of children and adolescents post-leukemia treatment (from a specialized service in oncologic treatment in a public hospital of Salvador - BA), as well as an association of excessive weight with a family history of obesity and increased waist circumference. However, no association was found between excessive weight and some characteristics of the patient (such as gender and age), of the disease (cell type of the ALL), or of the treatment received (protocol; cranial radiotherapy; corticosteroid therapy), suggesting that the development of excess weight in this population presents association with genetic and/or epigenetic characteristics, but not to factors directly related to ALL. Thus, a careful look at childhood obesity (which is known to increase the risk of cancer and numerous associated diseases), especially in a specific pediatric population such as leukemia survivors, has become a necessity and a significant challenge today.
Declaration of interest
All authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the local ethical research committee.
Informed consent
For this type of study, formal consent was required through the Informed Consent Form.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Hunger SP, Mullighan CG (2015) Acute lymphoblastic leukemia in children. N Engl J Med 373(16): 1541-1552.
- Brown AL, Lupo PJ, Danysh HE, Okcu MF, Scheurer ME, et al. (2016) Prevalence and predictors of overweight and obesity among a multiethnic population of pediatric acute lymphoblastic leukemia survivors: a cross-sectional assessment. J Pediatr Hematol Oncol 38(6): 429-436.
- Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, et al. (2013) Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 309(22): 2371-2381.
- Lupo PJ, Brown AL, Arroyo VM, Kamdar KY, Belmont JW, et al. (2019) DNA methylation and obesity in survivors of pediatric acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Genes Chromosomes Cancer 58(1): 52-59.
- Landier W, Skinner R, Wallace WH, Hjorth L, Mulder RL, Wong FL, et al. (2018) Surveillance for Late Effects in Childhood Cancer Survivors. J Clin Oncol 36: 2216-2222.
- Zhang FF, Kelly MJ, Saltzman E, Must A, Roberts SB, Parsons SK, et al. (2014] Obesity in pediatric ALL survivors: a meta-analysis. Pediatrics 133(3): e704-e715.
- Caze MO, Bueno D, Santos MEF (2010) Estudo referencial de um protocolo quimioterápico para leucemia linfocítica aguda infantile. Rev HCPA 30(1): 5-12.
- J Stary, Zimmermann M, Campbell M, Castillo L, Dibar E, Donska S, et al. (2014) Intensive Chemotherapy for Childhood Acute Lymphoblastic Leukemia: Results of the Randomized Intercontinental Trial ALL IC-BFM 2002. J Clin Oncol 32: 174-184.
- ALL IC-BFM 2009 A Randomized Trial of the I-BFM-SG for the Management of Childhood non-B Acute Lymphoblastic Leukemia. 2009 ago.
- Child growth standards.
- Fernández JR, Redden DT, Pietrobelli A, Alissson DB, et al. (2004) Waist circumference percentiles in nationally representative samples of African American, European-American, and Mexican American children and adolescents. J Pediatr 145(4): 439-444.
- Sociedade Brasileira de Nefrologia. Departamento Científico de Nefrologia. Manual de Orientação. Hipertensão arterial na infância e adolescência 2019.
- Diretriz Brasileira de Dislipidemia e Prevenção da Ateroesclerose. Arq Bras Cardiol 109(1): 76.
- Rocco ER, Mory DB, Bergamin CS, Valente F, Miranda VL, Calegare BFA, et al. (2011) Optimal cutoff points for body mass index, waist circumference and HOMA-IR to identify a cluster of cardiometabolic abnormalities in normal glucose-tolerant Brazilian children and adolescents 55(8): 638-645.
- Greulich WW, Pyle SI (1959) Radiographic atlas of skeletal development of the hand and wrist. 2. ed. Stanford: Stanford University Press 256 p.
- IBM SPSS Statistics.
- Atlas de obesidade infantil. Brasília: Secretaria de Atenção Primária à Saúde, do Ministério da Saúde; 2019.
- Bloch KV, Klein CH, Szklo M, Kuschnir MCC, Abreu GA, Barufaldi LA, et al. (2016) ERICA: Prevalences of hypertension and obesity in Brazilian adolescents. Rev Saúde Pública 50(1): 9s.
- Alves JGB, Pontes CMA, Lins MM (2009) Excesso de peso em crianças e adolescentes sobreviventes de leucemia linfoide aguda-estudo de coorte. Bras. Hematol. Hemoter 31(6): 427-431.
- Asner S, Ammann RA , Ozsahin H, Beck-Popovic M , N X von der Weid (2008) Obesity in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Pediatr blood cancer 51(1):118-122.
- Breene RAL, Williams RM, Hartle J, Gattens M, Acerini CL, et al. (2011) Auxological changes in UK survivors of childhood acute lymphoblastic leukaemia treated without cranial irradiation. Br J Cancer. 104(5): 746-749.
- Lughetti L, Bruzzi P, Predieri B, Paoluccci P (2012) Obesity in patients with acute lymphoblastic leukemia in childhood. Italian Journal of Pediatrics 38: 4.
- Schrover IM, Spiering W, Leiner T, Visseren FLJ (2016) Adipose Tissue Dysfunction: Clinical Relevance and Diagnostic Possibilities. Horm Metab Res 48(4): 213-225.
- Karakurt H, Sarper N, Kiliç SC, Gelen SA, Zengir E (2012) Screening Survivors of Childhood Acute Lymphoblastic Leukemia for Obesity, Metabolic Syndrome, and Insulin Resistance. Pediatr Hematol and Oncol 29: 551-561.
- Tonorezos ES, Vega GL, Sklar CA, Chou JF, Moskowitz CS, et al. (2012) Adipokines, Body Fatness, and Insulin Resistance Among Survivors of Childhood Leukemia. Pediatr Blood Cancer 58(1): 31-36.
- Touyz L, Cohen J, Neville KA, Wakefield CE, Garnett SP, et al. (2017) Changes in body mass index in long-term survivors of childhood acute lymphoblastic leukemia treated without cranial radiation and with reduced glucocorticoid therapy. Pediatr Blood Cancer 64: e26344.
- Oliveira AM, Oliveira AC, Almeida MS, Oliveira N, Adan L (2007) Influence of the family nucleus on obesity in children from northeastern Brazil: a cross-sectional study. BMC Public Health 7: 235.
- Anzman SL, Birch LL (2009) Low inhibitory control and restrictive feeding practices predict weight outcomes. J Pediatr 155: 651-656.
- Cohen J, Wakefield CE, Fleming CAK, Gawthorne R, Tapsell LC, et al. (2012) Dietary intake after treatment in child cancer survivors. Pediatr Blood Cancer 58: 752-757.
- Razzouk BI, Rose SR, Hongeng S, Wallace D, Smeltzer MP, Zacher M, et al. (2007) Obesity in Survivors of Childhood Acute Lymphoblastic Leukemia and Lymphoma. J Clin Oncol 25: 1183-1189.
-
Caroline Kupsch Medrado*, Lucas Rodrigo Gomes de Carvalho and Ana Marice Teixeira Ladeia. Excessive Weigth in Survivors of Acute Lymphoblastic Leukemia: What’s Going on with Children and Adolescent in Northeast Brazil?. Endo & Diab Opn Acc J. 1(3): 2024. EDOAJ.MS.ID.000511.
-
Acute Lymphoblastic Leukemia, Obesity, Childhood Cancer Survivors, excessive weigth. Abbreviations: ALL = Acute Lymphoblastic Leukemia; GBTLI = Brazilian Group of Childhood Leukemia Treatment; BFM = Berlin-Frankfurt-Münster-backbone
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






