 Research Article
Research Article
Outcome of Surveillance Stool Culture Guided Selection of Antibiotics during Febrile Neutropenia in Patient with Acute Leukaemia?
Aklima Khanam1, Shafiqul Islam2, Mohammmad Nazmul Islam3, Fahmida Ahmed4, Fatema Ahmed5, Md Shafiur Rahman6, Mesbah Uddin Ahmed7* and Farzana Rahman8
1Department of Haematology, Dhaka Medical College Hospital, Bangladesh
2Department of Haematology, Bangabandhu Sheikh Mujib Medical University, Bangladesh
3Department of Medicine, Sylhet MAG Osmani Medical College Hospital, Bangladesh
4Department of Haematology, Imperial Hospital Limited, Bangladesh
5Department of Haematology, Bangladesh
6Assistant Director, Hospital and Clinic, DGHS, Bangladesh
7Department of Microbiology, Bangladesh University of Health Sciences, Bangladesh
8Department of Haematology, Bangabandhu Sheikh Mujib Medical University, Bangladesh
Mesbah Uddin Ahmed, Department of Microbiology, Bangladesh University of Health Sciences, Bangladesh.
Received Date: December 19, 2019; Published Date: January 10, 2020
Abstract
Background:Most patients with acute leukaemia become repeatedly neutropenic for prolonged periods and therefore are at risk of becoming febrile at some point.
Aim: This study aimed at observing the outcome of surveillance stool culture guided selection of antibiotic during febrile neutropenia in acute leukaemia patient.
Methodology: This was a case control study conducted in the department of Haematology from March 2017 to February 2018. 30 cases and 30 controls diagnosed case of acute leukaemia patient admitted into Haematology department were enrolled in the study. Surveillance stool culture guided antibiotics was used in cases and empirical antibiotics was given in control during febrile neutropenia. The comparison between two groups was done regarding outcome of fever. Categorical comparisons were performed using chi-square test. Continuous variables were expressed as mean values ± standard deviation and compared using Student’s t-test/unpaired t-test. For all statistical tests, P-value less than 0.05 was considered as statistically significant.
Results: The mean age was found 33.4±16.9 years in cases and 29.8±14.1 years in control. Males were found 17(56.7%) in cases and 15(50.0%) in controls. Females were 13(43.3%) in cases and 15(30.0%) in controls. 25(83.3%) patients had E. coli and 5(16.7%) had Klebsiella in their surveillance stool culture in case group. Colistin was more sensitive 29(96.7%) followed by meropenem 23(76.7%), amikacin 23(76.7%), pipercillintazobactum 22(73.3%). 25(83.3%) patients were MDRO colonizer and 5(16.7%) were not MDRO colonizer., 60.0% of MDRO colonizer patients had no history of prophylactic antibiotic and 40.0% of MDRO colonizer has history of using prophylactic antibiotic. In case group majority 19(63.3%) patients had defervescence of fever ≤1 day and in control group 21(70.0%) had defervescence of fever >1 day. The mean defervescence of fever was found 2.0±1.9 days in cases and 3.3±2.1 days in controls. Early defervescence of fever (≤1 day) was significantly (p<0.05) higher in case group than control group. The mean duration of antibiotic used was found 5.8±2.0 days in cases and 7.7±3.4 days in controls. Duration of antibiotic used was significantly (p<0.05) longer in control group than case group. 8(26.7%) patients in cases and 16(53.3%) in controls needed for second line antibiotics. Conclusion: Prevalence of MDRO colonization in gut in patient with acute leukaemia is alarmingly high. Selection of antibiotic from individual stool antibiogram leads to earlier defervescence of fever, reducing the need for second line antibiotic in patient acute leukaemia.
Keywords: MDRO colonization; Febrile Neutropenia; Acute leukaemia
Introduction
Stool surveillance guided antibiotic usage leads to earlier fall of fever in higher number patients, also reduced the need for second line antibiotics in patients undergoing all SCT [1]. Antibiotic resistance is no longer restricted to hospital is prevalent in the community as well. The carbepenem resistant encerobacbericae carriage rate is higher India. Patient with haematological malignancy are immunocompromised and frequently developed profound neutropenia due to disease condition and or chemotherapeutics and they routinely got prophylactic antibiotic hence high risk for carrying multidrug resistance microorganism. Selection of antibiotics for individual depends on routine culture and sensitivity, surveillance culture and sensitivity, prior use of antibiotics, risk factor for developing infection related complication. This study observed the outcome of surveillance stool culturebased selection of antibiotics during febrile neutropenia as well as detected the prevalence of MDRO colonizer in stool in patient with acute leukaemia were admitted in department of Haematology of BSMMU which is a referral center. Surveillance stool culture guided selection of antibiotic during neutropenic fever which lead to early fall of fever, reduced the duration of antibiotic use, reduced the need for second line antibiotic thus help in early clinical recovery of patient and reduced the cost of treatment. The study might also help to develop empirical antibiotic policy for febrile neutropenia patient in Haematology department.
Methodology
• Study design: This was a experimental case control study from April 2017 to March 2018.
• Place of study: The study was done in the Department of Haematology of Bangabandhu Sheikh Mujib Medical University (BSMMU), Shahabag, Dhaka, Bangladesh.
• Study period: From April 2017 to March 2018.
• Study population: Acute leukaemia patients admitted in department of Haematology, BSMMU, Dhaka.
• Sampling method: Random sampling method was used. Every patient was given a number. Every odd patient was selected as case and even patient was selected as control. Due to time limitation 30 cases and 30 controls were taken in this study.
Inclusion criteria
Acute leukaemia patient with febrile neutropenia Patient able to give informed written consent.
Exclusion criteria
Patient unable to give informed written consent. Acute leukaemia patient without febrile neutropenia.
Research equipments
• Preformed structured data collection sheet
• Demography of the patient.
• Laboratory investigations.
• Surveillance stool culture and sensitivity report.
Study Procedure
This study conducted in department of Haematology, BSMMU from April 2017 to March 2018. Every patient was given an ID No. Every odd patient was selected as case and every even patient was selected as control. 30 cases and 30 controls diagnosed case of acute leukaemia patient with febrile neutropenia was admitted in department of Haematology were enrolled in study following inclusion and exclusion criteria. The patient was categorized in two groups. Group-1 (no stool surveillance culture was done), group-2 (surveillance stool culture was done). In group 2 patient morning stool sample were collected after admission in department of haematology with a clean plastic container having spoon and sent to the department of microbiology within the least possible time. No transport media was used. Stool was then inoculate in routine aerobic culture media. Organism was identified after gram stain and biochemical test for specific organism. Sensitivity was tested in muller hilton media. Different strains of organism were not identified. Normal flora and pathogenic flora were not categorized. Group 1 patient received empirical antibiotic when they developed neutropenic fever and Group 2 patient received antibiotic according to their stool antibiogram. In both group when the patient were not responding to initial antibiotic therapy (within 72 hours) either the antibiotic was changed, or another antibiotic was added. The primary end point of this study was defervescence of fever after initial antibiotic therapy. Secondary end points were need for second line antibiotic, duration of antibiotic use, prevalence MDRO colonization in surveillance stool culture. Informed written consent was taken from the subjects. Participants had every right to discontinue themselves from the study at any point of time. Ethical issues were addressed to every patient. Special precaution was taken to personal history. Ethical clearance was obtained from institutional review board (IRB) OF BSMMU. Quality assurance measurements was recorded with a semi structured questionnaire.
Data collection procedure
This study was a hospital-based, case control study which was conducted in the Departments of Haematology, Bangabandhu sheikh mujib medical university, Dhaka. The study was approved by the Institutional review board and all patients gave their informed consent to take part in this investigation. The duration of the study was 12 months and included 30 cases & 30 controls of acute leukaemia patients admitted in Haematology department meeting the inclusion & exclusion criteria. Complete history and physical examination of all patients were undertaken and the entire document necessary to confirm the diagnosis was collected.
Data analysis
The collected data were edited by the principle investigator on the same day of collection. Data obtained was recorded then entered into the computer using microsoft excel and analyzed with SPSS version 23.0 software package with help of a statistician. Qualitative variables (sex) of these studies were expressed as percentage. Quantitative variable (age) was expressed as mean ± standard deviation. Categorical comparisons were performed using chi-square test. Continuous variables were expressed as mean values ± standard deviation and compared using Student’s t-test/ unpaired t-test. For all statistical tests, P value less than 0.05 was considered as statistically significant.
Procedure of maintaining confidentiality
For safeguarding confidentiality and protecting anonymity each of the patients was given a special ID no which was followed during examination and each and every step of the procedure. A signed written informed consent was taken from the patient convincing that privacy of the patient was maintained.
Ethical consideration
Before starting this study, the research protocol was submitted and approved by the Institutional review board of BSMMU, Dhaka. All participants were informed about the objectives, methodology and purpose of the study in an easy understandable way. All information regarding benefits and hazards was delivered to the all participants & those who agree to participate were included in the study. Verbal and written consents were taken from all participants without any influences prior to sample collection. If the participants have any question or any other problems, they were requested to contact. Data obtained from the study was used only for the research purpose and the confidentiality of all study information was maintained strictly. Participants can discontinue themselves from the study any time even after giving consent.
Quality assurance
The study was done in the Department of Haematology under the guidance of specialist haematologists and patients stool sample were sent to microbiology department of BSMMU for surveillance culture and sensitivity in BSMMU.
Results
Table 1 shows majority patients belonged to age ≤40 years in both groups, which was 23(76.7%) in cases and 22(73.3%) in controls. The mean rank of age was found 32.02 years in cases and 28.98 in controls. The difference was not statistically significant (p>0.05) between two groups Figure 1.
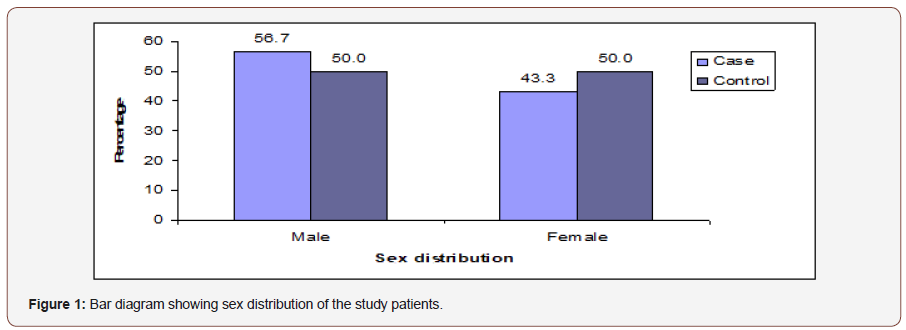
Table 1: Distribution of the study patients by age (n=60).
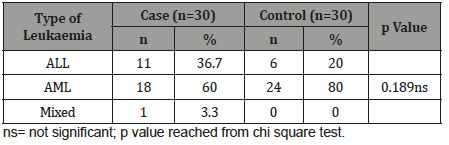
Table 2: Distribution of the study patients according to type of leukaemia (n=60).

Table 2 shows majority patients had AML in both groups, which was 18(60.0%) in cases and 24(80.0%) in controls. Eleven (36.7%) patients had ALL in cases and 6(20.0%) in controls. Only 1(3.3%) patient had mixed leukaemia in case group but not found in control group. The difference was not statistically significant (p>0.05) between two groups.
Table 3:Distribution of the study patients by age (n=60).
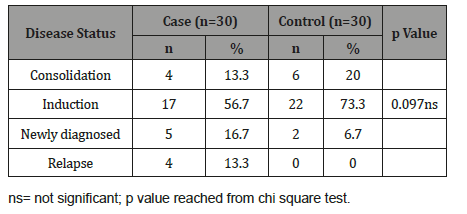
Table 3 shows majority patients were found induction phase in both groups, which was 17(56.7%) in cases and 22(73.3%) in controls. Consolidation was 4(13.3%) in cases and 6(20.0%) in controls. Newly diagnosed was 5(16.7%) and 2(6.7%) in cases and controls respectively. Relapse was 4(13.3%) in cases but not observed in controls group. The difference was not statistically significant (p>0.05) between two groups (Figure 2 & 3).

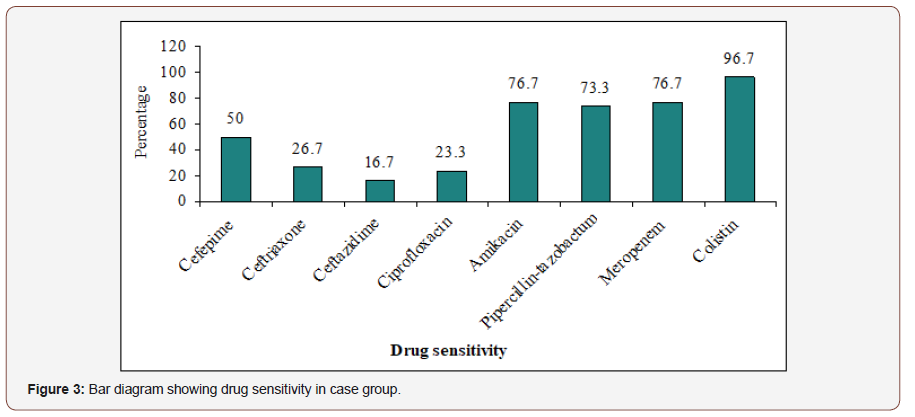
Colistin was the most sensitive 29(96.7%) antibiotic followed by meropenem 23(76.7%), amikacin 23(76.7%), pipercillintazobactum 22(73.3%). Ceftazidime is the least sensitive 16.7% antibiotic. Other results are depicted in the picture. Table 4 shows that Cefepime, Ceftriaxone, Ceftazidime, Ciprofloxacin, Amikacin, Pipercillin-tazobactum, Meropenem and Colistin drug resistant were not statistically significant (p>0.05) between E. coli and Klebsiella group Figure 4.
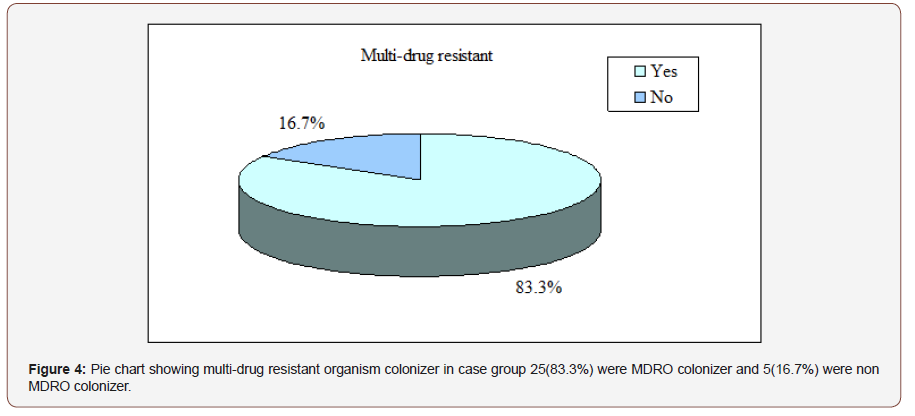
Table 4:Association between drug resistant with surveillance stool organism in case group (n=30).
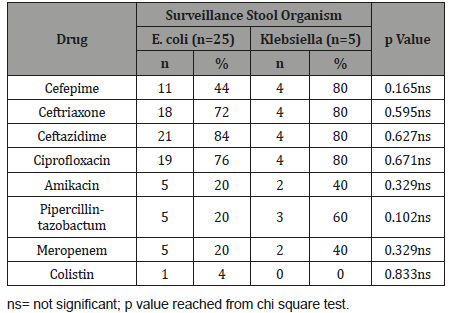
Table 5 shows relation between uses of prophylactic antibiotic with multi-drug resistant in case group, it was observed that 60.0% of multi-drug resistant patients had no history of prophylactic antibiotic and 40.0% of multi-drug resistant patients had history of using prophylactic antibiotic. The association was not statistically significant (p>0.05).
Table 5:Association between use of prophylactic antibiotic with multidrug resistant in case group (n=30).
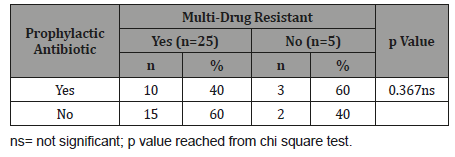
Table 6 shows that multi-drug resistant with type of leukaemia in case group, it was observed that AML was found in 16(64.0%) patients, ALL in 8(32.0%) and mixed leukaemia in 1(4.0%) among them who are multi-drug resistant. The association was not statistically significant (p>0.05).
Table 6:Association between MDRO colonization with type of leukaemia in case group (n=30).
Table 7 shows multi-drug resistant with disease status in case group, it was observed that 15(60.0%) patients received induction chemotherapy, 3(12.0%) patients received consolidation chemotherapy, 4(16.0%) patients was newly diagnosed & received no chemotherapy and 3(12.0%) patients received re-induction chemotherapy among who are multi-drug resistant in case group. The association was not statistically significant (p>0.05).
Table 7:Association between multi-drug resistant with disease status in case group (n=30).
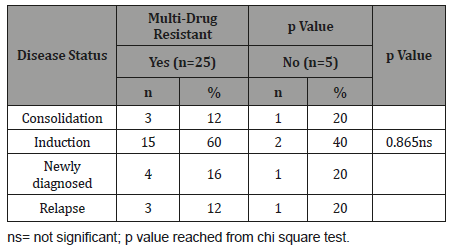
Table 8 shows 20(66.7%) patients in cases and 25(83.3%) patients in controls had ANC onset neutropenic fever <0.50×103/ μl. The difference was not statistically significant (p>0.05) between two groups.
Table 8:Distribution of the study patients according to ANC onset neutropenic fever (n=60).
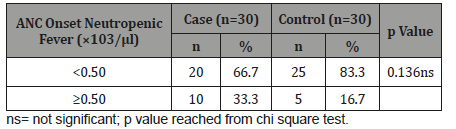
Table 9 shows in case group majority 19(63.3%) patients had defervescence of fever ≤1 day and in control group 21(70.0%) had defervescence of fever >1 day. The mean defervescence of fever was found 2.0±1.9 days in cases and 3.3±2.1 days in controls. Early defervescence of fever (≤1 day) was significantly (p<0.05) higher in case group than control group.
Table 9:Distribution of the study patients according to defervescence of fever after used of initial antibiotic therapy (n=60).
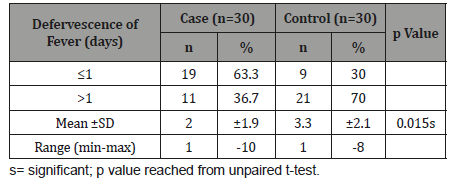
Table 10:Distribution of the study patients according to duration of antibiotic used (n=60).
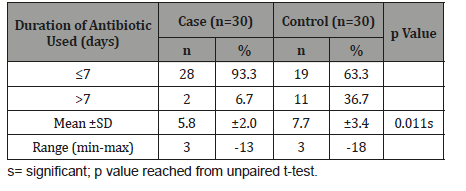
Table 10 shows in both groups majority patients used antibiotic ≤7 days, which was 28(93.3%) in cases and 19(63.3%) in controls. The mean duration of antibiotic used was found 5.8±2.0 days in cases and 7.7±3.4 days in controls. Duration of antibiotic used was significantly (p<0.05) longer in control group than case group Figure 5.
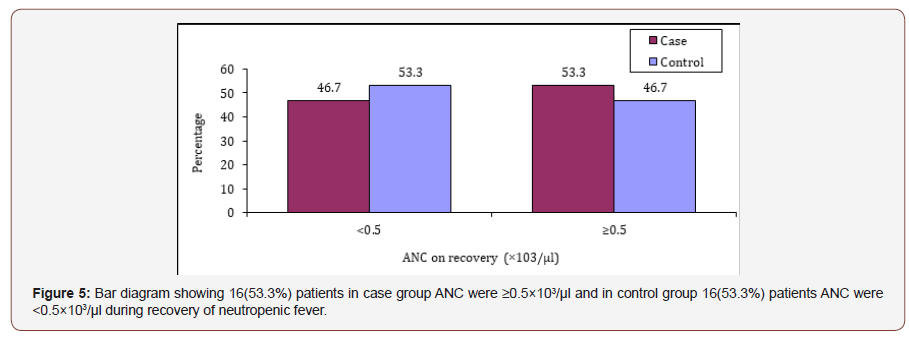
Table 11 shows that 24(80.0%) patients were found developed neutropenic fever after chemotherapy in case group and 29(96.7%) in control group. The differences was not statistically significant (p>0.05) between two groups.
Table 11:Distribution of the study patients according to receiving chemotherapy.
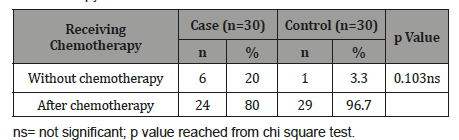
Table 12 shows that 4(25.0%) patients in ALL, 22(61.1%) in AML and 1(100.0%) in mixed leukaemia developed neutropenic fever on day 11-15 after chemotherapy. The difference was not statistically significant (p>0.05) among three groups.
Table 12:Association between onset neutropenic fever after chemotherapy with type of leukaemia (n=53).
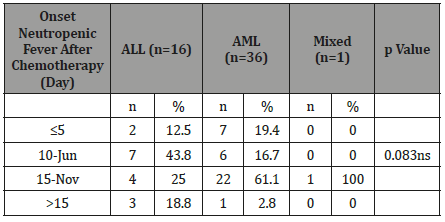
Table13:Distribution of the study patients according to response to antibiotic therapy (n=60)..
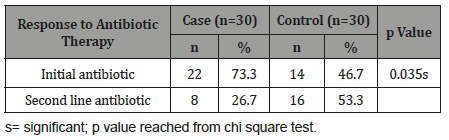
Table 13 shows 8(26.7%) patients in cases and 16(53.3%) in controls group needed second line antibiotic therapy. The differences was statistically significant (p<0.05) between two groups.
Discussion
Patient with haematological malignagcy are immune compromised and frequently developed neutropenic fever due to disease condition and or chemotherapeutics and they routinely get prophylactic antibiotic hence high risk for carrying multidrug resistance microorganism. This is a study carried out with an aim to observe the outcome of surveillance stool culture-based selection of antibiotics during febrile neutropenia. A total 60 acute leukaemia patients after admission in department of Hematology who developed febrile neutropenia with or without chemotherapy between April 2017 to March 2018 were enrolled in this study. The present study findings were discussed and compare with the previously published relevant studies. In this study it was observed that 76.7% patients belonged to age ≤ 40 years in cases and 73.3% patients in controls. The mean rank of age was found 32.02 years in cases and 28.98 years in controls. The difference was not statistically significant (p>0.05) between two groups. Paul, et al. [1] observed in their study in median age was 26 years (range 3-57) years. In this study majority patients 56.7% were male in cases (surveillance based) and 50% in control group (Empirical) difference was not statistically significant (p>0.05) between two groups. Paul, et al. [1] showed that 71.57% were male and 28.42% were female. In this study it was observed that majority patients 60% were suffering from AML in cases (surveillance based) and 80% in control (Empirical) group. The difference was not statistically significant (p>0.05) between two groups. Paul, et al. [1] found 39.44% patients were AML, 24.21% were ALL, 10% were CML, 9.47% Were Aplastic Anaemia, 6.31% were MDS, 10.52% were other who received allogenic bone marrow transplant. In this study, it was observed that majority patients received remission of induction therapy which was 17(56.7%) in cases and 22(73.3%) in controls. 4(13.3%) patients in case and 6(20.0%) in control group received consolidation therapy. 5(16.7%) and 2(6.7%) in cases and controls respectively received no chemotherapy.
The difference was not statistically significant (p>0.05) between two groups. In our study it was observed that majority patients 25(83.3%) had E. coli and 5(16.7%) had klebsiella in their surveillance stool culture. Those patients who grew E. coli in their surveillance stool culture showed 84% resistant to ceftazidime ,76% to ciprofloxacin, 72% to ceftriaxone, 44% to cefepime, 20% to Amikacin, Pipercillin- tazobactum, Meropenem and 4% resistant to colistin. Among those who had klebsiella in their surveillance stool culture showed 80% resistant to Ceftazidime, Ciprofloxacin, Ceftriaxone, Cefepime, 60% resistant to Pipercillin- tazobactum, 40% to Meropenem and no resistant to colistin. Drug resistant in between E. coli & klebsiella group were not statistically significant (p>0.05), in our study, 25 (83.3%) were multi drug resistant organism colonizer and 5(16.7%) were not multidrug resistant organism colonizer.
Barman, et al. [2] in her study screened stool samples of 61 patients for carbapenem resistant Enterobactriaceae. Out of which 33(54.09) were carbapenem resistant and 28 (45.90%) were carbapenem sensitive Enterobactriaceae. Thacker, et al. [3] found in their study 197 (34.4%) patients were colonized with two organisms and 30(5.2%) by three organism 334(58.4%) showed ESBL Enterobacteriaceae, of which 165(49.5%) were ESBL sensitive to beta lactum with beta-lactamase inhibitors combination and 169(50.5%) were resistant to combination. 116(20.2%) were resistant to carbapenem (CRE). 65(11.4%) patient had enterococci resistant to vancomycin. Only 63(21%) patients were colonized by a sensitive organism in their baseline surveillance cultures. Daw et al. (1988) found 48(63.15%) patient developed E. coli, Klebsiella 26(34.21%) patient, Entrobacter cloacae 21(27.63%), Pseudomonas aeruginosa 8(10.52%), Enterobacter 8(10.52%) out of 76 patients in their surveillance stool culture. Wingard, et al. [4] observed E. coli and Pseudomonas aeruginosa were the most frequent organism resistant to antibiotic, isolated and together they accounted for 48% of all resistant organism. In our study 10(40%) patient has history of prophylactic antibiotic use among those who were MDRO colonizer the association was not statistically significant (p>0.05) in between two groups. In our study majority patient 16(64%) belongs to AML, 8(32%) ALL group who are multidrug resistant the association was not statistically significant (p>O.05) in between two groups. In the present study majority patient 15(60%) received remission of induction therapy, 3(12%) received consolidation therapt, 4(16%) received no chemo therapy and 3(12%) received re-induction chemotherapy among those who are MDRO colonizer. The association was not statistically significant (p>0.05) in between two groups. In our study it was observed that 20(66.7%) patient in case and 25(83.3%) patient in control group ANC were <0.5x103 /μL during onset of neutropenic fever. In the present study majority patient 19(63.3%) had defervescence of fever ≤ one day in case group & 21(70%) patient in control group had defervescence of fever more than one day. Early defervescence of fever ≤ one day was statistically significant (p≤ 0.05) higher in case group then control group. Paul, et al. [1] observe 67(56.8%) patients in case group (surveillance-based selection) and 18(37.5%) patients in control group had defervescence of fever ≤one day and the different was statistically significant (p≤0.05). In the current study, we found majority patients had to used antibiotic for ≤7 days, which was 28(93.3%) in cases and 19(63.3%) in controls. The mean duration of antibiotic used was found 5.8±2 days in case and 7.7±3.4 days in control group. Duration of antibiotic used was significantly longer (p=0.011) in control group then case group. Paul, et al. [1] found in his study the medium duration total antibiotic used 17.5 days in group 1 (empirical) and 18 days in group 2 (surveillance-based selection) and p value was not statistically regarding duration of antibiotic used. In the present study it was found 16 (53.3%) patients ANC were ≥0.5x103μ L in case group and 16 (53.3) patients ANC were <0.5x103μ L in control group. The different was not statistically regarding (p>0.05). In the current study it was observed that 24(80%) patients in case group, 29(96.7%) in control group developed neutropenic fever after chemotherapy. The different was not statistically (p>0.05). In the present study it was observed that 22(61.1%) AML patient and 4(25%) ALL patients developed neutropenic fever on day 11 to day 15 after chemotherapy. The different was not statistically (p>0.05). In our study it was found that 22(73.3%) patients in case and 14(46.6%) patients in control group respond to initial antibiotic therapy after developing fever neutropenic fever on the different was statistically significant (p<0.035). Paul, et al. [1] found in his study defervescence of fever to first line antibiotic therapy occurred in 23 (48%) in group 1 (Empirical based) and in group 2 (Surveillance based selection) it occurred in 78 (66%) patients (p=0.05). In the present study it was observed that in case group 8(26.7%) patients required second line antibiotic, compared to 16(53.3%) patients in control group. The different was statistically significant (p<0.05) between two groups. Paul, et al. [1] observed in his study that 34.5% patients required second line antibiotic among those whom surveillance based antibiotic selection was given compared to 54% patients in whom empirical antibiotic was given during febrile neultropenia without surveillance stool culture. The different was statistically significant (p<0.0294). In our study it was observed that 28(93.3%) patients recovered from febrile neutropenia in both case and control group and the different was not statistically significant (p>0.05) between two groups. Paul, et al. [1] also found in his study there was no significant different in infection related mortality between two groups.
Conclusion
Prevalence of MDRO colonization in stool surveillance specially to CRE and other broad-spectrum antibiotic use to treat neutropenic fever is alarmingly high in patient with acute leukaemia which has grave implication that can affect the treatment outcome. Selection of antibiotic from individual stool antibiogram leads to earlier defervescence of fever in higher number of patients, reduce the need for second line antibiotic, reduce duration of use of antibiotic during neutropenic fever in patient with acute leukaemia.
Acknowledgment
None.
Conflict of Interest
No conflict of interest.
References
- Paul D, Ganesh A, Bhat V, Bonda A, Zanwar S, Bhat V (2016) Impact of surveillance stool culture guided selection of antibiotics in allogenic hematopoietic stem cell transplant patients. Blood 128(22): 3389.
- Barman P, Chopra S, Chaema L (2015) Stool antibiogram as an antibiotic therapy determinant in Haematological malignancy. Journal of Patient safety and infection control 47: 2-3.
- Thacker N, Perira N, Banavail S, Narula Vora T, Chinnaswansy C, et al. (2014) Alarming prevalence of community acquired multidrug resistant organism and implications for therapy: A prospective study. Indian J Cancer 51(4): 442-446.
- Wingard RJ, Dick J, Charache P, Saral R (1986) Antibiotic resistant bacteria in surveillance stool cultures of patients with prolonged neutropenia. Antimicrob Agents Chemother 30(3): 435-439.
-
Mesbah Uddin Ahmed, Aklima Khanam, Shafiqul Islam, Mohammmad Nazmul Islam, Fahmida Ahmed, et al. Outcome of Surveillance Page 8 of 8 Stool Culture Guided Selection of Antibiotics during Febrile Neutropenia in Patient with Acute Leukaemia. Curr Tr Clin & Med Sci. 1(3): 2020. CTCMS.MS.ID.000515.
-
MDRO colonization, Febrile Neutropenia, Acute leukaemia, Surveillance, Fever, Antibiotic, Haematology, Antibiotic therapy, Malignagcy, Chemotherapy
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






